Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Planning and Governance of COVID-19 Procurements to Increase the National Medical Stockpile

Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- This audit is one of five performance audits conducted under phase one of the ANAO’s multi-year strategy that focuses on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic.
- The Department of Health (Health), with the assistance of the Department of Industry, Science, Energy and Resources (DISER), undertook emergency procurements of personal protective equipment (PPE), ventilators and COVID-19 test kits for the National Medical Stockpile (NMS).
- The Australian Parliament and public require assurance that public resources were used properly and that the procurement requirement has been met.
Key facts
- The NMS is a reserve of medicines, vaccines, antidotes and PPE for use in response to a public health emergency, to be deployed as a supplement to state and territory stockpiles.
- At the outset of the COVID-19 pandemic the NMS was valued at $123 million.
What did we find?
- The procurement requirement for PPE and medical equipment was met or exceeded. Elements of Health’s procurement planning for the NMS could be improved.
- Health’s pre-pandemic procurement planning for the NMS was partially effective.
- Health’s and DISER’s planning and governance arrangements for the procurements in response to the COVID-19 pandemic were effective.
- Procurement of PPE for the NMS was approximately aligned with overall national health system demand.
What did we recommend?
- The Auditor-General made four recommendations to Health aimed at basing NMS procurement decisions on key strategic risks; collaborating with states and territories to document procurement priorities; developing a mechanism for sharing stockpile information between jurisdictions; and establishing protocols for emergency NMS procurements.
$3.23 billion
Total funding provided to Health between March and May 2020 to procure PPE and medical equipment
54
Number of contracts for PPE, medical equipment and COVID-19 test kits as at 31 August 2020
1.3 billion
Total items of PPE procured for the NMS as at 31 August 2020
Summary and recommendations
Background
1. Since its emergence in late 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic that is impacting on human health and national economies. From February 2020 the Australian Government commenced the introduction of a range of policies and measures in response to the emergence of COVID-19 that included:
- travel restrictions and international border control and quarantine arrangements;
- delivery of substantial economic stimulus, including financial support for affected individuals, businesses and communities; and
- support for essential services and procurement of critical medical supplies.
2. The National Medical Stockpile (NMS) is a reserve of pharmaceuticals, vaccines, antidotes and personal protective equipment (PPE) for use during the national response to a public health emergency that could arise from natural causes or terrorist activities. It is meant to supplement state and territory supplies in a health emergency. Between 3 March and 1 May 2020 $3.23 billion in funding was provided to the Australian Government Department of Health (Health) to procure medical supplies, namely PPE and medical equipment, for the NMS. Procurement activity peaked in April 2020, with the last contract for NMS supplies prior to 31 August 2020 entered into on 14 August 2020.
3. The Department of Industry, Science, Energy and Resources (DISER) began assisting Health with the COVID-19 NMS procurements on 2 March 2020. On 18 March 2020 the Acting Secretary of Health decided, under paragraph 2.6 of the Commonwealth Procurement Rules (CPRs), that the CPRs would not apply to the COVID-19 NMS procurements. Paragraph 2.6 allows the accountable authority to decide this in a range of circumstances, including to protect human health.
Rationale for undertaking the audit
4. The COVID-19 pandemic and the pace and scale of the Australian Government’s response impacts on the risk environment faced by the Australian public sector. This audit is one of five performance audits conducted under phase one of the ANAO’s multi-year strategy that will focus on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic.1
5. A challenging procurement environment, as well as the decision to not apply the CPRs, created additional risks to the proper use of public resources and achievement of procurement outcomes for the COVID-19 NMS procurements. The Australian Parliament and public require assurance that the procurement requirement has been met through the planning and governance arrangements that Health and DISER established in conducting the procurements.
Audit objective and criteria
6. The audit examined whether the COVID-19 NMS procurement requirement was met through effective planning and governance arrangements.
7. To form a conclusion against the audit objective, the following high level criteria were adopted:
- Was pre-pandemic procurement planning for the NMS effective?
- As part of the Australian Government’s COVID-19 response, was the planning and governance of the NMS procurements effective?
- Was the COVID-19 NMS procurement requirement for PPE and medical equipment met?
Conclusion
8. The COVID-19 NMS procurement requirement for PPE and medical equipment was met or exceeded. Elements of Health’s procurement planning for the NMS could be improved.
9. Health’s pre-pandemic procurement planning for the NMS was partially effective. Procurement planning was partially risk-based. Agreement with states and territories about stockpiling responsibilities was not documented and stockpile information was not adequately shared. There were no protocols for emergency procurements.
10. Health’s and DISER’s NMS procurement planning and governance arrangements in response to the COVID-19 pandemic were effective. Both entities had elements of a plan for meeting the requirement, established fit for purpose governance arrangements and considered risks.
11. The COVID-19 NMS procurement requirement was not clearly specified for PPE, swabs and COVID-19 tests. Procured quantities for the NMS were approximately aligned with overall national health system demand estimates for all items where demand modelling was undertaken, suggesting the procurement requirement was met or exceeded.
Supporting findings
Pre-pandemic procurement planning for the National Medical Stockpile
12. Health’s procurement planning for the NMS was partially risk-based. A strategic plan for the NMS did not consider procurement in detail, but did establish an overarching framework for key risks to be considered in management decisions, including procurement decisions. A Replenishment Plan set out procurement priorities that were focused on chemical, biological, radiological or nuclear (CBRN) threats and an influenza pandemic and did not address other potential health threats. Procurement planning documents did not provide a risk-based rationale for the quantity of PPE to be procured and held within the NMS and Health did not consider potential risks to PPE supply chain security during an emergency.
13. NMS procurement planning was not adequately coordinated with the states and territories in light of the objective to ‘supplement’ and work ‘in concert’ with state and territory stockpiles. Health does not have a documented agreement with the states and territories about stockpiling and there was a lack of regular and systematic information sharing about stockpiles with the states and territories.
14. Strategic planning for the NMS did not adequately prepare for emergency procurements. High level plans for responding to a disease occurrence do not provide specific guidance on conducting emergency NMS procurements and, despite the NMS’s core function as an emergency mechanism, Health had not developed specific protocols for conducting these procurements or for coordinating the multi-jurisdictional procurement response.
Planning and governance of COVID-19 National Medical Stockpile procurements
15. Health’s planning for the COVID-19 NMS procurements was fit for purpose. It did not develop a strategic or operational procurement plan but elements of a plan — such as definition of objectives, timeframes and procurement method — were incorporated in documentation. DISER’s operational planning for the procurement activities was also fit for purpose. It did not develop an overarching operational plan for its involvement but taskforces developed, used and shared process maps, templates and checklists to guide procurement activities.
16. Health’s and DISER’s internal and cross-departmental governance arrangements for the COVID-19 NMS procurements were fit for purpose. Respective roles between Health and DISER were not documented but were broadly understood. Both departments used a flexible taskforce approach to manage the procurements, involved procurement advisory services and actively engaged executive management in decision-making. There was a process for managing conflicts of interest in both departments, however, a requirement for specific conflict of interest declarations for the NMS procurements was introduced late and incompletely adhered to.
17. Health and DISER assessed and treated risks to the proper use and management of public resources in the COVID-19 NMS procurements and to procurement outcomes. Health did not conduct an overarching assessment of risk in relation to COVID-19 NMS procurement activity and risk treatments for individual procurements were not well documented. Both departments considered procurement risks in a number of their implementation activities.
18. When conducting the COVID-19 NMS procurements, Health applied the CPRs appropriately. Health officials informed the delegate of the use of paragraph 10.3(b) of the CPRs when seeking approval to commit funds through limited tender and sought the approval of the Acting Secretary of Health to invoke paragraph 2.6 to not apply the CPRs to the procurements. No alternative procurement framework for the COVID-19 NMS procurements was specified by the Acting Secretary. The Acting Secretary revoked the application of paragraph 2.6 when it was no longer necessary.
Meeting the COVID-19 National Medical Stockpile procurement requirement
19. In formulating the NMS procurement requirement, demand estimates and supply chain issues were considered by Health and DISER. However, due to the dynamic situation and late and partial information about existing national stocks of PPE, only the ventilator procurement requirement was specified clearly. In the absence of a specified procurement requirement, Health and DISER officials understood the requirement was to procure as much PPE as possible, as quickly as possible.
20. The NMS procurement requirement for invasive ventilators was exceeded. In the absence of a specific procurement target for PPE and swabs, the ANAO compared procurements of PPE and swabs to national health system demand estimates and found that the NMS procurement requirement for PPE and swabs was met, or exceeded once procurements by other actors including the states and territories are taken into account. The ANAO was unable to determine if the procurement requirement for COVID-19 tests was met due to no specified requirement or comparable demand estimates.
Summary of entity responses
21. Health’s and DISER’s summary responses to the report are provided below and their full responses are at Appendix 1. State and territory health departments’ responses to a report extract are also shown at Appendix 1.
Department of Health
The Department of Health (the department) notes the findings in the report and agrees with the recommendations relating to COVID-19 procurement for the National Medical Stockpile (NMS).
As for many people across Australia and the world, 2020 has been an extraordinary year which has seen a 1-in-100 year pandemic ravage Australia’s economy and put incredible pressure on Australia’s health system, especially its health professionals. The department has been at the forefront of the Australian Government’s response to the COVID-19 pandemic, including being focused on procuring the necessary personal protective equipment (PPE) and medical equipment and supplies to support Australia’s national and collaborative response to the COVID-19 pandemic.
Since the start of the pandemic to 30 October 2020, the department dispatched from the NMS:
- Over 78 million masks (both surgical and P2/N95 respirators), including:
- Over 43 million to states and territories; and
- Nearly 19 million to aged care.
- Over 12 million gloves;
- Over 5 million gowns; and
- Over 4.6 million goggles/face shields.
As part of this national health response, the traditional role of the NMS pivoted to provide additional assistance to ensure critical supplies could be procured and utilised in support of the frontline response. Unlike what we are sadly seeing internationally, our national response has seen a significant reduction in the impact of the novel coronavirus, notwithstanding the tragic passing of 907 people in Australia (as at 23 November 2020).
It was pleasing to note the ANAO found that the procurement requirement for PPE and medical equipment was met or exceeded, and procurement of PPE for the NMS was approximately aligned with overall national health system demand. Australia has not, during this pandemic, been in a position where clinically recommended PPE has not been able to be supplied to a health worker. This is not the case for many other countries in the world.
I am very proud of the Department of Health’s contribution to this pandemic response and the extension of the NMS to support the health response has been a key part of this.
The department notes the ANAO has identified areas where improvements can be made, including pre-pandemic planning, collaboration and establishing emergency procurement protocols for the NMS.
The department will work through each of the areas identified by the ANAO and notes the NMS Review, which is already underway, will also take these findings into account along with other Government initiatives. Once the review is complete, the department will seek a decision from Government on the role of the NMS into the future.
For the first time in its history, the National Incident Room has been continuously operating for 12 months and the department continues to support the COVID-19 pandemic response. The department recognises that part of the response is taking into account the lessons that can be learnt on how things can be done better for the next day and the future. Even the smallest improvements to communication and procedures can make a huge difference during the reality of a national crisis.
Department of Industry, Science, Energy and Resources
The department notes the audit’s recommendations relating to the Department of Health, and the key messages for all Australian Government entities in respect of governance and risk management.
The department acknowledges the report findings which confirm — inter alia — that the procurement requirement for personal protective equipment (PPE) and medical equipment was met or exceeded, and that both the department’s and the Department of Health’s procurement planning and governance arrangements were effective.
The COVID-19 pandemic posed many challenges. The department was pleased to support the Department of Health in procuring vital medical supplies to keep Australians safe.
I thank the Australian National Audit Office for its report and for the important work it is doing to provide assurance to the Parliament and Australian people about the proper use of public resources.
Recommendations
Recommendation no.1
Paragraph 2.23
Health’s business as usual procurement planning for the NMS be based on an analysis of strategic risks and threats, including a range of potential health emergencies, and the risk to the surety of supply chains for stockpiled items, including personal protective equipment.
Department of Health response: Agreed.
Recommendation no.2
Paragraph 2.34
Health seek jurisdictional agreement about, and document, the respective objectives of the Commonwealth and state and territory stockpiles and the roles and responsibilities of each jurisdiction, including for stockpiling specific items.
Department of Health response: Agreed.
Recommendation no.3
Paragraph 2.40
Health establish a mechanism for regular sharing of information between jurisdictions about stockpile inventories that will function in both business as usual and emergency conditions.
Department of Health response: Agreed.
Recommendation no.4
Paragraph 2.46
Health put in place a strategic procurement, management and distribution plan for the NMS that includes protocols for emergency procurements.
Department of Health response: Agreed.
Key messages from this audit for all Australian Government entities
Below is a summary of key messages that have been identified in this audit and may be relevant for the operations of all Australian Government entities.
Governance and risk management
1. Background
Introduction
1.1 Since its emergence in late 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic that is impacting on human health and national economies. On 21 January 2020 the Australian Government listed COVID-19 as a disease of pandemic potential under the Biosecurity Act 2015.2 The World Health Organisation declared COVID-19 to be a ‘public health emergency of international concern’ on 30 January 2020.
1.2 From February 2020, the Australian Government commenced the introduction of a range of policies and measures in response to the emergence of COVID-19. On 18 March 2020, in response to the pandemic in Australia, the Governor-General of the Commonwealth of Australia declared that a human biosecurity emergency exists.3
1.3 The Australian Government’s health and economic response has included:
- travel restrictions and international border control and quarantine arrangements;
- delivery of substantial economic stimulus, including financial support for affected individuals, businesses and communities; and
- support for essential services and procurement of critical medical supplies for the National Medical Stockpile (NMS).
1.4 4With the release of the 2020–21 Budget on 6 October 2020, the Australian Government reported it had committed $507 billion in overall support since the onset of the pandemic, including $272 billion over five years (2019–20 to 2023–24) in direct economic and health support.
The National Medical Stockpile
1.5 The National Medical Stockpile Strategic Plan 2015–19 states that the purpose of the NMS is to be a ‘strategic reserve of pharmaceuticals, vaccines, antidotes and personal protective equipment (PPE) for use during the national response to a public health emergency which could arise from natural causes (risks) or terrorist activities (threats).’ The NMS is intended to supplement state and territory supplies in a health emergency. In ‘non-emergency conditions, or business as usual’, the operational goal of the NMS is to ‘maintain capability for immediate deployment within an emergency’, using a ‘cost effective and risk appropriate system for operations.’
1.6 The Australian Government Department of Health (Health) manages the NMS. The Australian Health Protection Principal Committee (AHPPC) advises the Australian Health Ministers’ Advisory Council on health protection matters and mitigates emerging health threats related to infectious diseases, the environment and natural and human made disasters.5 The AHPPC provides policy oversight for the NMS.
1.7 The NMS is a potential response measure in a variety of national health response plans, including: the Australian Health Management Plan for Pandemic Influenza (AHMPPI); the National Health Emergency Response Arrangements; the Health Chemical Biological Radiological Nuclear Incidents of National Consequence; the Emergency Response Plan for Communicable Disease Incidents of National Significance; and, more recently, the Australian Health Sector Emergency Response Plan for Novel Coronavirus (COVID-19) (the COVID-19 Plan).
1.8 The NMS was established in 2002 as a reserve of medical supplies for use against potential chemical, biological, radiological or nuclear (CBRN) threats.6 Since its establishment the purpose and use of the NMS has changed to reflect evolving public health risks and national security threats. After outbreaks of Severe Acute Respiratory Syndrome (SARS) in 2002 and H5N1 influenza (avian flu) in 2004 in East Asia, the Australian Government allocated $124 million to the NMS for the purchase of anti-viral medicines. In 2005–06 the Australian Government released the AHMPPI and provided $135 million to the NMS to expand its capacity to respond to an influenza pandemic, including through the purchase of antivirals. The NMS was valued at $117 million at 30 June 2019 (Figure 1.1) and $123 million at 31 December 2019 (Figure 1.2).
Figure 1.1: Purchases, deployments, impairments and value of the NMS, 2004–2019

Note: All values at 30 June. Impairment refers to a permanent reduction in the value of the NMS due to damage, expiry or other loss of functionality of NMS supplies. This does not include items that have been deployed or sold.
Source: Australian National Audit Office (ANAO) analysis of Department of Health annual reports.
1.9 At 31 December 2019 the NMS contained PPE valued at about $11 million (nine per cent of the total value) and medical equipment valued at $28,000 (less than one per cent).
Figure 1.2: Value of product categories held in the NMS, 31 December 2019

Source: ANAO analysis of NMS inventory reconciliation.
1.10 In 2009–10 an outbreak of swine flu in Australia led to the first large scale deployment of the NMS; about 900,000 courses of antivirals and 2.3 million items of PPE were distributed to healthcare workers and Australian border agencies. In January 2020, 3.5 million P2/N95 respirators (P2 masks) were distributed from the NMS as part of the Australian Government’s response to a bushfire emergency in parts of Australia; this was the first time the NMS had been used for a natural disaster. As part of the COVID-19 response, between 29 January and 28 August 2020, 87.4 million items of PPE and medical equipment were deployed from the NMS to state and territory governments and public hospitals; other frontline health workers, including general practices and community pharmacies; residential aged care facilities and disability settings in the event of an outbreak; and Commonwealth agencies. Figure 1.3 shows the number of items deployed to five categories of recipient.
Figure 1.3: Number of NMS items deployed (millions) by recipient type, 29 January to 28 August 2020
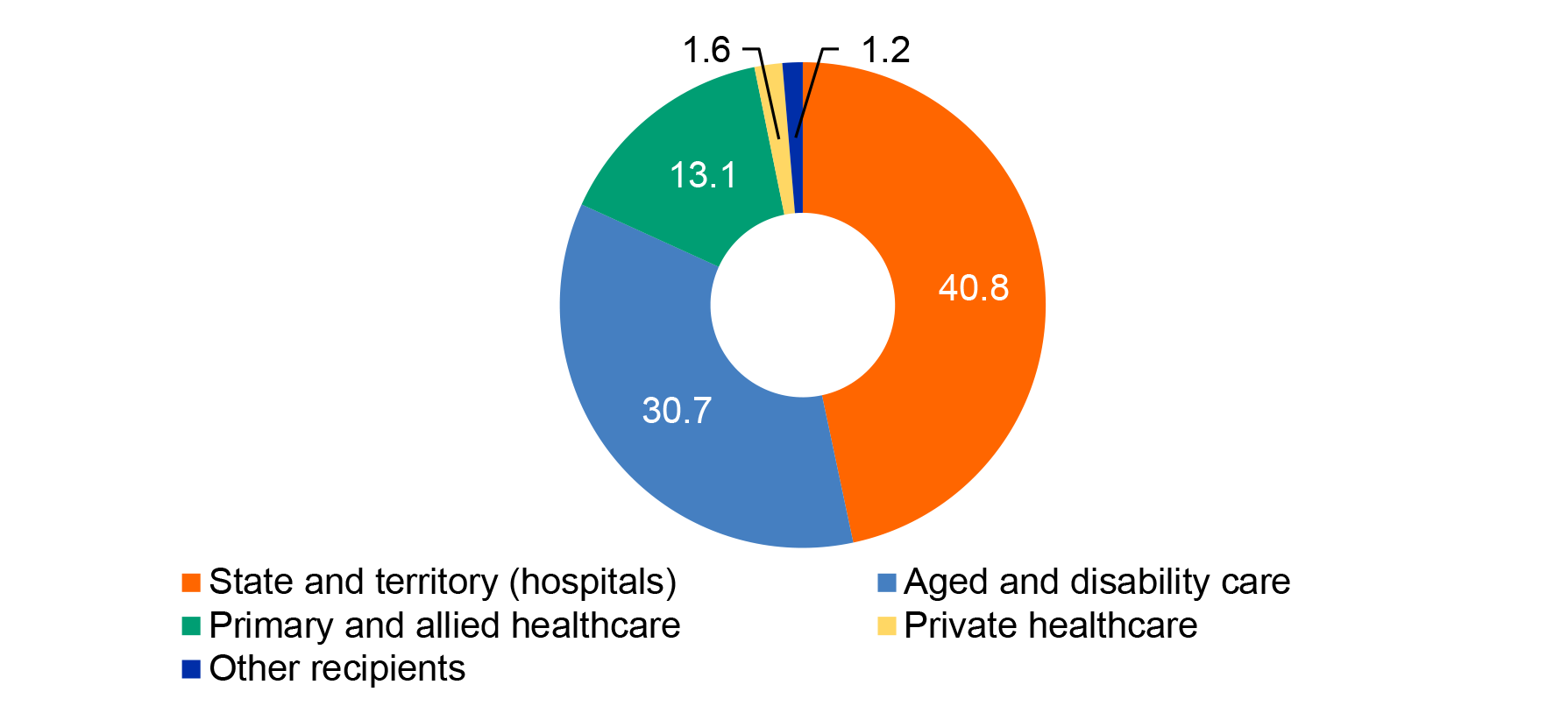
Note: ‘Private healthcare’ includes private hospitals and pathology. ‘Aged and disability care’ includes public and private residential aged care facilities. ‘Other’ includes Commonwealth agencies and testing facilities. Excludes PPE and medical equipment humanitarian assistance deployments, pharmaceuticals including antiviral medication, CBRN items and reagents.
Source: ANAO analysis of Health deployment data. The ANAO has not verified the completeness and accuracy of this data.
Procurements for the National Medical Stockpile in response to the COVID-19 pandemic
1.11 A timeline for the activation of the NMS as part of the Australian Government’s response to COVID-19 is shown at Figure 1.4.
Figure 1.4: Timeline of COVID-19 NMS procurement, to 31 August 2020
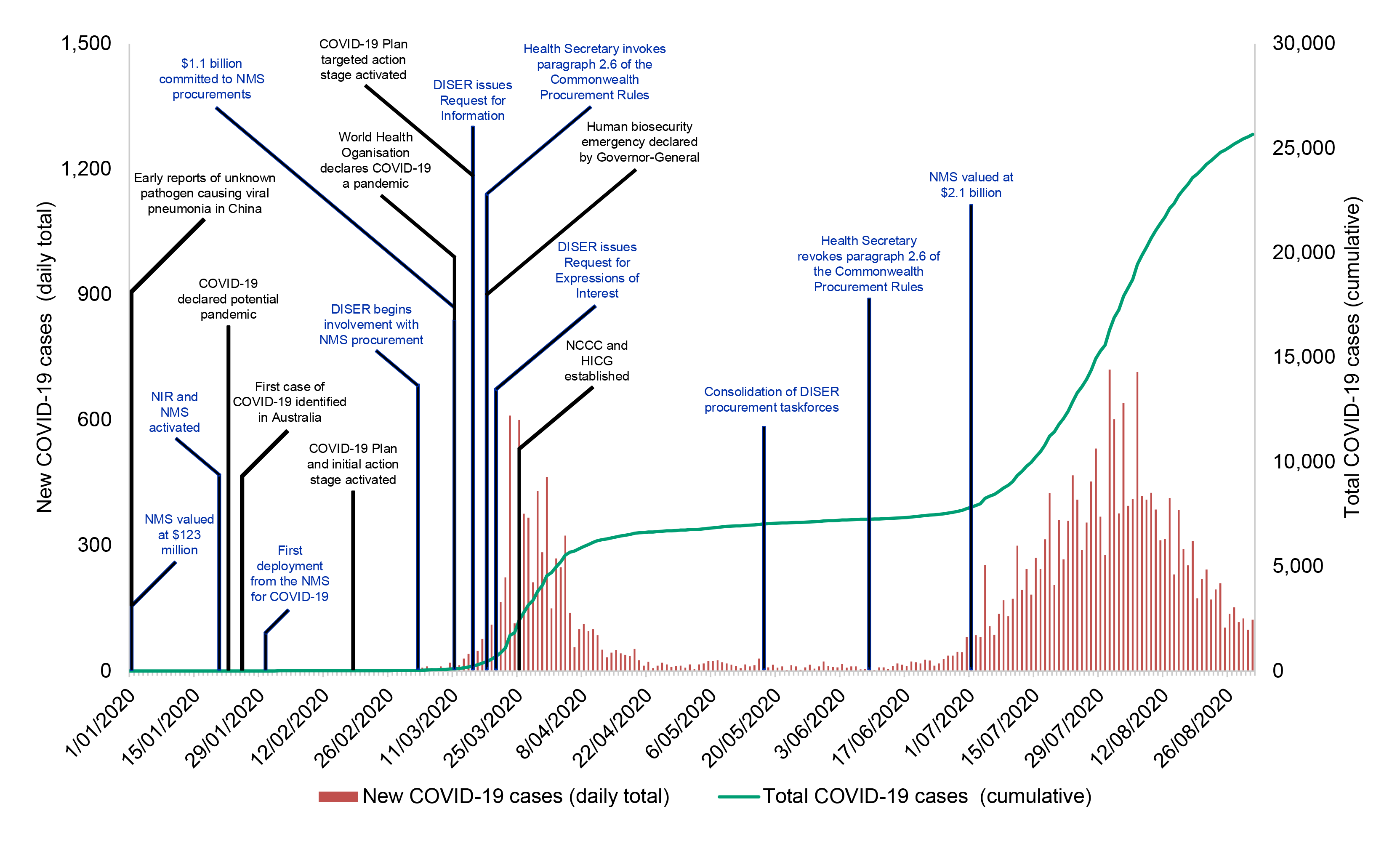
Source: ANAO analysis of Health documentation, public information and daily confirmed cases of COVID-19 in Australia from Australia: Coronavirus Pandemic Country Profile, Our World in Data, AUS, 2020, available from https://ourworldindata.org/coronavirus/country/australia?country=~AUS [Accessed 8 September 2020].
1.12 The National Incident Room was activated for COVID-19 on 20 January 2020. In late January Health turned its attention to procurement of essential medical supplies for the NMS. The Department of Industry, Science, Energy and Resources (DISER) began assisting Health with the COVID-19 NMS procurements on 2 March. Procurement activity peaked in April, with the last contract for NMS supplies prior to 31 August 2020 entered into on 14 August.
1.13 Between 3 March and 1 May 2020 $3.23 billion in funding was provided to Health to procure medical supplies for the NMS. This included $1.88 billion in various Advances to the Finance Minister on 3 March, 9 March, 3 April, and 9 April; and $1.35 billion from other funding measures.7 At 30 June 2020 the NMS was valued at $2.1 billion, 16 times its value at 31 December 2019 (refer Figure 1.2).
1.14 As potential gaps in national supply became evident, Health identified different priority products for NMS procurement and domestic production (refer Table 1.1).
Table 1.1: Priority products for procurement and domestic production, March 2020
|
Dates |
Priority 1 |
Priority 2 |
Priority 3 |
|
Priority products as at 9 March 2020 |
Masks (surgical and P2)
|
Gowns Gloves Goggles Hand sanitiser |
Waste bag closure devices (ties) Clinical waste bags Blood and fluid spill kits Mask fit test kits Thermometers |
|
Priority products as at 24 March 2020 |
Masks (surgical and P2) Ventilators Test kits and swabs Gowns Gloves Goggles Hand sanitiser |
Waste bag closure devices (ties) Clinical waste bags Blood and fluid spill kits Mask fit test kits Thermometers |
|
Source: ANAO analysis of Health and DISER documentation.
Whole-of-government response to the procurement
1.15 Health, as the accountable agency for the procurement, was the final decision-maker on all procurements and funded, negotiated, executed and managed contracts with suppliers. DISER’s role in the procurements included: identifying areas of supply chain vulnerabilities; sourcing, triaging and assessing offers to supply PPE and other medical supplies to the NMS; conducting due diligence on some offers of assistance; and drafting some contracts.
1.16 A number of other entities were involved in the COVID-19 NMS procurements, including through grants and logistical support to manufacturers and suppliers. These entities included the Department of Defence; Department of Finance; Department of Foreign Affairs and Trade; Department of Home Affairs; Department of Infrastructure, Transport, Regional Development and Communications; Commonwealth Scientific and Industrial Research Organisation (CSIRO); and Australian Trade and Investment Commission.
Procurement framework for the COVID-19 procurements
1.17 The keystone of the Australian Government’s procurement policy framework is the Commonwealth Procurement Rules (CPRs) issued by the Finance Minister under section 105(b) of the Public Governance, Performance and Accountability Act 2013 (the PGPA Act). Commonwealth officials must comply with the CPRs when conducting a procurement. Accountable Authority Instructions also set out entity-specific operational rules to ensure compliance with the rules of the procurement framework.
- ‘Division 1’ rules apply to all procurements. Achieving value for money is the ‘core rule’, or underlying principle, of the CPRs.8
- ‘Division 2’ rules apply to procurements that are at or above a relevant procurement value threshold. These rules specify that procurements must be achieved through open tender except when certain conditions and exemptions apply.
1.18 On 18 March 2020 the Acting Secretary of Health determined that the CPRs did not apply to the COVID-19 NMS procurements by invoking paragraph 2.6 of the CPRs. This provision allows the accountable authority to decide, in a range of circumstances including to protect human health, that the CPRs do not apply. In addition, a number of the procurements conducted prior to 18 March 2020 were exempted from CPR Division 2 rules under paragraph 10.3(b) — for reasons of extreme urgency. Despite the use of these provisions, the proper use and management of public resources in the procurements remained relevant under the PGPA Act.9
Rationale for undertaking the audit
1.19 The COVID-19 pandemic and the pace and scale of the Australian Government’s response impacts on the risk environment faced by the Australian public sector. This audit is one of five performance audits conducted under phase one of the ANAO’s multi-year strategy that will focus on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic, and one of two performance audits focused on procurements for the NMS.
1.20 A challenging procurement environment, as well as the decision to not apply the CPRs, created additional risks to the proper use of public resources and achievement of procurement outcomes for the COVID-19 NMS procurements. The Australian Parliament and public require assurance that the procurement requirement has been met through the planning and governance arrangements that Health and DISER established in conducting the procurements.
1.21 This audit will assist all Commonwealth entities to consider the effectiveness of their arrangements in identifying and responding to the challenges and risks associated with the rapid implementation of initiatives.
Audit approach
Audit objective, criteria and scope
1.22 The audit examined whether the COVID-19 NMS procurement requirement was met through effective planning and governance arrangements.
1.23 To form a conclusion against the audit objective, the following high level criteria were adopted:
- Was pre-pandemic procurement planning for the NMS effective?
- As part of the Australian Government’s COVID-19 response, was the planning and governance of the NMS procurements effective?
- Was the COVID-19 NMS procurement requirement for PPE and medical equipment met?
1.24 The audit scope included planning and governance of COVID-19 NMS PPE and medical supply procurements to 31 August 2020. COVID-19 NMS pharmaceutical procurement was not considered. The ANAO is conducting a second audit, due to be tabled in 2021, which is examining implementation of the COVID-19 procurements and deployments of the NMS.
Audit methodology
1.25 The audit involved:
- reviewing entity documentation including contracts and correspondence;
- examining the business information system for NMS inventory management;
- interviewing officers from relevant business areas within Health and DISER;
- interviewing officers from state and territory health authorities; and
- reviewing seven submissions from organisations and individuals with an interest in PPE supply chains in Australia.
For the purpose of this audit, procured products are grouped into four broad categories: masks; other PPE; ventilators; and COVID-19 testing kits and components.10
1.26 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $424,000.
1.27 The audit team was Christine Chalmers, Zoe Pilipczyk, Irena Korenevski, William Richards, Matthew Rigter, Zhiying Wen, Song Khor, Ann MacNeill, Ammar Raza, Lesa Craswell, Rahul Tejani and Deborah Jackson.
2. Pre-pandemic procurement planning for the National Medical Stockpile
Areas examined
This chapter examines whether procurement planning for the National Medical Stockpile (NMS) was effective prior to the coronavirus disease 2019 (COVID-19) pandemic.
Conclusion
Health’s pre-pandemic procurement planning for the NMS was partially effective. Procurement planning was partially risk-based. Agreement with states and territories about stockpiling responsibilities was not documented and stockpile information was not adequately shared. There were no protocols for emergency procurements.
Areas for improvement
The ANAO made four recommendations to the Department of Health (Health) aimed at improving NMS procurement planning by basing decisions on key strategic risks; collaborating with states and territories to document respective procurement responsibilities; developing a mechanism for sharing stockpile information between jurisdictions; and establishing protocols for emergency procurements.
2.1 The Australian Health Management Plan for Pandemic Influenza (AHMPPI) specifies that the Australian Government is responsible for ensuring that the resources and systems required to mount an effective response to a pandemic are ‘readily available’ through the NMS, among other measures. According to the National Medical Stockpile Strategic Plan 2015–19 (the 2015–19 NMS Strategic Plan), the NMS is intended to ‘reduce security risks and support rapid responses, and [increase] Australia’s level of self-sufficiency for emergency items during times of high global and domestic demand and service delivery pressures.’
2.2 The ANAO examined pre-pandemic procurement planning for the NMS, comprising whether:
- there was risk-based procurement planning for the NMS;
- procurement planning for the NMS was adequately coordinated with the states and territories given its objective to be a ‘supplementary’ stockpile to state and territory stockpiles; and
- there was planning for emergency procurements.
Was there risk-based procurement planning for the National Medical Stockpile?
Health’s procurement planning for the NMS was partially risk-based. A strategic plan for the NMS did not consider procurement in detail, but did establish an overarching framework for key risks to be considered in management decisions, including procurement decisions. A Replenishment Plan set out procurement priorities that were focused on chemical, biological, radiological or nuclear (CBRN) threats and an influenza pandemic and did not address other potential health threats. Procurement planning documents did not provide a risk-based rationale for the quantity of personal protective equipment (PPE) to be procured and held within the NMS and Health did not consider potential risks to PPE supply chain security during an emergency.
2.3 Procurement planning should be documented and based on analysis of strategic risks and threats. The ANAO examined whether there was a procurement plan in place for the NMS prior to the COVID-19 pandemic emergency and risk was considered in procurement planning.
Was there a procurement plan?
2.4 The 2015–19 NMS Strategic Plan provided a high level strategy for the NMS. There is no current strategic plan, however Health advised the ANAO that it considered the 2015–19 NMS Strategic Plan to be still valid and guiding the operation of the NMS during the 2020 COVID-19 response and that a new policy proposal was in the process of being drafted prior to the start of 2020 for consideration in the May 2020–21 budget. Health advised that the development of a new strategic plan is subject to the outcomes of a review of the composition, modelling and coverage of the NMS, which was requested by the Australian Government in July 2020 and is due to report in June 2021.
2.5 While the 2015–19 NMS Strategic Plan addresses broad priorities and potential initiatives in relation to procurement, and provides an overarching risk framework for management of the NMS, it does not consider procurement in detail.
2.6 In the 2017–18 budget the Australian Government provided $85 million to the NMS over three years, including $75 million for the replenishment of products.11 In September 2017 a three-year Strategic Replenishment Plan (2017–18 to 2019–20) (the Replenishment Plan) was approved by the Chief Medical Officer (CMO). The Replenishment Plan allocated the budgeted funds under three broad categories of CBRN threat countermeasure items, antivirals and PPE.
Table 2.1: Budget for NMS replenishment, 2017–18 to 2019–20
|
|
2017–18 |
2018–19 |
2019–20 |
Total budget |
Per cent of total |
|
CBRN items |
$4,234,418 |
$3,738,650 |
$4,000,088 |
$11,973,157 |
16% |
|
Antivirals |
$17,998,780 |
$18,001,026 |
$17,996,829 |
$53,996,635 |
72% |
|
PPE |
$3,015,689 |
$2,992,119 |
$3,009,373 |
$9,017,181 |
12% |
|
Budget allocation |
$25,248,888 |
$24,731,795 |
$25,006,290 |
$74,986,972 |
100% |
Source: Department of Health, Three Year Strategic Plan — Replenishment of National Medical Stockpile (NMS) Items 2017–18 to 2019–20 — As of 5 September 2017, Health, 2017.
2.7 A minute to the CMO seeking approval of the Replenishment Plan indicated three procurement priorities:
- CBRN items ($12 million notional allocation for 19 CBRN items);
- Antivirals ($54 million notional allocation for treatment courses); and
- PPE ($9 million notional allocation for 12 million ‘combo P2/N95 respirators and surgical masks’ (combo masks)).
2.8 Any changes to the Replenishment Plan were to be provided to the delegate for approval. This occurred on several occasions in 2018–20, including a 13 July 2018 request to approve commitment of funds for the replenishment of combo masks.
Was risk considered in procurement planning?
2.9 Various audits and reviews of the NMS since 2007 have commented on consideration of risk in NMS procurement planning. Auditor-General Report No.53 2013–14 Management of the National Medical Stockpile noted modelling to support decision-making on PPE inventory levels had concluded that the PPE stockpile was sufficient for use in a pandemic of moderate impact but recommended that Health update the strategic management plan for the NMS to identify objectives, priorities and strategies and review the operational risk management plan to incorporate emerging risks.12
2.10 The ANAO examined the extent to which risk was discussed in meetings with various advisory bodies to Health that are identified in the NMS 2015–19 Strategic Plan. In the 2015–19 NMS Strategic Plan, the National Health Emergency Management Standing Committee (NHEMS) of the Australian Health Protection Principal Committee (AHPPC) and the CBRN Technical Panel were described as providing input on CBRN related plans and policies, including prioritisation of risks and selection of inventory.13 The NHEMS met on five occasions between March 2018 and June 2020, with an update on the NMS given at three meetings and CBRN threats discussed at two of these meetings. The CBRN Technical Panel did not meet in 2018–19 or 2019–20. Prior to the COVID-19 pandemic the NMS Advisory Group, the key advisory group to Health on the NMS, met most recently in July 2018 and March 2019.14 The meetings were not minuted, however, meeting agenda and papers do not indicate that risks and threats were to be discussed.
2.11 The ANAO also examined the extent to which risk was addressed in the 2015–19 NMS Strategic Plan and the Replenishment Plan. The 2015–19 NMS Strategic Plan provided a framework for risk consideration in NMS management and identified three levels of risk:
- Foundation risk — risks to the health system in sourcing required medical supplies in a health emergency;
- Strategic risk — risks that should be considered in identifying and prioritising response capability requirements; and
- Operational risk — risks to the management and deployment of stock to effectively enable the implementation of relevant response plans.
2.12 The NMS itself is a treatment for foundation risk. It addresses the risk to human health during a national health emergency posed by insufficient medical supplies.15
2.13 Procurement is most closely aligned to strategic risk in the 2015–19 NMS Strategic Plan. The key strategic risks relating to procurements of the NMS comprised:
- Product — the logical and justifiable selection of the most appropriate NMS products considering their importance, efficacy, safety and quality standards;
- Identification and assessment — the accurate identification and assessment of potential health emergencies, including where certain items may need to be stockpiled and to what level; and
- Supply — surety and timeliness of procured supply, including the location of manufacturing facilities and the potential for global demand surges.
Product
2.14 In 2017 the NMS held about nine million surgical and five million P2/N95 respirators (P2 masks). PPE such as gowns, gloves and goggles were not held in the NMS prior to the COVID-19 pandemic.
2.15 Neither the Replenishment Plan nor the 13 July 2018 commitment approval minute amending the Replenishment Plan provided a risk-based rationale for the approach to mask or other PPE procurement. The expenditure on antivirals is explained in the Replenishment Plan, however, the rationale for the CBRN prioritisation and quantity, and the allocation to PPE, is not explained. The 13 July minute indicated that budgeted expenditure on PPE was $7.8 million for 10.7 million masks over three years. A rationale for the 13 per cent reduction in expenditure on PPE compared to the Replenishment Plan was not provided. The minute explained that the stock of surgical masks in the NMS would expire in 2021–22 and there was no budget allocation to replace these, but that the combo masks could be used as a surgical mask if required.
Identification and assessment of potential health emergencies
2.16 In 2013 the House of Representatives Standing Committee on Health and Ageing, while commending the Commonwealth and state and territory governments on their influenza pandemic preparedness, noted that it was ‘concerned that planning for a national health emergency involving the spread of infectious disease appears to be solely focussed on pandemic influenza.’16
2.17 To mitigate the risk that potential health emergencies will not be accurately identified and assessed, the 2015–19 NMS Strategic Plan proposed ongoing threat assessments.
2.18 The possibility and implications of several types of CBRN attack were considered by the NHEMS in July 2018 and June 2019. Health advised the ANAO that threat assessments are also provided to Health and the NMS Advisory Group through annual briefings by the Australian Security Intelligence Organisation, the Defence Intelligence Organisation and the Australian Federal Police; daily briefings by the Australian Government Crisis Coordination Centre; and briefings when required by the Bureau of Meteorology and Emergency Management Australia. The ANAO found no evidence of discussion of the risk of a pandemic or the implications for stockpiling PPE.
2.19 Threat assessments to inform NMS procurement planning in the two years leading to the COVID-19 pandemic were primarily focused on CBRN threats. There is no evidence that the risk of a pandemic from a pathogen such as coronavirus informed NMS procurement priorities.
Surety of procured supply
2.20 The Global Health Security Index (GHSI) benchmarks health security capabilities for the 195 countries that are ‘States Parties’ to the World Health Organization 2005 International Health Regulations, with a particular emphasis on countries’ preparedness to counter infectious disease threats.17 In October 2019 Australia was ranked fourth out of 195 countries on the GHSI, which includes consideration of whether the country maintains and effectively deploys a stockpile of medical countermeasures. In relation to its stockpile of medical countermeasures, Australia ranked 24th out of 195 countries. Part of the rationale for Australia’s lower ranking on this category, relative to its overall ranking, was that, although Australia maintained a stockpile, it was highly reliant on products that are manufactured overseas. Prior to the pandemic, Australia produced very limited PPE domestically.
2.21 To mitigate the risk of supply chain disruptions during a global health emergency, the 2015–19 NMS Strategic Plan proposes collecting information for and conducting ‘supply risk assessments’. The Replenishment Plan and September 2017 minute to the CMO considered risks to supply of CBRN and influenza items, such as risk associated with the United States of America controlling the supply of CBRN products and risk to the supply of antivirals in the event of an influenza pandemic.
2.22 There is no evidence that the NMS considered the risk of reliance on overseas manufacturers for NMS stocks of PPE. Health advised the ANAO that it ‘is not responsible for domestic manufacturing policy or the creation of a commercial market for the wide range of PPE and various medical supplies that could potentially be required in any health response scenario.’
Recommendation no.1
2.23 Health’s business as usual procurement planning for the NMS be based on an analysis of strategic risks and threats, including a range of potential health emergencies, and the risk to the surety of supply chains for stockpiled items, including personal protective equipment.
Department of Health response: Agreed.
2.24 Health’s existing risk analysis, including partnering with relevant Government agencies, creates capacity to respond to a wide variety of potential health emergencies, noting the challenge of a novel coronavirus in this case, means by definition the specific pathogen and potential treatment were unknown.
2.25 Opportunities to consider strengthening these activities will be informed by the review of the NMS, along with other government initiatives, such as the Productivity Commission’s review of supply chains.
Was procurement planning coordinated with the states and territories?
NMS procurement planning was not adequately coordinated with the states and territories in light of the objective to ‘supplement’ and work ‘in concert’ with state and territory stockpiles. Health does not have a documented agreement with the states and territories about stockpiling and there was a lack of regular and systematic information sharing about stockpiles with the states and territories.
2.26 In the health sector, state and territory governments procure PPE to help meet health system demand. This may be held in public hospitals and in jurisdictional stockpiles. Private service providers, such as private hospitals and pathology laboratories, also procure PPE and other supplies. According to the 2015–19 NMS Strategic Plan, the NMS is intended to operate ‘in concert with medical stockpiles held by each state and territory’ by supplementing state and territory supplies in a health emergency. State and territory governments can formally request stock from the NMS in an emergency response, but are expected to maintain their own stockpiles. This co-dependency between the NMS and state and territory stockpiles means that planning for the NMS needs to be undertaken in close collaboration with the states and territories, which is acknowledged by the 2015–19 NMS Strategic Plan:
The Department cannot effectively manage and deploy the Stockpile without the cooperation and contribution of a range of other stakeholders. This means working directly and in concert with other Australian Government departments and state and territory health authorities and clinicians to ensure a consistent and comprehensive approach to stockpiling capability across Australia.
2.27 Under the AHMPPI, the Australian Government has a responsibility to ‘Coordinate development of policy, in consultation with states/territories regarding the inventory and deployment of the NMS (including conduct of modelling / research required to inform decisions).’18
2.28 A 2011 Health review of Australia’s response to the 2009 H1N1 (swine flu) pandemic found there was inconsistency between jurisdictions in stockpiling; that policies regarding the rationale, content, allocation and release of stockpiled items was not well communicated between the states and territories and the NMS; and that there was a lack of clarity regarding the respective responsibilities of the NMS and the states and territory stockpiles for providing PPE.19 The Health Industry Coordination Group (HICG), a body established by the Australian Government in March 2020 to facilitate procurement, transport, distribution and domestic production or repurposing of PPE and other medical supplies, noted that during the COVID-19 response in 2020 there were very different expectations for the role of the NMS across states, territories and industry.20
2.29 Health advised the ANAO that states and territories are responsible for stockpiling of PPE, including surgical masks, and other high turnover consumables based on four key reasons:
- the addendum to the National Health Reform Agreement 2020–25 (NHRA)21 indicates that the states and territories are responsible for system management of public hospitals, including hospital services22; it is implied from pricing models that providing hospital services includes the purchase of PPE23;
- state and territories governments can more readily cycle high use inventory, such as PPE, through the health system thereby reducing impairment and waste;
- the focus of NMS expenditure should be where the Australian Government possesses unique leverage in relation to higher cost items, such as P2 masks, which can be purchased in bulk to lower costs; and
- the focus of NMS expenditure should be where the Australian Government possesses unique authority; with respect to strategic CBRN items, only the Australian Government has the legislative authority to import products that are not registered for use in Australia.
2.30 Health also advised the ANAO that the intention to focus on P2 rather than surgical masks in the NMS is because of their potential usage, when compared to surgical masks, in a broader range of emergency settings, and because surgical masks are funded through existing funding arrangements between the states and territories and the Commonwealth under the National Pricing Model established by the Independent Hospital Pricing Authority.24
2.31 The primary mechanism for consulting with the states and territories about stockpiling for health emergencies is the NMS Advisory Group. ANAO analysis of meeting agenda and papers found that discussions are related to NMS holdings at a general level, as well as deployment and warehousing arrangements.
2.32 In December 2016 Health shared a policy paper on the ‘Use of PPE from the NMS’ with NMS Advisory Group members. The paper noted the quantities of masks being held within the NMS and Health’s intention to stockpile P2 masks only, and stated that ‘states and territories will be expected to supply surgical masks during a pandemic response.’ In 2019 Health developed a draft policy discussion paper on a national PPE stockpiling strategy to inform potential changes to the AHMPPI. The paper re-stated Health’s position regarding surgical and P2 masks, noting that its stock of P2 masks was limited and that there was a need for national planning on how stockpiles can support the non-hospital health sector during a pandemic. The paper noted that the outlined NMS mask strategy is consistent with initial discussions held between Commonwealth and state and territory representatives regarding a National Stockpiling Agreement. Although the draft 2019 policy paper was presented to the Communicable Diseases Network Australia (CDNA) it was not endorsed by the CDNA and has not been presented to the AHPPC.
2.33 While the NHRA establishes responsibilities for Australia’s public hospital system, to date, no documented agreement has been reached with the states and territories regarding a national medical supplies stockpiling strategy for a health emergency response, including specific roles and responsibilities for stockpiling. A National Stockpiling Agreement was foreshadowed in the 2015–19 NMS Strategic Plan but never eventuated. Health advised the ANAO that its position regarding mask and PPE stockpiling is ‘known and is a principle in place within the health system’, but that a stockpiling agreement will be developed as part of a review of the National Health Security Agreement. This was originally scheduled for 2020–21 but has been delayed due to the COVID-19 and other health emergencies; Health has advised the ANAO that consultation with the states and territories will be ‘absolutely essential’ to the review process.
Recommendation no.2
2.34 Health seek jurisdictional agreement about, and document, the respective objectives of the Commonwealth and state and territory stockpiles and the roles and responsibilities of each jurisdiction, including for stockpiling specific items.
Department of Health response: Agreed.
2.35 Health agrees that it is appropriate that all parties document objectives of their stockpiles. The Commonwealth notes that states and territories retain sovereignty of decision making and autonomy to prioritise on matters of budget. Collaboration will be required.
2.36 Health will, where appropriate and possible, continue to provide clarity on responsibilities, such as Commonwealth responsibility for chemical, biological, radiological and nuclear items, and jurisdictional responsibilities such as PPE, as per the National Health Reform Agreement.
2.37 Given the NMS’s role as a ‘supplementary’ stockpile, it is important for emergency planning and responsiveness that Health has a specific understanding of the items and quantities of medical supplies that states and territories hold in their stockpiles, and, conversely, that states and territories understand what is held in the NMS. A 2010–11 Department of Finance review of the NMS recommended better information sharing between Health and the states and territories regarding available inventory and distribution.25
2.38 There is no regular mechanism for collecting or collating data from states and territories on stockpile holdings. In 2016 and May 2019 Health attempted to gather information about items and brands held in jurisdictional stockpiles that were not Commonwealth owned. Following on from the 2019 investigation, at the June 2019 NHEMS meeting there was discussion of whether stock information could be shared among all jurisdictions; Health was to consider this proposal but it was not discussed at the next meeting of the NHEMS in October 2019.
2.39 Health’s information gathering in 2016 showed that New South Wales (NSW), Northern Territory (NT), Queensland (QLD) and Western Australia (WA) were stockpiling PPE. The results of the 2019 investigation, which was partly in response to requests from some states and territories to know more about items held in the NMS, showed that stockpiling across Australia was uneven, particularly in relation to gowns, goggles and gloves (Table 2.2). As this request for information about state and territory stockpiles did not provide or ask for quantities, once the COVID-19 pandemic was declared, it was unclear the extent to which the NMS in combination with other stockpiles would meet potential demand for PPE and other medical supplies.
Table 2.2: NMS and state and territory stockpiled items as reported to Health, May–July 2019
|
|
Jurisdictionsa |
||||||||
|
|
NMS |
ACT |
NSW |
NT |
QLDb |
SA |
TAS |
Vicc |
WA |
|
Masks |
✔ |
✔ |
✔ |
No stockpile |
|
✔ |
✔ |
No stockpile |
✔ |
|
Gowns/coveralls |
|
|
✔ |
✔ |
|
✔ |
✔ |
||
|
Waste bags |
✔ |
✔ |
|
|
✔ |
✔ |
✔ |
||
|
Thermometers |
✔ |
✔ |
|
|
✔ |
✔ |
|
||
|
Goggles/face shields |
|
✔ |
✔ |
|
|
✔ |
|
||
|
Gloves |
|
|
✔ |
|
|
✔ |
✔ |
||
|
Spill kits |
✔ |
✔ |
|
|
✔ |
|
|
||
|
Hand sanitiser |
|
|
✔ |
|
|
✔ |
|
||
|
Waste bag ties |
✔ |
|
|
|
✔ |
|
✔ |
||
|
Mask fit test kits |
✔ |
|
|
|
✔ |
|
|
||
|
Swabs |
|
|
✔ |
|
|
|
|
||
Note a: Does not include PPE and pharmaceuticals held in public hospitals.
Note b: Queensland Health advised the ANAO that in 2015 it commenced a process to increase stockholdings of essential PPE, resulting in the establishment of an ‘active stock management system’, which allowed for PPE in the holdings to be rotated within their expiry period and replenished, rather than a ‘static stockpile’. Queensland Health advised that when the request for stockpile holdings was received in 2019, it was limiting static stockpile holdings to minimise the wastage and disposal costs associated with expired stock. Queensland Health advised Health in 2019 that ‘the majority of our PPE stock is not specifically stockpiled; however we hold a buffer amount over and above normal day to day purposes for periods of unexpected high demand.’
Note c: The Victorian Department of Health and Human Services advised the ANAO that Victoria’s public health system operates in a ‘devolved governance model’ where individual health services are responsible for managing supplies of PPE for their staff and patients, including for emergency events, but that a state supply chain was established in response to COVID-19 to dispatch stock to health services.
Source: ANAO analysis of May 2019 Health ’survey’ of state and territory stockpiles.
Recommendation no.3
2.40 Health establish a mechanism for regular sharing of information between jurisdictions about stockpile inventories that will function in both business as usual and emergency conditions.
Department of Health response: Agreed.
2.41 The Commonwealth agrees that a universal stock holding information system would be beneficial and will progress a comprehensive arrangement with the states and territories.
Did strategic planning for the National Medical Stockpile adequately prepare for emergency procurements?
Strategic planning for the NMS did not adequately prepare for emergency procurements. High level plans for responding to a disease occurrence do not provide specific guidance on conducting emergency NMS procurements and, despite the NMS’s core function as an emergency mechanism, Health had not developed specific protocols for conducting these procurements or for coordinating the multi-jurisdictional procurement response.
2.42 Although emergency procurement is acknowledged as a possibility in the 2015–19 NMS Strategic Plan, health response plans such as the AHMPPI do not consider the potential need for procurements for the NMS in the early stages of a pandemic response. The 2015–19 NMS Strategic Plan states that in a health emergency where a required product is not stocked within the NMS an emergency procurement should be initiated where appropriate, but it does not provide further guidance regarding how to conduct that procurement.
2.43 Neither the AHMPPI, the Emergency Response Plan for Communicable Disease Incidents of National Significance, the 2015-19 NMS Strategic Plan, nor any other planning document sets out operational principles or specific protocols for an emergency procurement of medical supplies for the NMS or for a coordinated national response to procurement in business as usual or emergency conditions.
2.44 On 7 April 2020 Health acknowledged that in the COVID-19 pandemic response there was an urgent need for greater coordination between the Australian government agencies involved in PPE procurement and the states and territories, noting that all were competing for the same products. The HICG concluded that an uncoordinated approach to COVID-19 procurements across jurisdictions caused price rises in some instances, disruption to contracts and a lack of clarity on procurement priorities.
2.45 Having protocols in place prior to an emergency would help ensure that, once a threat is realised, attention can be focused on the emergency response without diverting resources to establishing systems and procedures. Emergency protocols would help ensure that procurements can occur as rapidly as possible and that key governance structures are in place at an early stage thereby optimising their value. Emergency protocols for the NMS might include up to date cross-jurisdictional and cross-entity contact lists, temporary inter and intra-governmental governance arrangements to be implemented immediately, a cross-jurisdictional and departmental communications plan and a strategy for communicating with industry and suppliers, advance identification of a framework for the procurements should paragraph 2.6 of the Commonwealth Procurement Rules be invoked, contract templates, channels and vetting arrangements for offers of assistance, checklists for initial procurement activities, record keeping and information management protocols, an up to date list of relevant standards and technical specifications for various types of supplies, and supplier panel arrangements including domestic suppliers.
Recommendation no.4
2.46 Health put in place a strategic procurement, management and distribution plan for the NMS that includes protocols for emergency procurements.
Department of Health response: Agreed.
2.47 Health agrees that it would be appropriate to put in place expanded documentation to record information in relation to emergency procurements that builds on the recently published ANAO and Department of Finance advice.
3. Planning and governance of COVID-19 National Medical Stockpile procurements
Areas examined
This chapter examines whether, as part of the coronavirus disease 2019 (COVID-19) pandemic response, the Department of Health (Health) and the Department of Industry, Science, Energy and Resources (DISER) had effective planning and governance arrangements for the COVID-19 National Medical Stockpile (NMS) procurements.
Conclusion
Health’s and DISER’s NMS procurement planning and governance arrangements in response to the COVID-19 pandemic were effective. Both entities had elements of a plan for meeting the requirement, established fit for purpose governance arrangements and considered risks.
3.1 Modelling in late February 2020, after the COVID-19 pandemic response was activated, indicated national health system demand estimates for masks would be between 250 million and 1.2 billion and Health system demand to 31 December 2020 established in late March for other PPE suggested there would be a need for as many as 161 million gowns and coveralls; 633 million gloves; and 57 million goggles. The government reported to the Australian Health Protection Principal Committee on 11 March 2020 that shortfalls in personal protective equipment (PPE) in jurisdictional stockpiles would be filled by the NMS. This resulted in a large-scale emergency procurement for the NMS, with the Australian government allocating $3.2 billion for the procurements at 31 August 2020 (refer paragraph 1.13).26 This procurement occurred in the context of disrupted global supply chains and a surge in demand and prices for PPE and other medical supplies.27
3.2 Fit for purpose governance and planning arrangements can mitigate risks to the proper use of public resources created by a challenging procurement environment.
3.3 The ANAO examined the planning and governance of the COVID-19 activities within the context of the urgent circumstances of the COVID-19 pandemic response, including whether:
- a strategic and operational plan for meeting the COVID-19 procurement requirement was developed;
- fit for purpose governance arrangements for the COVID-19 procurement activities were established;
- risks to the proper use and management of public resources in the procurements were identified, analysed and treated; and
- Health appropriately applied the Commonwealth Procurement Rules (CPRs) when conducting the COVID-19 NMS procurements.
Was a plan for meeting the COVID-19 procurement requirement developed?
Health’s planning for the COVID-19 NMS procurements was fit for purpose. It did not develop a strategic or operational procurement plan but elements of a plan — such as definition of objectives, timeframes and procurement method — were incorporated in documentation. DISER’s operational planning for the procurement activities was also fit for purpose. It did not develop an overarching operational plan for its involvement but taskforces developed, used and shared process maps, templates and checklists to guide procurement activities.
3.4 Strategic planning includes a consideration of objectives, the key activities involved and the operating environment, with the intention of providing clarity and transparency on the intended outcomes of the activity. Implementation planning is the process of determining how an initiative will be carried out. An implementation plan typically addresses key tasks, roles, responsibilities and timelines for milestones and deliverables, as well as record-keeping protocols.28 The ANAO considered whether Health, as the accountable agency for the procurements, developed a strategic and implementation plan that was fit for purpose and whether DISER, as an assisting agency for the procurements (refer paragraph 1.15), developed an implementation plan that was fit for purpose.
Did Health develop an effective plan for meeting the COVID-19 procurement requirement?
Strategic procurement planning
3.5 Health did not develop a single strategic procurement plan for the COVID-19 NMS procurements. However, elements of a plan were contained in various documents (Table 3.1).
Table 3.1: Strategic procurement planning components
|
Component |
Health activity |
|
A procurement objective expresses the goals and purpose of the procurement. |
On 11 March Health released a fact sheet that indicated that the objective of the NMS procurements was ‘effective protection of health professionals treating patients [helping] critical health staff avoid infection’ and the prevention of transmission of COVID-19 from patients. |
|
Procurement planning should consider the timeframes for the procurement. |
Health did not establish specific timeframes for the COVID-19 NMS procurement, but noted it should proceed as quickly as possible. |
|
Procurement planning involves selecting an appropriate procurement method, based on the procurement value. |
Health explicitly determined a limited tender procurement method in mid-March although limited tender procurement processes were well underway prior to this. |
|
Estimating the value of procurement helps with assessing procurement risk and determining the procurement method. |
Health did not estimate the total value of the COVID-19 procurements and has noted that this was not possible due to uncertainty over disease transmission and the changing price of key items on a daily basis. Funding was provided incrementally over three months from March 2020 in response to requests to government that outlined the potential costs of additional PPE based on prevailing prices at the time. |
|
Procurement planning should incorporate evaluation. |
Although no evaluation has been planned, Health advised the ANAO that an evaluation of the COVID-19 NMS procurements is being considered and will be conducted once Health’s active engagement in the COVID-19 response is concluded. |
Source: ANAO analysis of Health documentation and the Commonwealth Procurement Rules, April 2019.
Operational procurement planning
3.6 Health did not develop an operational plan for the COVID-19 NMS procurements to guide its activities or the work of DISER. Some elements of an operational plan were contained in various documents, including a process map dated 6 April 2020, which provided instructions to Health officials on the key actions to be taken as part of the procurement process. Some Health officials developed draft guidance for personal or taskforce use, which assigned responsibilities for specific tasks and provided instructions on processes, such as how to action contracts referred by DISER. A contract checklist that included directions for record keeping was developed, but not until most of the procurements had been completed in late May.
Did DISER develop an effective operational plan for meeting the COVID-19 requirement?
3.7 No overarching implementation plan was developed for the DISER procurement activities. Operational planning was managed by each procurement taskforce (refer Figure 3.1), with varying levels of effectiveness. A PPE taskforce developed an operational plan that included a statement of the taskforce’s goal; detailed instructions on the key actions to be undertaken by the taskforce in relation to product sourcing and contract preparation; staff responsibilities against specific PPE products and against particular work activities, including responsibility for approvals at key milestones; and instructions on record-keeping arrangements throughout the process, including shared documents to be updated throughout the work and naming conventions for key documents. This operational plan did not determine any specific timeframes for the activities, which was consistent with DISER’s view that the direction from Health was to ‘procure as much PPE…as possible, as quickly as possible.’ DISER’s mask, ventilator and COVID-19 test kit taskforces did not develop an operational plan of this nature, but implementation planning in the form of templates for email correspondence, process maps, a procurement checklist and staff allocation to specific work tasks were used to organise the work.
3.8 DISER taskforces had different focus areas but shared some planning and operational tools. As part of concluding activities and preparing to hand over remaining work, taskforces developed ‘closure reports’ that explained the overall objectives and strategies of the taskforce, the procurement activities undertaken, the location of records and key lessons learnt.
Were fit for purpose governance arrangements for the procurement activities established?
Health’s and DISER’s internal and cross-departmental governance arrangements for the COVID-19 NMS procurements were fit for purpose. Respective roles between Health and DISER were not documented but were broadly understood. Both departments used a flexible taskforce approach to manage the procurements, involved procurement advisory services and actively engaged executive management in decision-making. There was a process for managing conflicts of interest in both departments, however, a requirement for specific conflict of interest declarations for the NMS procurements was introduced late and incompletely adhered to.
3.9 In a rapidly evolving environment, governance arrangements need to be established that are fit for purpose and that support the entity in fulfilling its responsibilities, including the proper use of public resources. The accountable authority has a critical role in determining what is fit for purpose for the entity. To determine whether Health and DISER had fit for purpose governance arrangements for the COVID-19 NMS procurements, the ANAO reviewed whether:
- roles and responsibilities for the COVID-19 procurements were clearly assigned between and within Health and DISER;
- financial delegations were clear and adhered to;
- procurement advisory services were appropriately involved;
- executive management in both departments were appropriately engaged in procurement decision making; and
- there was a requirement for conflict of interest declarations.
Were roles and responsibilities clearly assigned across entities and within Health and DISER?
3.10 Effective governance and planning arrangements include clearly defined roles and responsibilities. Cross-boundary work requires agreement among stakeholders about the nature of the problems at hand and each party’s respective contribution to addressing these issues, such as through networked governance arrangements.
Roles and responsibilities across entities
3.11 On 2 March 2020 the Secretary of the Department of the Prime Minister and Cabinet requested that Health and DISER work together on medical, pharmaceutical and PPE stocks. DISER and Health subsequently confirmed that DISER would be responsible for leading a team focused on the procurement of masks and that Health would be the ‘ultimate procurer’.
3.12 Although there was a broad understanding of respective roles, there was no documented agreement on respective responsibilities. On 2 March DISER requested clarification from Health on the role and scope of its work. A proposed memorandum of understanding did not eventuate, but respective responsibilities were determined in subsequent days through emails and meetings. DISER sent its first draft contract for goods for Health’s review and action on 20 March, and on 25 March DISER wrote to Health to confirm DISER’s responsibilities in more detail, specifically in relation to DISER conducting due diligence reviews on suppliers prior to sending goods contracts to Health.29 Health also conducted due diligence on suppliers referred by DISER. In early April Health provided feedback to DISER in which it noted that ‘contracts received from DISER by Health are not always fit for purpose.’ This related to the contract template that DISER was using, with Health using a different contract template developed by its legal service provider, and Health concerns about needing to conduct further follow up with some suppliers thereby impeding rapid execution of contracts. After being advised of this, DISER used Health’s contract template.
3.13 DISER established the Health Industry Coordination Group (HICG) on 23 March to provide a single point of contact for industry and government and to reduce duplication and overlap of functions. In addition, the Health Industry Senior Officials Group was established in March to support efficient procurement of essential items to respond to the COVID-19 pandemic; this was chaired by DISER and attended by officials from the Commonwealth and all state and territory industry departments. Between 25 March and 6 May, the Commonwealth Minister for Industry, Science and Technology met state and territory manufacturing ministers and industry department officials on six occasions in order to, according to DISER, ‘understand PPE procurement arrangements occurring across Australia and focus on developing a coordinated approach to supporting local capability in PPE and other medical supplies.’
Roles and responsibilities within Health and DISER
3.14 Prior to the pandemic response, the NMS was managed by a section comprising seven staff within the Health Emergency Management Branch in the Office of Health Protection. In late January 2020 the National Incident Room was ‘stood up’ temporarily as a separate division, within which was formed the National Medical Stockpile and Finance Branch (NMS Branch), later the NMS Taskforce. An Assistant Secretary was appointed to lead PPE, ventilator and swab procurement work. On 22 July, after most of the procurements had been completed, the National Incident Room Division and Office of Health Protection amalgamated into one division, the Office of Health Protection and Response.
3.15 Test kit procurements were led by the Diagnostic Imaging and Pathology Branch (DIPB) within the Medical Benefits Division at Health, which had specific expertise in and direct engagement with the pathology sector. This division of labour reduced duplication of effort on test procurement. The procurements of swabs, a test kit component, was led by the NMS Branch. The DIPB and NMS Branch communicated regularly but DIPB developed its own procurement and documentation protocols.
3.16 A taskforce approach can be an effective means to develop governance and delivery arrangements in compressed timeframes. Health and DISER both implemented a taskforce approach for the NMS procurements (refer Figure 3.1), with DISER establishing this from the outset of its involvement on 2 March and Health establishing supplementary procurement teams to assist the NMS Branch in late March.
Figure 3.1: COVID-19 NMS procurements taskforce structure

Source: ANAO analysis of Health and DISER documentation.
3.17 Within Health, taskforces (called ‘procurement teams’ by Health) were used to manage surge resourcing requirements. In late March an initial procurement team was established to support the NMS Branch. As work outpaced capacity, by early April two additional procurement teams were established. The three procurement teams — each led by an Executive Level 2 official — drew personnel from across the department, including from the department’s Procurement Advisory Service (PAS). Health advised the ANAO that, at the peak of procurement activity, the NMS Branch, the three procurement teams, dedicated staff from the DIPB and a proposal triage team comprised 35 full time equivalent staff.
3.18 Although the DIPB specialised in COVID-19 test procurement, the NMS Branch and three procurement teams worked across all swab and PPE procurements and contracts on an as-needed basis without specialisation, leading to some duplication and overlap. Activities included due diligence, including of suppliers referred by DISER, and contract development and implementation. All DISER and Health taskforce referrals of draft contracts with suppliers for delivery of masks, PPE, ventilators, test kits and swabs were channelled through the Assistant Secretary, NMS Branch.
3.19 DISER advised the ANAO that at the peak of its involvement between 16–29 April, 173 full time equivalent staff were diverted to the taskforces supporting Health to procure supplies for the NMS, including 20 senior executive level staff. The taskforce governance model was adopted from the outset, with an initial taskforce focused on masks (as well as hand sanitiser, waste bag closure ties and clinical waste bags) established on 2 March and additional specialised taskforces established during March to focus on other product areas, comprising other PPE (gowns, gloves, googles, face shields, blood and fluid spill kits, mask fit test kits and thermometers), ventilators and test kit components (namely swabs), as these became relevant to the procurement requirement. This specialisation enabled taskforce members to gain an understanding of complex product categories, including standards and specifications.
3.20 The Australian government received offers to supply PPE and other medical supplies; some of these were made directly to Health while others were communicated through channels such as other Australian or state government departments, manufacturers, the HICG, the Therapeutic Goods Administration (TGA), ministers’ offices and responses to approaches to market advertised on AusTender by DISER.30 In late March both departments established coordinating taskforces for first pass due diligence of the offers of assistance. The coordinating taskforces could have added greater value if established from the outset to quickly process the large volume of offers. DISER also established the HICG to support the initial taskforces and provide greater coordination across departments.
3.21 All DISER taskforces were consolidated on 19 May 2020 into the COVID Response Taskforce, which had the goal of gradually moving all COVID-19 response activities back into business areas. In closure reports, DISER noted that:
The crisis has demonstrated that the Department can be agile in standing up taskforces quickly, and hopefully the Department uses this experience as a culture change to use taskforces more frequently. Taskforces are an important way to solve problems and move resources quickly. Assembly and disassembly needs to be quick. Otherwise, there is a risk that work will be led by one area and become a coordination exercise instead of a cross-departmental effort.
Were financial delegations clearly assigned and adhered to?
3.22 Commonwealth officials must not approve a proposed commitment of relevant money unless they have been delegated powers to do so. During the NMS procurements, financial delegations were clearly established by Health. There were four instruments of delegation in effect: 24 December 2019 to 28 March 2020; 29 March to 28 June 2020; 29 June to 4 August 2020; and from 5 August 2020.
3.23 Delegations were adhered to. Delegate approval was obtained primarily through commitment approval minutes, although several were indicated by an email between the delegate and the supplier. Delegates ranged from Executive Level 2 through to the Secretary. In all cases the accountable authority or an appropriate delegate provided the approval.
Were procurement advisory services appropriately involved?
3.24 Health and DISER have central procurement advisory services areas. In Health, this is PAS and in DISER, this is the Procurement and Financial Policy section (PFP). Both departments’ internal procurement policies specify that officials conducting procurements should consult with procurement areas for higher risk and higher value procurements, and comply with their advice.
3.25 Central procurement advisory service areas within the departments were used but could have been involved earlier by Health. On 11 March 2020 a PAS official contacted the Department of Finance requesting advice in relation to the application of paragraph 2.6 of the CPRs for COVID-19 related purchases (refer paragraph 3.52). However, the NMS Branch did not consult PAS about the COVID-19 NMS procurements until 16 March, when assistance was requested in relation to commitment approval minutes for three suppliers with whom negotiations were already well underway. On 19 March the head of the Financial Management Division, within which PAS resides, communicated to officials involved in the COVID-19 response that PAS was available to assist with NMS procurement activities, noting that the invocation of paragraph 2.6 did not ‘obviate the need to undertake appropriate due diligence in any procurement, and to assure yourself as a PGPA delegate that any spending decisions you make meet the PGPA Act requirements in respect of efficient and effective use of public funds.’31 Subsequent to this communication, PAS assisted the NMS Branch and temporary procurement teams with commitment approvals, contracts and financial reporting. In April, two PAS officials were directly appointed to a procurement team.
3.26 Within DISER, the PFP was involved in procurement activity from early March 2020, when the initial taskforce was established, and DISER advised the ANAO that two dedicated PFP officers were tasked to provide advice and assistance to the various taskforces from March to May, including assistance with four approaches to market. PFP involvement included preparation and review of draft contracts and, in late March 2020, development of a procurement checklist to be used by the DISER taskforces. During April the PFP communicated with Health to confirm DISER’s due diligence process and sought advice from the Department of Finance in relation to applicable procurement policies following the application of paragraph 2.6.
Was executive management actively engaged in decision making?
3.27 Rapid delivery requires active engagement by executive management. Senior management has a responsibility to ensure that it has adequate program visibility through quality reporting that does not provide a false sense of assurance.
3.28 Executive management within Health and DISER were actively engaged in providing direction on procurement processes; considering estimates of demand and the procurement requirement; communicating with other Commonwealth agencies, state and territory governments, suppliers, manufacturers, and other stakeholders; making decisions and approving commitments of public funds. The HICG included executive representation from both departments and DISER taskforces were each led by a senior executive.
3.29 Health and DISER executive management were kept informed of key risks, developments and decisions through regular reporting. In late March Health established a reporting framework that included daily updates of NMS inventory movements and the procurement pipeline to executive management and its Minister’s office. Between 6 March and 18 May DISER taskforces provided its Minister’s office and senior executives within Health and DISER with a daily update on the incoming supply of masks, other PPE, ventilators and test kits, as well as DISER’s progress on tasks, key achievements and upcoming priorities and emerging issues. The DISER ventilator taskforce also provided regular status updates to Health and DISER executives and to the Chief Scientist of Australia from late March 2020 until mid May 2020. DISER taskforces regularly corresponded with the Health executive to clarify requirements and provide progress updates.
Was there a requirement for conflict of interest declarations?
3.30 A conflict of interest occurs where a person’s personal interests, affiliations or relationship prejudices impact on their impartiality, or might be perceived by a reasonable person as potentially prejudicing their impartiality, or result in an incompatibility with the duties owed to an entity undertaking a procurement. The CPRs state that officials undertaking procurement must recognise and deal with actual, potential and perceived conflicts of interest and the Public Governance, Performance and Accountability Act 2013 (PGPA Act) states that an official who has a material personal interest that relates to the affairs of the entity must disclose details of the interest.32 Entities should identify any procurement circumstances that involve elevated risk of conflicts and require that declarations be made and managed before the person begins the work.33
3.31 Health’s policy requires staff to declare any conflicts of interest upon engagement with the department and when there has been a change in employee circumstances. DISER’s policy requires staff to undertake awareness training on conflicts of interest upon engagement and annually, and disclose any conflicts annually or if there is a change of circumstances.
3.32 Health requested that its staff working on the COVID-19 NMS procurements create a new conflict of interest declaration or update their existing declaration in mid May 2020. This was late in the procurement process and not all staff had complied by July. DISER advised staff involved in the expression of interest process and evaluation of several approaches to market to complete a conflict of interest declaration on 25 March and 15 April 2020 but not all staff involved in the procurements updated or completed a declaration.
Were risks to the proper use and management of public resources in the procurement identified, analysed and treated?
Health and DISER assessed and treated risks to the proper use and management of public resources in the COVID-19 NMS procurements and to procurement outcomes. Health did not conduct an overarching assessment of risk in relation to COVID-19 NMS procurement activity and risk treatments for individual procurements were not well documented. Both departments considered procurement risks in a number of their implementation activities.
3.33 The CPRs indicate that entities must establish processes to identify, analyse, allocate and treat risk when conducting a procurement and the effort directed to risk assessment and management should be commensurate with the scale, scope and risk of the procurement.34 Health and DISER provide internal guidance and templates to staff for achieving value for money procurements through a risk-based approach. The ANAO examined whether Health and DISER:
- identified and managed risks to the proper use of public resources by establishing a risk tolerance or appetite level for the procurements;
- completed a risk assessment for the procurements;
- developed mitigation strategies for any risks that exceeded the risk appetite; and
- implemented mitigation strategies where required.
Did Health identify risks to the proper use of public resources in the procurements?
Establishment of a risk tolerance/appetite level
3.34 Health’s enterprise risk appetite statement states that:
Specifically, the department is eager to engage with higher levels of risk and look for innovation, in relation to its policy development and delivery outcomes where the potential rewards may provide improvements to the health and well-being of the Australian public. Conversely, the department has little to no risk appetite for engaging with risk that could harm its people or the Australian public.
3.35 Health’s enterprise risk appetite has not been adjusted since the beginning of the COVID-19 pandemic. Health’s risk tolerance for the COVID-19 NMS procurements aligns with Health’s enterprise risk tolerance of ‘medium’ for program delivery and governance.
Risk assessment
3.36 Health’s procurement plan template requires an overall risk profile to be established for each procurement in the planning and sourcing stage, using a procurement risk profile template. A consequence and risk treatment must be outlined for all risks that are identified as unacceptable.
3.37 Health did not complete an overarching risk profile for the COVID-19 procurements of the NMS. Health advised the ANAO that, in the urgent circumstances, risk, including risk to human life, was considered at the individual contract level, as evidenced in most commitment approval minutes. Health advised the ANAO that discussions with the Secretary were used to ‘raise and consider individual risk as appropriate.’ In an ANAO review of a sample of 54 commitment approval minutes across 54 executed contracts, risk was explicitly mentioned in 48 minutes.35 Four minutes referred to a medium risk profile, two to a ‘low to medium’ risk profile, 38 minutes to a low risk profile and four did not specify the risk profile. Sixteen of the 48 minutes provided a justification for the risk rating.
Mitigation strategies
3.38 As Health did not complete a risk assessment for the COVID-19 NMS procurements, no mitigation strategies pertaining to all procurements were specified. Ten of 54 commitment approval minutes examined included one or more risk treatments for specific procurements, and several minutes indicated that risk would be mitigated through the contract negotiation process.
Did DISER identify risks to the proper use of public resources in the procurements?
Establishment of a risk tolerance/appetite level
3.39 DISER did not establish a risk tolerance or appetite level specific to the NMS procurements, but applied its existing enterprise risk tolerance to the work. The risk tolerance for DISER’s procurement-related business functions is medium.
Risk assessment
3.40 A Risk Management Plan (Risk Plan) covering the work of the DISER taskforces involved in the procurements of the NMS was started on 6 March, adapted throughout the procurement activities, and finalised at the end of April. In developing the Risk Plan, advice was sought and received from DISER’s risk management and legal teams. Fifteen risks were identified — nine rated medium and five rated high. Several controls were identified against all 15 risks. Seven risks were rated higher than DISER’s enterprise risk tolerance after treatment, but were accepted.
3.41 In a separate process, the test kit taskforce completed a risk management plan on 15 March. This plan identified four risks, with several controls provided against each risk. All risks were considered acceptable after application of these controls. The taskforce completed additional operational risk assessments for undertaking a request for information and supporting the domestic manufacture of test kits.
Mitigation strategies
3.42 In total, 118 controls were proposed as part of the DISER Risk Plan.36 Fifty of the proposed controls were applied to the seven risks that exceeded the risk tolerance.37
Did Health and DISER appropriately manage risks to the proper use of public resources in the procurements?
3.43 A number of procurement-related activities undertaken by Health considered risks to the proper use of public resources and achieving the procurement outcomes.
- On 15 March Health recommended the government support domestic production to mitigate the risk of interrupted overseas supply chains. In a minute to the Acting Secretary on 30 April Health officials stated that the COVID-19 pandemic highlighted the need for a domestic manufacturing capability and capacity to protect the current and future health of Australians.
- On 29 March Health’s Secretary reduced the financial delegation for the First Assistant Secretary in order to increase the level of oversight by senior executive management.
- Health taskforces received internal and external legal support and advice that included a variety of services, such as assistance with negotiation, due diligence and contract development.
- Due diligence on suppliers was conducted to reduce the risk that they would be unable to supply the contracted goods or the goods would not meet a quality standard. On 1 April Health consolidated information on procurement progress into a tracking sheet to monitor the status of proposals received.38 In a 27 May version of the tracking sheet, a reason was provided for 30 suppliers not proceeding to a contract, nine of which were a result of due diligence identifying potential risks with the offer.
- Other measures intended to address the procurement risks were progressively developed. On 9 April Health advised DISER to avoid making promises to suppliers as NMS needs might change; preferably deal with known providers; use payment schedules; and receive quotes that were inclusive of freight.
- Due diligence processes undertaken by Health included consideration of quality specifications in relation to TGA requirements and registration on the Australian Register of Therapeutic Goods.
3.44 By June 2020 Health was implementing 70 COVID-19 measures across the department. In order to provide ‘real time’ and ongoing risk, fraud and assurance advice to the executive, the Corporate Assurance Branch developed a live assurance work plan for these measures across five themes, including ‘procurements and contracts’.39 In July Health reviewed the Minderoo Foundation and Beijing Genomics Institute pathology related contracts, which had been assessed as high risk on 25 May.40 The review identified a number of positive practices, including executive oversight, effective record keeping, financial and budget reporting, legal due diligence and product safety certifications. The review also noted areas for improvement, including the lack of a general register of conflict of interest declarations and contract management plans. These findings may be of relevance to the department in the future.
3.45 DISER also considered risks to the proper use of public resources through a number of its implementation activities.
- Like Health, DISER conducted due diligence on suppliers to reduce the risk that they would be unable to supply the contracted goods or the goods would not meet a quality standard. On several occasions in late March and early April, DISER advised the Health executive of the due diligence procedures that would be followed by the procurement teams.
- DISER’s taskforces sought internal legal advice regarding a number of activities related to the NMS procurements, covering contractual arrangements, company structures, product standards and more general contract terms and conditions.
- A procurement checklist guided officials to document the contract terms, background, value for money assessment, risks and mitigation strategies.
- In addition to engaging with industry stakeholders through forums such as a supply chain roundtable and the HICG, DISER sought to obtain market intelligence and broaden the pool of potential suppliers by approaching the market, via AusTender, on four occasions, comprising:
- a Request for Information on domestic production capabilities relevant to a range of medical PPE (15 March) ;
- a request for Expression of Interest for the supply of swabs suitable for COVID-19 sample collection (20 March); and
- two Requests for Information for Australian production capability for components of COVID-19 test kits (3 and 9 April 2020).
- As an additional measure aimed at reducing risk associated with the quality of contracted goods, on 8 May DISER commissioned a review of 14 contracted suppliers. The final report was provided to Health and DISER on 13 May. The review noted that given the low maturity of some suppliers and lack of independent testing of manufacturing quality, Health may need to rapidly establish quality and materials tracking capability to ensure that it is accepting high quality PPE.
3.46 In mid May 2020 DISER executive agreed to pause elements of the 2019–20 Annual Assurance and Audit Plan, and consider how internal audit could focus on providing assurance over the department’s COVID-19 taskforce and other response activities. An overarching framework for internal audit of COVID-19 activities was agreed, and two internal audits relating to the NMS procurements were initiated under that framework — procurement activity relating to the COVID-19 response and a governance fundamentals review.41 The reports were completed on 3 and 17 August 2020, respectively. The reports will provide value to the department for future activities.
Did Health appropriately apply the Commonwealth Procurement Rules when conducting the COVID-19 NMS procurements?
When conducting the COVID-19 NMS procurements, Health applied the CPRs appropriately. Health officials informed the delegate of the use of paragraph 10.3(b) of the CPRs when seeking approval to commit funds through limited tender and sought the approval of the Acting Secretary of Health to invoke paragraph 2.6 to not apply the CPRs to the procurements. No alternative procurement framework for the COVID-19 NMS procurements was specified by the Acting Secretary. The Acting Secretary revoked the application of paragraph 2.6 when it was no longer necessary.
3.47 Paragraph 10.3 of the CPRs provides that an entity is only permitted to conduct a procurement at or above the relevant procurement threshold through limited tender in defined circumstances, while paragraph 2.6 provides for the CPRs to not apply to procurement activity in certain circumstances.42 Forty six of 54 COVID-19 NMS procurements considered in the audit were valued more than the relevant threshold of $80,000 (GST inclusive), with the highest being $799.6 million (excluding GST). All COVID-19 NMS procurements were conducted by some form of limited tender or on a non-competitive basis.
3.48 In August 2020, in the context of the COVID-19 pandemic response, the Department of Finance advised Commonwealth entities that the CPRs include mechanisms that ‘enable more streamlined processes to engage suppliers more urgently’43, namely:
- paragraph 10.3(b) — which states that ‘A relevant entity must only conduct a procurement at or above the relevant procurement threshold through limited tender in the following circumstances:…when, for reasons of extreme urgency brought about by events unforeseen by the relevant entity, the goods and services could not be obtained in time under open tender’; and
- paragraph 2.6 — which states that ‘These CPRs do not apply to the extent that an official applies measures determined by their Accountable Authority to be necessary for the maintenance or restoration of international peace and security, to protect human health, for the protection of essential security interests, or to protect national treasures of artistic, historic or archaeological value.’
3.49 Nearly two thirds of Australian Government entities are required to report on AusTender any contracts they have awarded with a value above prescribed reporting thresholds. Across all reporting agencies, AusTender records indicate that paragraph 10.3(b) was used 131 times and paragraph 2.6 was used 909 times in 2018–19, including twice by Health.44 In 2019–20 the use of paragraph 2.6 increased by 41 per cent to a total of 1,280 instances. Usage can be attributed to three Commonwealth entities primarily: Department of Defence (1,002 instances in 2019–20), Health (157 instances) and Department of Home Affairs (13 instances).
3.50 The ANAO examined whether Health obtained proper approval to approach the market through limited tender for the COVID-19 NMS procurements; whether Health sought and followed advice regarding the application of paragraph 2.6 to the procurements; and the framework that applied to the NMS procurements following the application of paragraphs 10.3(b) and 2.6.
Did officials obtain proper approval to approach the market through limited tender?
3.51 Health advised the ANAO that delegate approval to approach the market in a limited way under paragraph 10.3(b) of the CPRs was not sought in the early days of the procurement activity. However, commitment approval minutes for two contracts executed prior to 18 March 2020, when paragraph 2.6 of the CPRs was invoked, advised the delegate about the use of paragraph 10.3(b) to conduct a limited tender.
3.52 Paragraph 2.6 allows the Secretary of Health to decide that the CPRs do not apply to measures necessary to protect human health. The Acting Secretary agreed in writing to invoke paragraph 2.6 for all procurements under the COVID-19 response on 18 March 2020 and the use of paragraph 2.6 was referred to in financial commitment approval minutes for most COVID-19 NMS procurements. DISER was notified of the use of paragraph 2.6 on 20 March 2020. The application of paragraph 2.6 for the COVID-19 procurements was revoked by the Acting Secretary of Health on 9 June 2020.
Did Health seek and follow advice about the application of paragraph 2.6?
3.53 The minute that sought approval from the Acting Secretary to invoke paragraph 2.6 of the CPRs indicated that advice on its usage had been sought from the department’s Chief Financial Officer and the Department of Finance.
3.54 On 10 March 2020 Health’s Legal and Assurance Division advised senior management that when using paragraph 2.6 they would need to identify the particular measures relating to the procurement that require departure from the CPRs; determine the extent of the departure from specific requirements of the CPRs to address the measure; and document and obtain approval by the Secretary on such measures. Legal services advised that assistance be sought from Health’s PAS regarding the use of this approach.
3.55 On 11 March 2020 the Financial Management Division wrote to the Department of Finance asking if it was required to notify it of its intention to invoke paragraph 2.6 in the COVID-19 procurements. In its response, the Department of Finance advised Health officers that no notification was needed but that:
- under paragraph 2.6, the accountable authority has the option to not apply the CPRs at all, apply only Division 1 or not apply specific sections, such as reporting;
- when invoking paragraph 2.6, relevant entities will need to identify the particular measures relating to the procurement that require departure from the CPRs, determine the extent of departure from specific requirements of the CPRs to address the measure and document and obtain approval from the accountable authority on such measures; and
- when exercising paragraph 2.6, officials continue to be bound by the broader requirements of the PGPA Act, including the proper use and management of public resources, where ‘proper’ means efficient, effective, economical and ethical.
3.56 The minute that sought approval to invoke paragraph 2.6 (refer Appendix 3) advised the Health Acting Secretary that:
- a ‘broad’ ‘exemption’ from the CPRs was ‘necessary and appropriate’ because of the ‘rapidly evolving situation’ and ‘the Department’s requirement to respond, particularly to supply constraints of medical equipment’ and the more limited exemption under paragraph 10.3(b) would become ‘more difficult to rely on’;
- the measures relating to the procurements that required departure from the CPRs were ‘procurements under the COVID-19 response’;
- PGPA Act obligations to ‘document the process’, obtain ‘necessary financial approvals’ and meet ‘reporting obligations outside the CPRs such as Senate Orders (e.g. Senate Order 13…)’ were still in effect; and
- no specific notification requirement to Finance was required but that Finance had recommended that Health ‘maintain clear documentation around the exemption process and procurements relying on exemptions’.
3.57 When determining the extent of departure from specific requirements of the CPRs, the minute broadly explained that Health’s Financial Management Division ‘would work with relevant divisions to prioritise expedient and effective procurement for the protection of human health, whilst promoting other procurement options to ensure CPR compliance, good governance and robust public administration.’ In a minute to the Health Acting Secretary submitted on the following day, officials asked her to note the proposal to procure masks through limited tender ‘or other appropriate direct engagement processes’ with ‘a small number of organisations identified as having the capacity, capability and interest to meet Health’s requirements’.
3.58 The minute did not specifically advise the Acting Secretary of the Department of Finance’s advice that the accountable authority has the option to not apply the CPRs at all, apply only Division 1 or not apply specific sections, such as reporting; or that when paragraph 2.6 has been invoked, officials continue to be bound by the requirements of the PGPA Act to use and manage public resources properly.
What procurement framework applied to the COVID-19 NMS procurements?
3.59 Until the invocation of paragraph 2.6 of the CPRs on 18 March 2020, the procurement framework for the COVID-19 NMS procurements was the CPRs.
3.60 The ‘other procurement options’ and ‘other appropriate direct engagement processes’ mentioned in the 18 and 19 March minutes were not explained, and no alternative framework for conducting the procurements was specified. Although it did not clearly state this, Health and DISER officials interpreted the minute setting aside the CPRs to be a ‘blanket’ provision, with the extent of departure from the specific requirements of the CPRs to be in full. However, correspondence from DISER to Health in late March advised that ‘to the extent practical in the circumstances, the Department will follow the requirements of Division 1 of the CPRs where they represent better practice and are applicable and practicable.’ From 18 March and until 9 June, when the invocation of paragraph 2.6 was revoked by the Acting Secretary, there was no alternative procurement framework in place.
3.61 In any case, the accountable authority remained obliged, under paragraph 15(1)(a) of the PGPA Act, to govern the entity in a way that promotes the proper use and management of public resources for which the accountable authority is responsible. Section 8 of the PGPA Act defines ‘proper’ to mean efficient, effective, economical and ethical use or management of public resources.45
3.62 When invoking paragraph 2.6, other Australian Government entities have specified an alternative framework when determining the extent of departure from specific requirements of the CPRs. For example, in August 2017 the Secretary of the Department of Home Affairs (Home Affairs) invoked paragraph 2.6 of the CPRs in its procurements of garrison support and welfare services for Manus Island46 and determined the extent of departure from specific requirements of the CPRs by stating that the department would still comply with rules relating to value for money (CPRs, Part 4); efficient, effective, economic and ethical procurement (CPRs, Part 6); accountability and transparency in procurement (CPRs, Part 7); and procurement risk (CPRs, Part 8). The Department of Defence (Defence) states in its Defence Procurement Policy Manual that invocation of paragraph 2.6 for a Defence procurement means that the procurement is usually exempt from the operation of Division 2 rules of the CPRs.47 The Manual further notes that:
Even if a procurement is exempt from Division 2 of the CPRs, Defence officials are still required to undertake their procurements in accordance with Division 1 of the CPRs. In addition, Defence officials are still required to comply with all applicable Defence Procurement Policy Directives contained in this manual.
4. Meeting the COVID-19 National Medical Stockpile procurement requirement
Areas examined
This chapter examines whether the coronavirus disease 2019 (COVID-19) National Medical Stockpile (NMS) procurement requirement was formulated on the basis of sound analysis and whether the procurement requirement was met.
Conclusion
The COVID-19 NMS procurement requirement was not clearly specified for personal protective equipment (PPE), swabs and COVID-19 tests. Procured quantities for the NMS were approximately aligned with overall national health system demand estimates for all items where demand modelling was undertaken, suggesting the procurement requirement was met or exceeded.
4.1 On 11 March 2020 the Department of Health (Health) announced that it was seeking to increase Australia’s supply of PPE and pharmaceuticals held in the NMS in order to protect health professionals by preventing the transmission of COVID-19 from patients. The ANAO reviewed:
- whether the COVID-19 procurement requirement was developed on the basis of sound analysis; and
- whether the COVID-19 NMS procurement requirement for PPE and medical equipment was achieved.
Was the COVID-19 National Medical Stockpile procurement requirement formulated on the basis of sound analysis?
In formulating the NMS procurement requirement, demand estimates and supply chain issues were considered by Health and DISER. However, due to the dynamic situation and late and partial information about existing national stocks of PPE, only the ventilator procurement requirement was specified clearly. In the absence of a specified procurement requirement, Health and DISER officials understood the requirement was to procure as much PPE as possible, as quickly as possible.
4.2 The Australian Health Sector Emergency Response Plan for Novel Coronavirus (COVID-19) (COVID-19 Plan) states that an estimate of the anticipated impact of COVID-19 will be used to guide the allocation and conservation of resources and to develop strategies to supplement likely shortfalls.48 As a supplementary stockpile, the NMS procurement requirement, in terms of quantity of goods, is the difference between likely national health system demand for essential medical supplies and stock-in-hand (existing inventory and confirmed orders) within the NMS, state and territory stockpiles or stocks of other health system procurerers known to Health.
4.3 The ANAO reviewed whether the NMS procurement requirement, in terms of quantity, was:
- formulated by Health on the basis of accurate and timely information about existing stock;
- formulated by Health on the basis of estimates of demand; and
- specified by Health so as to guide procurement activities and decisions.
In formulating the procurement requirement, did Health use accurate and timely information about existing stock?
4.4 The ANAO reviewed Health’s and DISER’s activities to analyse NMS stock-in-hand; state and territory and other health system procurers’ stock-in-hand and intended procurements; and the capability of existing supply chains to continue to supply the Australian market.
Information about National Medical Stockpile inventory
4.5 An inventory information management system should provide accurate and timely information about stock levels, status (condition and expiry information) and location. This information informs management, procurement and deployment decisions, and the accuracy and timeliness of this information becomes particularly critical during a health emergency when rapid decisions about procurement and deployment may be required.
4.6 Since 2010 Health has used an information management system called jIMMY to record additions, disposals, impairment and changes to inventory records for the NMS. External warehouse contractors’ weekly reports and invoices are used to manually enter information. This information includes product type, brand, number of items and cartons, manufacturer, product code, supplier, batch number and expiry date. jIMMY is a Microsoft Access database that is maintained outside the regular Health IT network. It is used to generate monthly financial and inventory management reports.
4.7 Auditor-General Report No.53 2013–14 Management of the National Medical Stockpile identified delays and inaccuracies arising from the manual updating process, particularly during periods of relatively high volume disposals and procurements in 2013. The ANAO recommended that Health review its information management arrangements for the transfer of stockpile data. Health agreed with the recommendation, noting that new logistics arrangements would include new data management systems.49 Health considers the recommendation to be ‘completed’.
4.8 The ANAO examined jIMMY, including its functionality during the pandemic response. The ANAO found that the system does not have controls that are designed to ensure the confidentiality, integrity and availability of data; and that this impeded its usefulness and reliability during the response.
- There is no system interface to support automated data transmission between NMS warehouses, or between the warehouse contractors and jIMMY.
- There are irregularities with back-ups and Health has done none of the common system assurance processes (security and change management) to ensure the integrity of the data that is held within the system.50 As a result, data integrity cannot be confirmed.51
- Health has not performed an Information Security Registered Assessors Program (IRAP) assessment52 and has not provided evidence of any other reviews to ensure the system is well controlled.
- Manual data entry is not independently quality assured at the time of entry, but is reconciled with Health’s financial information system monthly and compared to stock takes once annually.
4.9 These significant control issues mean that reports and data from the system cannot be relied upon without corroborating evidence, in either business as usual or emergency conditions.
4.10 As was previously demonstrated on a smaller scale in 2013 (refer paragraph 4.7), in COVID-19 emergency conditions the system, which is reliant on a single laptop and manual data entry, could not be kept up to date in times of rapid stock movements. Manual updating of jIMMY was stopped when it became clear that manual data entry could not keep up. It therefore could not be used as a reliable source of information about NMS stocks during this period. Health advised the ANAO that it considers that information about NMS inventory during the COVID-19 response was effectively managed and assured through other means such as tracking warehouse dispatch records on a daily basis and other ‘reconciliations’. From 31 March 2020 the NMS Taskforce provided Health executive management and government with approximate information about total NMS stock, procurements and dispatches by product type using warehouse reports about deployments, executed contracts and Health email correspondence with suppliers.
4.11 The ANAO’s 2019–20 financial statements audit of Health has identified deficiencies in processes and controls for recording and managing the NMS. In particular, the ANAO raised a moderate finding regarding Health’s inventory management system for the NMS, stating that the inventory management system supporting the NMS is not fit for purpose during a pandemic. The ANAO made four recommendations aimed at improving the capability of the inventory management system to provide timely and accurate data, including during health emergencies.
Information about state and territory and other procurers’ stockpiles and procurement activities
4.12 On 20 January 2020 Health requested information from the Department of Defence and state and territory health authorities about the current stock of P2/N95 respirators (P2 masks) and antivirals held in emergency stockpiles. At the Australian Health Protection Principal Committee (AHPPC) meeting on 7 March, states and territories were asked to advise the Commonwealth of their estimated COVID-19 PPE stocks and requirements and on 13 March the Acting Secretary wrote to the state and territory health authorities requesting this information. At National Cabinet it was agreed that this information would be provided on a monthly basis and Health issued requests on a monthly basis to August 2020.
4.13 Information was not immediately provided to Health by all jurisdictions. On 26 March the Minister for Health indicated to the Australian Government that without recent stock information from the states and territories, the Commonwealth was unable to accurately predict the quantities required to support the system. Known data was collated into a national preparedness tracker by 23 April but at 1 May the status of gowns and gloves still required confirmation.
4.14 To determine the stock of ventilators available to treat COVID-19 patients in the national health system, Health and DISER relied upon data from the Australian and New Zealand Intensive Care Society (ANZICS).53 DISER had difficulty determining state and territory procurements of ventilators and consolidated information on ventilator consumables held in hospitals was not available. However, from 1 May Health was able to access the Critical Health Resource Information System (CHRIS) to monitor demand for and capacity of Australian intensive care units (ICUs).54
4.15 From 30 March 2020 Health was informed by a testing platform manufacturer of the amount of testing stock being requested by pathology laboratories using that platform55, as well as constraints on testing consumables, and DISER also reviewed daily testing rates to inform swab procurement. Health advised the ANAO that it held regular meetings with the Medical Technology Association of Australia56 from the end of March to understand testing platforms and supply disruptions, and testing supplies were also discussed on a regular basis by the Public Health Laboratory Network57, which met 21 times between late February and end June 2020. DISER noted that due to the variation in testing methods and arrangements for procurements of pathology supplies across states and territories, it had a high level of engagement with state and territory procurement officials, which DISER advised the ANAO was effective in terms of clarifying demand and needs for COVID-19 tests.
Information about supply chains
4.16 As at 21 March 2020 Global Trade Alert estimated that 46 export curbs on medical supplies had been introduced by 54 governments since the start of 2020.58 Australian demand for PPE and testing consumables has historically been served by international supply chains. COVID-19 procurements of medical supplies by states, territories and the Commonwealth also heavily relied upon international supply chains, with the Health Industry Coordination Group (HICG) estimating that approximately 85 per cent of contracts for procurements for the NMS, in terms of value, were with Australian based businesses importing from overseas.
4.17 The Coronavirus (COVID-19) in Australia – Pandemic Health Intelligence Plan (Pandemic Health Intelligence Plan) notes that careful monitoring of international and national supply chains would be required to inform policy decisions.59 The Secretary of the Department of the Prime Minister and Cabinet identified Health and DISER as the lead agencies for the assessment of supply chains in relation to medical, pharmaceutical and PPE stocks. In response, DISER monitored, reported and addressed supply chain issues for masks, other PPE, ventilators and test kits. This work included logistical support and grant funding to domestic mask and PPE manufacturers; liaison with Australian ventilator manufacturers to understand potential supply chain issues; and a market investigation of COVID-19 testing supplies.
4.18 Investigation of the market to supply COVID-19 tests identified risks associated with many Australian pathology laboratories using ‘closed’ ribonucleic acid (RNA) extraction platforms with branded RNA extraction kits and reliance on a single overseas manufacturer for both swabs and high-volume testing platforms. On 23 March 2020 Health provided a briefing to the AHPPC on the security of existing supply chains for COVID-19 testing and reported that the major suppliers were currently meeting Australian demand. Two Australian companies capable of manufacturing open-platform RNA extraction kits were identified, with Health providing an interest-free loan agreement to one to establish manufacturing capability.
Did Health establish demand estimates?
4.19 Robust national health system demand estimates were essential to establishing the NMS procurement requirement. This was particularly important in the NMS procurements because, as noted by the HICG, there was no overall transparent picture of supply and demand needs for PPE nationally, making the coordination of procurement strategies challenging.
4.20 A meeting between Health and DISER officials on 22 April 2020 established respective roles for supply-demand modelling efforts. Health and DISER progressed a number of demand estimates over the course of the pandemic response for masks, other PPE, ventilators and COVID-19 tests (refer Table 4.1 and Appendix 4).
Table 4.1: Demand estimates for essential medical supplies
|
Organisation |
Relevant product category |
Users considered in demand estimates |
Commissioning entity/body |
Date first output (2020) |
|
Doherty Institute |
Masks |
Hospitals and general practice clinics |
Health |
28 February |
|
Quantium |
Masks and other PPE |
Range of potential usersa |
Health |
30 March |
|
DISER |
Swabs |
Pathology laboratories |
DISER |
15 April |
|
ANZICS |
Ventilators |
ICUs |
Health / DISER |
22 April |
|
McKinsey and Company/ DISER b |
Masks, other PPE, ventilators |
Hospital and community healthcarec |
HICG |
28 April |
|
Doherty Institute |
COVID-19 tests and test kit components |
Pathology laboratories |
Health |
19 June |
Note a: Quantium provided weekly PPE models to Health; models provided varied according to different policy settings regarding PPE use. This included scenarios where PPE was only provided to hospitals. Other scenarios included provision of PPE to aged care workers or the implementation of universal masking in Victoria.
Note b: DISER advised the ANAO that ‘while McKinsey was commissioned to develop the model, DISER owned and updated the model.’
Note c: Community health includes general practice clinics, pathology, aged care, ambulance and allied health care clinics.
Source: ANAO analysis of modelling used by Health and DISER to estimate COVID-19 demand for medical supplies.
4.21 Modelling expected usage of PPE and other medical products required a number of assumptions to be made including about the level of interventions, the rate of spread of the virus, hospitalisation rates and how products are used. Demand estimates throughout the procurement period were based on a wide range of assumptions and affected by real life events. For example, an initial estimated demand of 800 million to 1.2 billion surgical masks was reduced in April to less than 200 million due to the status of COVID-19 at that time.
Was a procurement requirement specified to guide procurement activities and decisions?
4.22 Timely communication of the requirement to officers involved in procurement is important for prioritising procurement activity and making decisions. The procurement requirement comprises information about the goods to be procured, their quality specifications and the quantities required.
4.23 Procurements were focused on masks from late January, but in March expanded to other types of PPE, COVID-19 tests and intensive care consumables. DISER was kept informed of product requirements by Health and adjusted its procurement activities and governance structure in response.
4.24 The HICG has noted in a ‘closure report’ that there was an initial lack of clarity around required approvals through the Therapeutic Goods Administration (TGA) and application of standards outside of TGA arrangements where products that do not make therapeutic claims are regulated as general consumer items. The HICG also found a general lack of clarity on the relevant standards and technical specifications for various types of PPE and other medical supplies. In interviews with officials from state and territory health authorities, the ANAO found that expectations for minimum standards varied by jurisdiction, potentially impacting on the willingness of states and territories to use NMS stocks.
4.25 The degree to which the quantities required were clearly specified varied by product category.
- Masks and other PPE — at the outset the goal was to procure as much PPE as possible, as quickly as possible. In the absence of information about state and territory stockpiles and procurement activity, the NMS requirement for masks and other PPE was based on national demand estimates, when these became available. From February 2020 onwards, these demand estimates fluctuated in the uncertain environment.
- Ventilators — the ventilator NMS procurement requirement was specified in late March 2020 and was clear. Taking into account an intensive care unit (ICU) capacity limitation, DISER and Health determined that 3,327 additional ventilators were required to meet ICU surge capacity.
- Test kits — there was no procurement requirement, in terms of quantity, established for COVID-19 tests and swabs. Health advised the ANAO that it sought to procure enough test kit components to support six weeks of testing in line with specifications in the Pandemic Health Intelligence Plan. Health has also advised the ANAO that ‘The approach to the procurement of pathology supplies was to allow usual commercial supply arrangements established by public and private pathology providers to operate, with close monitoring by Health, and procure a strategic reserve for the NMS for use in period of high demand and/or shortages or disruptions to commercial supply arrangements.’ Health did not specify to DISER a required quantity for swabs and DISER generated its own estimates.
Was the National Medical Stockpile procurement requirement met?
The NMS procurement requirement for invasive ventilators was exceeded. In the absence of a specific procurement target for PPE and swabs, the ANAO compared procurements of PPE and swabs to national health system demand estimates and found that the NMS procurement requirement for PPE and swabs was met, or exceeded once procurements by other actors including the states and territories are taken into account. The ANAO was unable to determine if the procurement requirement for COVID-19 tests was met due to no specified requirement or comparable demand estimates.
4.26 The ANAO examined:
- what the COVID-19 NMS procurement requirement, in terms of quantity, was and what has been procured; and
- whether NMS procurements met the requirement.
What was the National Medical Stockpile procurement requirement and what was procured?
4.27 As a supplementary stockpile, the procurement requirement for the NMS was the difference between estimated national health system demand and the quantity of NMS stock held at the start of the pandemic response (baseline stock) combined with what other actors in the system had or would procure.
4.28 shows the demand estimates for masks, gowns, goggles, gloves and swabs using the lowest and highest demand estimates that were generated before 30 June 2020 and that were based on at least nine months of demand to 31 December 2020 (refer Appendix 4), noting that a wide range of different assumptions, including the assumption of an unmitigated outbreak, informed these estimates.60 Estimates for COVID-19 tests, which were generated from 19 June 2020, were for a five-month period only and are therefore not included in the analysis.
4.29 Table 4.2 also shows baseline levels of NMS PPE stock at 30 January 2020 and state and territory PPE stock-in-hand as it was known by Health at 1 July 2020. The specific quantity of stocks independently held by other procurers for the national health system, such as hospitals and private pathology laboratories, were largely unknown to Health and are not shown here. This is particularly relevant for COVID-19 tests as high reliance was placed by Health on normal commercial supply chains established by private and state pathology laboratories to procure the necessary testing supplies.
4.30 No clear NMS procurement requirement was developed for masks and other PPE, swabs or tests. An NMS procurement requirement, in terms of quantity, was clearly established for ventilators only. This was possible because ICU capacity placed an upper limit on what could be procured and information about existing national stocks was good. In the absence of a specified NMS procurement requirement for all items except ventilators, national health system demand estimates were used by Health and DISER to guide procurement activity for PPE.
Table 4.2: Demand, supply and procurements of PPE, ventilators, swabs and tests (millions)
|
|
Demand estimatesa (January–July 2020) |
Baseline NMS stock (at 21 January 2020) |
State and territory stock (at 1 July 2020) |
NMS procurements (to 31 August 2020) |
|
|
|
(lowest) |
(highest) |
|
|
|
|
Surgical masks |
58 |
1,200 |
9 |
109 |
468.7b |
|
P2 masks |
32 |
251 |
12 |
50 |
135.3 |
|
Gowns / coveralls |
22 |
302 |
– |
15 |
54.8 |
|
Goggles / face shields |
6 |
57 |
– |
46 |
44.4 |
|
Gloves |
70 |
1,313 |
– |
194 |
565.3c |
|
Swabs |
10 |
11 |
– |
2 |
10.7 |
|
COVID-19 tests |
n/a |
n/a |
– |
– |
6.4d |
|
Ventilatorse |
7,500 |
8,000 |
– |
4,638f |
4,540g |
Note a: Demand estimates shown in this table used a wide range of different assumptions, but in all cases reflected between nine to 12 months of estimated national health system demand. The highest and lowest estimates of demand to 31 December 2020 are shown. For more information refer to Appendix 4. Other procured goods not included in these totals included hand sanitiser, thermometers, spill kits, chemical reagents for test kits and COVID-19 diagnostic systems.
Note b: Deeds of standing offer were negotiated with two domestic manufacturers of masks and mask quantities produced or to be produced by these suppliers are not included in these totals. Health reports of total mask supplies procured at 30 September 2020, which were 595 million surgical and 166 million P2 masks, included product obtained from these manufacturers.61
Note c: Contracted quantities of gloves were reported by Health to be substantially lower at 30 September 2020 (261 million). This was reflected in an October 2020 contract variation with the largest gloves supplier. A provision in the original contract for optional further supply of 300 million gloves in October, November and December 2020 was removed from the amended contract.
Note d: Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) tests only. Figure excludes one million procured point-of-care serology tests.
Note e: All quantities are in millions except for invasive ventilators.
Note f: As at 1 July 2020 states and territories held 5,838 invasive ventilators. Excluding 1,200 ventilators that were estimated to be needed for treating critically ill patients with illnesses other than COVID-19, this left a balance of 4,638 invasive ventilators available for treating COVID-19 patients.
Note g: Invasive ventilators only. Excludes 5,000 non-invasive ventilators that were also procured.
Source: ANAO analysis of Health and DISER documentation, including demand models, NMS reconciliations, reports of state and territory stock levels and executed contracts.
4.31 Health has undertaken 54 COVID-19 NMS procurements between 1 February and 31 August 2020, with a total value of $3.01 billion at 31 August 2020, excluding some freight (refer Appendix 5).62
Did National Medical Stockpile procurements meet requirements?
4.32 In the absence of an NMS procurement requirement for all items except ventilators, it is not possible to determine if Health met what was required to supplement the medical supplies held by the states and territories and other actors within the national health system. Figure 4.1 and Figure 4.2 compare actual procurements with the full range of demand estimates (refer Table 4.2). This does not take into account existing state and territory stocks and procurements, or procurements by other actors within the system.
Figure 4.1: Comparison of NMS procured quantities to overall national health system demand estimates —masks and PPE, at 31 August 2020
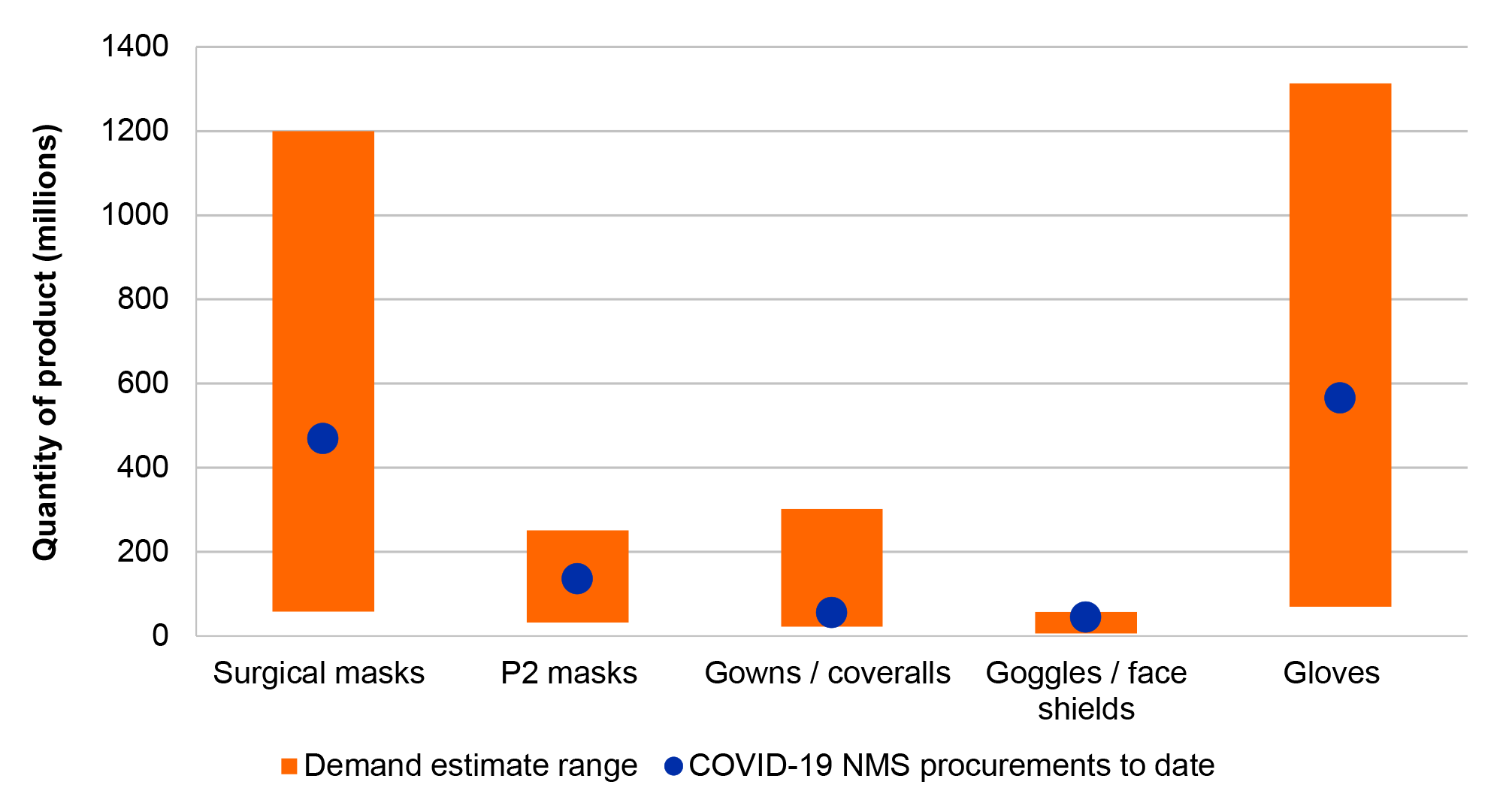
Source: ANAO analysis of Health demand and supply documentation and contracts executed.
Note: Contracted quantities of gloves were reported by Health to be substantially lower at 30 September 2020 (261 million). This was reflected in an October 2020 contract variation with the largest gloves supplier. A provision in the original contract for optional further supply of 300 million gloves in October, November and December 2020 was removed from the amended contract.
Figure 4.2: Comparison of NMS procured quantities to procurement requirement / overall national health system estimates — ventilators, swabs and COVID-19 tests, at 31 August 2020
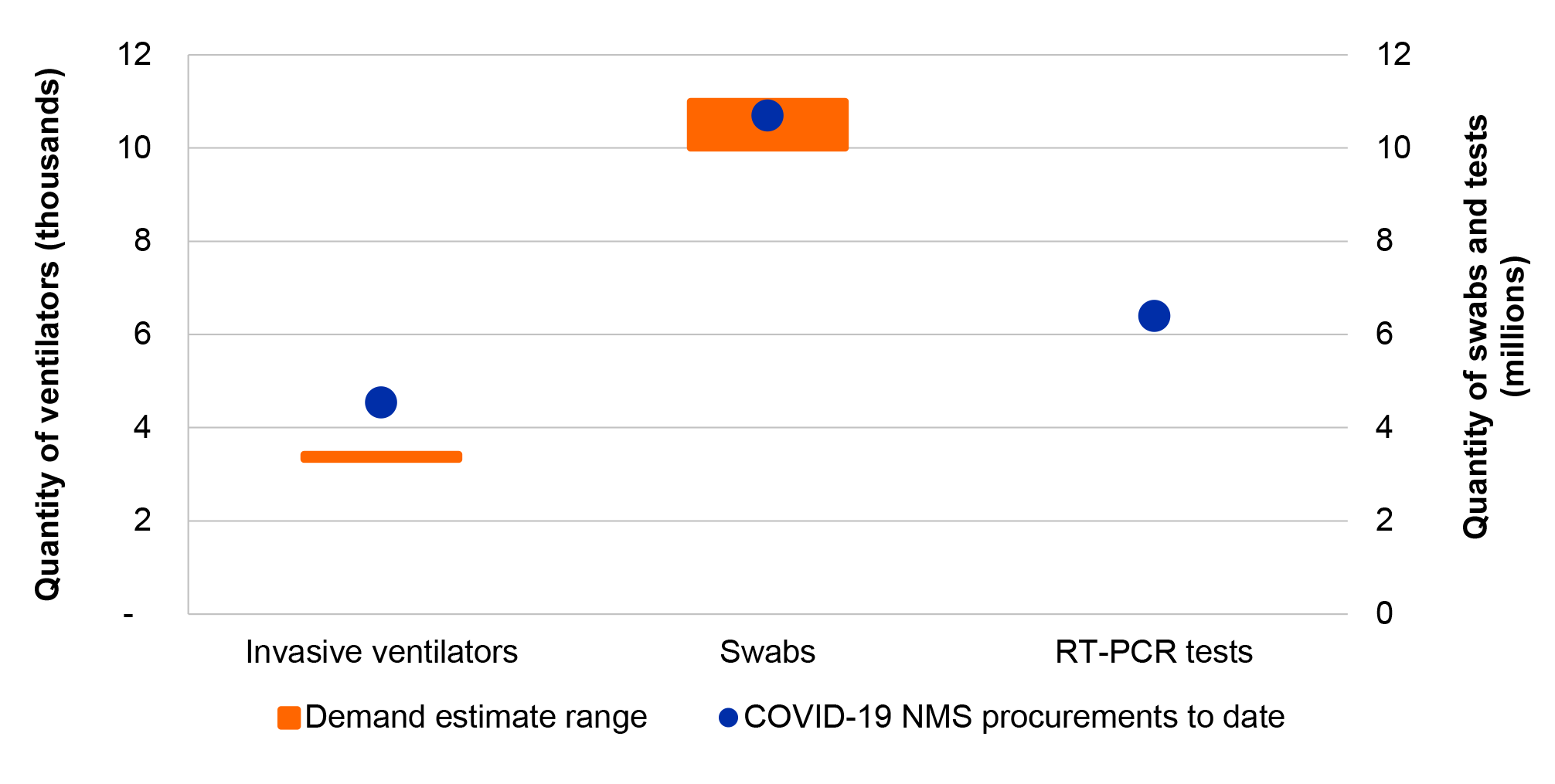
Note: There was no demand estimate for COVID-19 tests. Graph shows the actual NMS procurement requirement for invasive ventilators rather than a national demand estimate.
Source: ANAO analysis of Health demand and supply documentation and contracts executed.
4.33 With respect to ventilators, Figure 4.2 shows procurements for invasive ventilators only. In addition to these, Health acquired 5,000 non-invasive ventilators from a single Australian manufacturer at a cost of $36.5 million to supplement the supply of invasive ventilators and mitigate the risk that Australia could not procure sufficient invasive ventilators to meet demand. The Chief Scientist of Australia and the Department of Defence investigated the potential to convert these to invasive ventilators but Health advised that this was not pursued because sufficient procurement of the preferred invasive ventilators was achieved. Health has also advised that conversion remains a possibility ‘in the unlikely event of local need’ or for deployment to other countries as part of an overseas humanitarian assistance program.
4.34 With respect to tests, Figure 4.2 shows procurements for RT-PCR COVID-19 tests only. In addition to these, one million point-of-care serology tests were acquired for the NMS at a cost of $18.9 million.63 Subsequent post-market validation on behalf of the TGA found these tests performed below advertised specifications and should not be used for the diagnosis of acute COVID-19 infections. Health advised that these tests were not purchased for the purpose of, and should not be used to, diagnose acute infection of COVID-19, but may have a role to play in population level surveillance studies to further understand the level of COVID-19 infection in the Australian community should infection levels increase in future. It also advised that, at the time of purchase, there was the potential that there may have been a severe shortage of PCR tests, as occurred in other countries, and point-of-care serology tests may have been of clinical value in that context.
4.35 On 1 May Health advised DISER that sufficient supplies of all goods had been secured or were being negotiated, and that no further assistance with procurement would be required from DISER. It was the HICG’s understanding that at 15 May 2020, when it was disbanded, Australia had sufficient stock on hand and confirmed supply of all medical supplies to meet demand to December 2020, with the possible exception of gloves. In a June minute to the Acting Secretary of Health asking her to revoke the setting aside of the Commonwealth Procurement Rules for the COVID-19 procurements, it was noted that:
As the Department has successfully procured significant medical supplies, goods, and services to protect human health, and the COVID-19 emergency appears to have stabilised in Australia, the urgency of further procurement has reduced.
4.36 As at June 2020 the Australian Government’s intention was to maintain sufficient supply in the NMS to continue to support the needs of frontline healthcare workers, including in the event of a surge of cases, while also ensuring that there was not a significant surplus of supplies incurring storage and disposal costs. Cost management proposals included termination or transfer of agreements with international suppliers that had not yet delivered.
Appendices
Appendix 1 Entity responses
Australian Government Department of Health


ANAO comment on the Department of Health response
The first paragraph of the response refers to a 24 November 2020 letter in which the Associate Secretary of the Department of Health sought formal consideration that some information in the proposed report be omitted under paragraphs 37(2)(a) and 37(2)(e) of the Auditor-General Act 1997 (the Act). The Auditor-General did not form an opinion, based on the evidence provided, that there were public interest grounds under section 37 of the Act to omit this particular information from a public report. However, some of this particular information was excluded from the public report as the Auditor-General was comfortable that it did not have a material impact on the audit findings and conclusion.
Australian Government Department of Industry, Science, Energy and Resources

Australian Capital Territory Health Directorate

New South Wales Ministry of Health

Northern Territory Department of Health

Tasmania Department of Health

Victoria Department of Health and Human Services


Western Australia Department of Health

Appendix 2 National Medical Stockpile product category descriptions
|
Masks |
|
|
The first priority for NMS procurements was masks. The two main types are surgical and P2 masks (otherwise known as N95 respirators). Surgical masks are disposable, loose-fitting masks that cover the nose, mouth and chin. These are further differentiated by Standards Australia into level one, two and three masks, depending on their resistance to penetration by synthetic blood, with level three having the highest bacterial filtration efficiency and suitable for surgical procedures. P2 masks are tight-fitting masks that filter out harmful particles and that should be fit-tested before use. Seven components, or inputs, are involved in mask manufacture. |
|
|
Surgical mask |
P2/N95 respirator |
|

|

|
|
Tight-fitting respirators must seal to the wearer’s face. A mask fit test kit can be used to measure leakage around the face seal. |
|
|
Other PPE |
|
|
On 9 March 2020, Health identified other PPE as priority medical supplies, especially gowns, goggles and gloves. These products are designed to protect the wearer from the spread of disease, illness and infection. Surgical gowns may be used for any contamination risk level (level 1 to 4) and surgical isolation gowns are used for medium to high risk levels; these are regulated by the Therapeutic Goods Administration (TGA). Non-surgical gowns are used for low to minimum risk levels. The main types of gloves include surgical (sterile, precise sizing range, powder free) and examination (non-sterile protective barrier providing weaker chemical protection). The TGA maintains a register (Australian Register of Therapeutic Goods) which lists the 57 surgical glove products and 257 patient examination glove products that can be lawfully supplied in Australia. |
|
|
Surgical gown |
Surgical gloves |
|

|

|
|
Eye protection can be provided by safety goggles, safety glasses, eye shields or face shields. |
|
|
Goggles |
Face shield |
|

|

|
|
Other products grouped within PPE include thermometers, blood and fluid spill kits, mask fit test kits, clinical waste bags, waste bag closure devices (ties) and hand sanitiser. Thermometers include digital, digital infrared tympanic and liquid crystal forehead thermometers. Blood and fluid spill kits are either single use, or multiple use, packages that contain cleaning equipment (such as mops, cleaning bucket and cleaning agents) that help manage spills in areas where cleaning materials may not be readily available. Mask fit test kits test the fit of respirators for efficacy and can be digital or manual. |
|
|
Ventilators |
|
|
A ventilator is used to help or replace a patient’s respiratory function, completing the process of inhalation and exhalation. In March 2020, Health estimated that six per cent of patients who contract COVID-19 require ventilation, with half requiring treatment using invasive ventilators. There are two types of ventilators:
|
|
|
Non-invasive ventilator |
Invasive ventilator |
|

|

|
|
Test kits |
|
|
Early identification of COVID-19 cases through testing is a component of the public health response. The majority of testing in Australia has been conducted using the Reverse Transcription Polymerase Chain Reaction (RT-PCR) method. This method involves a three-stage process, with each stage involving specialised products.
|
|
|
RT-PCR test |
Swab |
|

|
|
|
To secure the supply of testing consumables against temporary shortages, Health identified a need to procure a stockpile of swabs, chemical reagents and machinery. Point-of-care serology tests are another type of test. Using blood samples obtained from finger pricks, these tests detect SARS-CoV-2 antibodies and can provide results in less than 15 minutes. In May 2020, the TGA advised that ‘Accurate identification of a COVID-19 infection based on serology results…requires an understanding of the antibody response profile which is currently not well defined. It is known that these tests can fail to detect COVID-19 if testing is performed in the acute phase of the infection prior to the development of detectable antibodies.’a |
|
|
Point-of-care serology test |
|
|

|
|
Note a: Therapeutic Goods Administration, Post-market evaluation of serology-based point of care tests [Internet], TGA, 13 August 2020, available from https://www.tga.gov.au/post-market-evaluation-serology-based-point-care-tests [accessed 4 September 2020].
Appendix 3 Minute invoking paragraph 2.6 of the Commonwealth Procurement Rules for the COVID-19 NMS procurements


Appendix 4 Supply and demand estimates for the National Medical Stockpile procurement requirement
Table A.1: Supply and demand estimates (Lower range demand)
|
|
Surgical mask |
P2 mask |
Gown/ cover all |
Goggles/ face shield |
Gloves |
Hand sanitiser |
Invasive ventilatora |
Swab |
COVID-19 test |
|||||||
|
# |
Type |
Name |
Source |
Entity |
Date (2020) |
Start (2020) |
End (2020) |
Minimum (millions)b |
||||||||
|
D1 |
Demand |
Overall usage of masks (moderate / high usage) |
Doherty Institute |
Health |
28/02 |
1/01 |
31/12 |
250 |
32 |
– |
– |
– |
– |
– |
– |
– |
|
D2 |
Demand |
Ventilator estimate |
Chief Scientist |
DISER |
22/03 |
22/03 |
31/12 |
– |
– |
– |
– |
– |
– |
8,000 |
– |
– |
|
D3 |
Demand |
PPE demand (restricted / broad usage) |
Quantium |
Health |
30/03 |
1/03 |
31/12 |
58 |
45 |
22 |
6 |
70 |
2 |
– |
– |
– |
|
D4 |
Demand |
Ventilator estimate (‘target capacity’) |
Health |
Health |
31/03 |
22/03 |
31/12 |
– |
– |
– |
– |
– |
– |
7,500 |
– |
– |
|
D5 |
Demand |
PPE projections update |
Quantium |
Health |
6/04 |
1/03 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D6 |
Demand |
PPE demand (3 scenarios of usage) — Draft |
Quantium |
Health |
6/04 |
1/03 |
31/12 |
60 |
51 |
45 |
12 |
100 |
2 |
– |
– |
– |
|
D7 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
9/04 |
1/04 |
31/12 |
110 |
115 |
– |
– |
– |
– |
– |
– |
– |
|
D8 |
Demand |
Estimate of swab demand |
DISER |
DISER |
15/04 |
15/04 |
31/08 |
– |
– |
– |
– |
– |
– |
– |
3 |
– |
|
D9 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
1/05 |
1/03 |
Varied |
831 |
80 |
– |
– |
– |
– |
– |
– |
– |
|
D10 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
1/05 |
1/05 |
31/12 |
526 |
134 |
86 |
66 |
1,590 |
8 |
6 |
– |
– |
|
D11 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
7/05 |
1/03 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D12 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
7/05 |
7/05 |
31/12 |
446 |
127 |
82 |
63 |
1,493 |
7 |
6 |
– |
– |
|
D13 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
14/05 |
14/05 |
31/12 |
118 |
34 |
30 |
13 |
160 |
3 |
– |
– |
– |
|
D14 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
14/05 |
14/05 |
31/12 |
446 |
1227 |
82 |
63 |
1,493 |
7 |
6 |
– |
– |
|
D15 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
20/05 |
20/05 |
31/12 |
420 |
127 |
77 |
60 |
1,406 |
7 |
15 |
– |
– |
|
D16 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
25/05 |
25/05 |
31/12 |
420 |
120 |
77 |
60 |
1,406 |
7 |
15 |
– |
– |
|
D17 |
Demand |
Test kit modelling summaryc |
DISER |
DISER |
31/05 |
1/01 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
10 |
– |
|
D18 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
5/06 |
4/06 |
31/12 |
95 |
30 |
28 |
7 |
128 |
2 |
– |
– |
– |
|
D19 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
11/06 |
1/03 |
31/12 |
122 |
41 |
37 |
9 |
173 |
3 |
– |
– |
– |
|
D20 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
18/06 |
17/06 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D21 |
Demand |
Model future laboratory testing demand |
Doherty Institute |
Health |
19/06 |
1/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
46 |
|
D22 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
25/06 |
24/06 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D23 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
26/06 |
1/03 |
31/12 |
193 |
87 |
171 |
12 |
458 |
8 |
– |
– |
– |
|
D24 |
Demand |
National inventory needs assessment — incomplete |
State health authorities |
Health |
1/07 |
1/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
4 |
– |
|
D25 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
1/07 |
24/06 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D26 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
3/07 |
1/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D27 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
9/07 |
8/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D28 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
21/07 |
21/07 |
31/12 |
314 |
79 |
60 |
46 |
1,043 |
6 |
15 |
– |
– |
|
D29 |
Demand |
Universal masking demand |
Quantium |
Health |
23/07 |
20/07 |
31/12 |
133 |
– |
– |
– |
– |
– |
– |
– |
– |
|
D30 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
30/07 |
29/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D31 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
6/08 |
5/08 |
21/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D32 |
Demand |
Test kit modelling summaryc |
DISER taskforce |
DISER |
9/08 |
1/01 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
11 |
– |
|
D33 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
13/08 |
12/08 |
11/11 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D34 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
20/08 |
19/08 |
18/11 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
S1 |
Supply |
Stock-in-hand (NMS) — Masks |
EY Stock take |
Health |
30/06/2019 |
9 |
13 |
– |
– |
– |
– |
– |
– |
– |
||
|
S2 |
Supply |
Stock-in-hand (NMS) — Masks |
Health |
Health |
24/01/2020 |
9 |
12 |
– |
– |
– |
– |
– |
– |
– |
||
|
S3 |
Supply |
Ventilators nationwide estimate |
ANZICS |
DISER |
27/03/2020 |
– |
– |
– |
– |
– |
– |
4,173 |
– |
– |
||
|
S4 |
Supply |
Stock-in-hand (state) — Incomplete |
State health authorities |
Health |
23/04/2020 |
14 |
7 |
3 |
1 |
78 |
0 |
– |
1 |
– |
||
|
S5 |
Supply |
Stock-in-hand (NMS) |
EY Stock take |
Health |
30/06/2020 |
183 |
42 |
7 |
6 |
39 |
0 |
5,400 |
5 |
1 |
||
|
S6 |
Supply |
Stock-in-hand (state) — Incomplete |
State health authorities |
Health |
1/07/2020 |
109 |
50 |
15 |
46 |
194 |
5 |
5,838 |
2 |
– |
||
|
|
Capacity |
ICU beds |
ANZICS |
DISER |
27/03/2020 |
– |
– |
– |
– |
– |
– |
6,454 |
– |
– |
||
|
|
COVID-19 NMS procurements to date |
ANAO analysis |
Health |
31/08/2020 |
469 |
135 |
55 |
44 |
565 |
11 |
4,540 |
11 |
6 |
|||
|
|
Stated NMS procurement requirement (if applicable) |
– |
– |
– |
– |
– |
– |
3,327 |
– |
– |
||||||
Note a: Ventilator estimates D2 and D4 were based on an intensive care unit surge capacity limitation. Other ventilator estimates assumed that the COVID-19 infection rate would remain at levels consistent with those at the time of the estimate.
Note b: All quantities in millions except ventilators.
Note c: Estimates of COVID-19 tests were done by DISER to inform the procurement of swabs.
Source: ANAO analysis of Health and DISER documentation, including executed contracts.
Table A.2: Supply and demand estimates (Higher range demand)
|
|
Surgical mask |
P2 mask |
Gown/cover all |
Goggles/ face shield |
Gloves |
Hand sanitiser |
Invasive ventilatora |
Swab |
COVID-19 test |
|||||||
|
# |
Type |
Name |
Source |
Entity |
Date (2020) |
Start (2020) |
End (2020) |
Maximum (millions)b |
||||||||
|
D1 |
Demand |
Overall usage of masks (moderate / high usage) |
Doherty Institute |
Health |
28/02 |
1/01 |
31/12 |
1,200 |
50 |
– |
– |
– |
– |
– |
– |
– |
|
D2 |
Demand |
Ventilator estimate |
Chief Scientist |
DISER |
22/03 |
22/03 |
31/12 |
– |
– |
– |
– |
– |
– |
8,000 |
– |
– |
|
D3 |
Demand |
PPE demand (restricted / broad usage) |
Quantium |
Health |
30/03 |
1/03 |
31/12 |
119 |
205 |
161 |
57 |
633 |
11 |
– |
– |
– |
|
D4 |
Demand |
Ventilator estimate (‘target capacity’) |
Health |
Health |
31/03 |
22/03 |
31/12 |
– |
– |
– |
– |
– |
– |
7,500 |
– |
– |
|
D5 |
Demand |
PPE projections update |
Quantium |
Health |
6/04 |
1/03 |
31/12 |
232 |
116 |
81 |
28 |
586 |
4 |
– |
– |
– |
|
D6 |
Demand |
PPE demand (3 scenarios of usage) — Draft |
Quantium |
Health |
6/04 |
1/03 |
31/12 |
220 |
117 |
79 |
28 |
590 |
4 |
– |
– |
– |
|
D7 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
9/04 |
1/04 |
31/12 |
194 |
195 |
– |
– |
– |
– |
– |
– |
– |
|
D8 |
Demand |
Estimate of swab demand |
DISER |
DISER |
15/04 |
15/04 |
31/08 |
– |
– |
– |
– |
– |
– |
– |
5 |
– |
|
D9 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
1/05 |
1/03 |
Varied |
887 |
165 |
– |
– |
– |
– |
– |
– |
– |
|
D10 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
1/05 |
1/05 |
31/12 |
532 |
244 |
196 |
178 |
1,824 |
9 |
3,500 |
– |
– |
|
D11 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
7/05 |
1/03 |
31/12 |
518 |
149 |
139 |
49 |
843 |
– |
– |
– |
– |
|
D12 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
7/05 |
7/05 |
31/12 |
452 |
237 |
191 |
174 |
1,727 |
9 |
3,500 |
– |
– |
|
D13 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
14/05 |
14/05 |
31/12 |
150 |
149 |
121 |
40 |
635 |
11 |
– |
– |
– |
|
D14 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
14/05 |
14/05 |
31/12 |
452 |
237 |
191 |
174 |
1,727 |
9 |
3,500 |
– |
– |
|
D15 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
20/05 |
20/05 |
31/12 |
452 |
229 |
191 |
174 |
1,727 |
9 |
3,500 |
– |
– |
|
D16 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
25/05 |
25/05 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
D17 |
Demand |
Test kit modelling summaryc |
DISER |
DISER |
31/05 |
1/01 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
11 |
– |
|
D18 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
5/06 |
4/06 |
31/12 |
119 |
135 |
113 |
33 |
621 |
10 |
– |
– |
– |
|
D19 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
11/06 |
1/03 |
31/12 |
168 |
182 |
149 |
35 |
846 |
14 |
– |
– |
– |
|
D20 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
18/06 |
17/06 |
31/12 |
– |
– |
111 |
– |
– |
– |
– |
– |
– |
|
D21 |
Demand |
Model future laboratory testing demand |
Doherty Institute |
Health |
19/06 |
1/07 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
– |
68 |
|
D22 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
25/06 |
24/06 |
31/12 |
138 |
168 |
157 |
26 |
851 |
14 |
– |
– |
– |
|
D23 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
26/06 |
1/03 |
31/12 |
227 |
251 |
302 |
30 |
1,313 |
22 |
– |
– |
– |
|
D24 |
Demand |
National inventory needs assessment — incomplete |
State health authorities |
Health |
1/07 |
1/07 |
31/12 |
94 |
33 |
52 |
34 |
494 |
1 |
– |
4 |
– |
|
D25 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
1/07 |
24/06 |
31/12 |
138 |
168 |
185 |
26 |
849 |
14 |
– |
– |
– |
|
D26 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
3/07 |
1/07 |
31/12 |
134 |
161 |
178 |
26 |
812 |
14 |
– |
– |
– |
|
D27 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
9/07 |
8/07 |
31/12 |
131 |
159 |
175 |
26 |
804 |
13 |
– |
– |
– |
|
D28 |
Demand |
HICG Medical PPE demand-supply dashboard |
McKinsey/ DISER |
DISER |
21/07 |
21/07 |
31/12 |
320 |
188 |
170 |
157 |
1,276 |
7 |
3,500 |
– |
– |
|
D29 |
Demand |
Universal masking demand |
Quantium |
Health |
23/07 |
20/07 |
31/12 |
2,064 |
– |
– |
– |
– |
– |
– |
– |
– |
|
D30 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
30/07 |
29/07 |
31/12 |
478 |
167 |
208 |
33 |
904 |
15 |
– |
– |
– |
|
D31 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
6/08 |
5/08 |
21/12 |
470 |
165 |
203 |
33 |
895 |
15 |
– |
– |
– |
|
D32 |
Demand |
Test kit modelling summaryc |
DISER taskforce |
DISER |
9/08 |
1/01 |
31/12 |
– |
– |
– |
– |
– |
– |
– |
11 |
– |
|
D33 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
13/08 |
12/08 |
11/11 |
283 |
57 |
89 |
15 |
329 |
5 |
– |
– |
– |
|
D34 |
Demand |
PPE demand (3 scenarios of usage) |
Quantium |
Health |
20/08 |
19/08 |
18/11 |
270 |
53 |
87 |
32 |
315 |
5 |
– |
– |
– |
|
S1 |
Supply |
Stock-in-hand (NMS) — Masks |
EY Stock take |
Health |
30/06/2019 |
9 |
13 |
– |
– |
– |
– |
– |
– |
– |
||
|
S2 |
Supply |
Stock-in-hand (NMS) — Masks |
Health |
Health |
24/01/2020 |
9 |
12 |
– |
– |
– |
– |
– |
– |
– |
||
|
S3 |
Supply |
Ventilators nationwide estimate |
ANZICS |
DISER |
27/03/2020 |
– |
– |
– |
– |
– |
– |
4,173 |
– |
– |
||
|
S4 |
Supply |
Stock-in-hand (state) — Incomplete |
State health authorities |
Health |
23/04/2020 |
14 |
7 |
3 |
1 |
78 |
0 |
– |
1 |
– |
||
|
S5 |
Supply |
Stock-in-hand (NMS) |
EY Stock take |
Health |
30/06/2020 |
183 |
42 |
7 |
6 |
39 |
0 |
5,400 |
5 |
1 |
||
|
S6 |
Supply |
Stock-in-hand (state) — Incomplete |
State health authorities |
Health |
1/07/2020 |
109 |
50 |
15 |
46 |
194 |
5 |
5,838 |
2 |
– |
||
|
|
Capacity |
ICU beds |
ANZICS |
DISER |
27/03/2020 |
– |
– |
– |
– |
– |
– |
6,454 |
– |
– |
||
|
|
COVID-19 NMS procurements to date |
ANAO analysis |
Health |
31/08/2020 |
469 |
135 |
55 |
44 |
565 |
11 |
4,540 |
11 |
6 |
|||
|
|
Stated NMS procurement requirement (if applicable) |
– |
– |
– |
– |
– |
– |
3,327 |
– |
– |
||||||
Note a: Ventilator estimates D2 and D4 were based on an intensive care unit surge capacity limitation. Other ventilator estimates assumed that the COVID-19 infection rate would remain at levels consistent with those at the time of the estimate.
Note b: All quantities in millions except ventilators.
Note c: Estimates of COVID-19 tests were done by DISER to inform the procurement of swabs.
Source: ANAO analysis of Health and DISER documentation, including executed contracts.
Appendix 5 COVID-19 National Medical Stockpile procurements at 31 August 2020
COVID-19 National Medical Stockpile procurements at 31 August 2020

Note a: Some contracts are for goods across multiple categories. Where this occurs contracts have been allocated to a category according to the relative quantity or value of the goods.
Note b: Contract values are based on executed contracts at 31 August 2020. All values are in Australian dollars (AUD) excluding GST and rounded to the nearest million dollars. Suppliers awarded contracts of less than $1 million in value are not shown. Many contracts did not include freight costs which were invoiced separately. Dissolved contracts are not included or shown.
Note c: Deed of standing offer.
Source: ANAO analysis of DISER contract referrals and contracts awarded by Health.
Footnotes
1 Further details on the ANAO’s COVID-19 multi-year audit strategy can be found at: https://www.anao.gov.au/work-program/covid-19
2 Biosecurity (Listed Human Diseases) Amendment Determination 2020, 21 January 2020.
3 Biosecurity (Human Biosecurity Emergency) (Human Coronavirus with Pandemic Potential) Declaration 2020, 18 March 2020.
4 Department of the Treasury, Economic response to the Coronavirus [Internet], 25 May 2020, available at: https://treasury.gov.au/sites/default/files/2020-05/Overview-Economic_R… [accessed 17 June 2020].
5 AHPPC membership is comprised of the Chief Health Officers from each state and territory, representatives from several Australian Government departments and agencies (including Emergency Management Australia and the Australian Defence Force) and technical experts and advisors. It is chaired by the Commonwealth Chief Medical Officer.
6 CBRN countermeasure supplies include antibiotics, vaccines, antidotes, decorporation agents for radioactive contamination and personal protective equipment.
7 Health drew against four Advances to the Finance Minister in 2019–20, comprising $100 million (3 March), $200 million (9 March), $800 million (3 April) and $780 million (9 April) [Auditor-General Report No.36a 2019–20 Advances to the Finance Minister for the period 1 July 2019 to 24 April 2020, p.13]. Health then drew a further $700 million for ‘the purchase of personal protective equipment’ against the Coronavirus Economic Response Package Act No 2 2020, which received Royal Assent on 24 March 2020. Health subsequently obtained the Prime Minister’s authority to make further financial commitments of up to $650 million to supplement supplies of PPE and other medical supplies for the NMS, noting that the fiscal and underlying cash impacts of this additional authority to commit would be finalised with the Finance Minister at a later time when they were better able to be fully quantified. The Prime Minister agreed to this on 1 May 2020.
8 Department of Finance, Commonwealth Procurement Rules, Finance, 20 April 2019, paragraphs 4.4–4.6. Achieving value for money means that officials must be satisfied that the procurement is non-discriminatory; uses public resources efficiently, effectively, economically and ethically; is transparent; has considered risk; and is commensurate with the scale and scope of the business requirement.
9 Section 21 of the PGPA Act states: ‘The accountable authority must govern the entity in accordance with paragraph 15(1)(a) in a way that is not inconsistent with the policies of the Australian Government. Section 15 states: ‘The accountable authority of a Commonwealth entity must govern the entity in a way that promotes the proper use and management of public resources for which the authority is responsible…’ Section 8 of the PGPA Act defines ‘proper’ as when used in relation to the use or management of public resources, means efficient, effective, economical and ethical.
10 Included within the category of other PPE is gowns, gloves, face shields, goggles, thermometers, blood and fluid spill kits and mask fit test kits.
11 $10.4 million was allocated to support ongoing operational and warehousing activities of the NMS including logistics, annual disposal of expired items and annual insurance payments.
12 Auditor-General Report No.53 2013–14 Management of the National Medical Stockpile, p. 24.
13 The NHEMS includes representation from each state and territory, New Zealand and several Commonwealth agencies. The CBRN Technical Panel is a sub-group of the NHEMS that provides advice on the medical aspects of a CBRN response.
14 Membership of the NMS Advisory Group is comprised of state and territory health department representatives and it is chaired by Health. Meetings occur annually and outputs are considered by the AHPPC.
15 Australian Government Department of Health, National Medical Stockpile Strategic Plan 2015-19, p.11. ‘The primary risk to the Stockpile is the foundation risk — the capacity to source required supplies in a health emergency. Australia’s geographic location and lack of local production capability in specialist pharmaceuticals and protective equipment would be major hurdles in sourcing essential supplies during an emergency. The existence of the Stockpile, as a national asset and strategic reserve of supplies for use in a health emergency response, is an important mitigation option to reduce the impact of the foundation risk.’
16 House of Representatives Standing Committee on Health and Ageing, Diseases have no borders: Report on the inquiry into health issues across international borders, Department of House of Representatives, Canberra, 2013, p.101, paragraph 5.39.
17 Center for Health Security, Johns Hopkins Bloomberg School of Public Health and the Nuclear Threat Initiative, 2019 GHS Index Country Profile for Australia [Internet], GHSI, October 2019, available from https://www.ghsindex.org/country/australia [accessed 16 July 2020]. Health advised the ANAO that the GHSI (indicator 4.5) is referencing the 2016 Emergency Response Plan for Communicable Disease Incidents of National Significance (CD Plan) which was replaced with a new CD Plan in 2018.
18 Australian Government Department of Health, Australian Health Management Plan for Pandemic Influenza, 2019, p.199.
19 Department of Health and Ageing, Australia’s Health Sector Response to Pandemic (H1N1) 2009: Lessons Identified, 2011. Available from http://www.health.gov.au [accessed 31 July 2020].
20 Membership of the HICG included representatives from DISER, Health, the Medical Technologies Association of Australia, the Australian Chamber of Commerce and Industry and the Advanced Manufacturing Growth Centre.
21 The NHRA is an agreement between the Australian Government and all state and territory governments which serves as ‘the key mechanism for the transparency, governance and financing of Australia’s public hospital system.’ Through this agreement, ‘the Australian Government contributes funds to the states and territories for public hospital services. This includes services delivered through emergency departments, hospitals and community health settings.’ Australian Government Department of Health, About the NHRA [Internet], available from https://www.health.gov.au/ [accessed 20 October 2020].
22 Addendum to National Health Reform Agreement 2020–25 [Internet], available from http://www.federalfinancialrelations.gov.au/ [accessed 8 October 2020], clause 10.
23 Independent Hospital Pricing Authority, National Hospital Cost Data Collection, Data Request Specifications v1.0, Round 22 (Financial Year 2017-18) [Internet], available from https://www.ihpa.gov.au/publications/national-hospital-cost-data-collec… [accessed 8 October 2020].
24 The Independent Hospital Pricing Authority (IHPA) determines the price and cost for health care services provided by public hospitals, to inform decision making in relation to the funding of public hospitals. IHPA members are appointed by the Australian government with the agreement of the states and territories. Independent Hospital Pricing Authority, Pricing Authority [Internet], IHPA, available from https://www.ihpa.gov.au/who-we-are/pricing-authority [accessed 20 October 2020].
25 Australian Government, Review of the National Medical Stockpile, February 2011.
26 The funding allocation does not include funding for costs associated with labour involved in conducting the procurements or additional resources supplied by Health or other Australian government departments and agencies. It also does not include logistical or financial support (loan agreements or grants) provided to various domestic manufacturers to scale up capability and capacity to manufacture PPE, ventilators and test kit components.
27 World Health Organisation, ‘Shortage of personal protective equipment endangering health workers worldwide’, media release, Geneva, 3 March 2020.
28 Department of Finance, Commonwealth Procurement Rules, April 2019, p. 16.
29 DISER’s proposed due diligence reviews included whether products were fit for purpose and conformed to industry standards; the company’s ability to supply and deliver on time; and whether the company was registered with the Australian Taxation Office for goods and services tax, if applicable.
30 TGA is Australia’s regulatory authority for therapeutic goods, responsible for assessing and monitoring therapeutic goods to ensure they are of an acceptable standard. Therapeutic Goods Administration, About the TGA [Internet], TGA, available from https://www.tga.gov.au/about-tga [accessed 20 October 2020].
31 Public Governance, Performance and Accountability Act 2013.
32 Department of Finance, Commonwealth Procurement Rules, April 2019, paragraph 6.6, and the Public Governance, Performance and Accountability Act 2013, section 29.
33 ANAO, Management of conflicts of interest in procurement activity and grants programs, June 2020, Canberra. This ANAO paper was published after most of the procurements had been completed.
34 Department of Finance, Commonwealth Procurement Rules, Finance, 2019, paragraph 8.2.
35 At least one commitment approval minute was considered for each executed COVID-19 contract to 31 August 2020 for PPE, medical equipment and test kits. Two commitment approval minutes were examined for one contract and one commitment approval minute covered two contracts.
36 Number of proposed controls for each risk ranged from one to 21.
37 Controls included due diligence checks on companies undertaken prior to recommendation for product supply; ensuring appropriate records are kept and practices documented and provided to Health; value for money assessments applied for all proposals received; interactions with stakeholders are recorded and cross shared with external agencies; standard operating procedures for specific business processes are in place; regular briefings to the executive and clear advice provided to the Minister; robust planning and clearly defined governance structures, including the mobilisation of DISER’s workforce to deliver priority; and a lessons learned process is in place. The ANAO has not assessed whether these controls were consistently applied.
38 154 suppliers were listed on this tracking sheet as at 10 August 2020.
39 The other four themes identified were fraud and compliance, planning and delivery, budget and financial management and record keeping.
40 The Minderoo Foundation is a philanthropic organisation. The review report indicates that the Minderoo Foundation contacted DISER in March 2020 to offer support in procuring and transporting medical equipment and PPE necessary to respond to the COVID 19 pandemic. The Acting Secretary of the Department of Health agreed to reimburse the Minderoo Foundation for the costs of purchase and transport of medical equipment and supplies provided by Minderoo Foundation and the Minderoo Foundation procured laboratory equipment and test kits from the Beijing Genomics Institute as well as PPE items from other suppliers. The procurement was conducted by the Minderoo Foundation’s wholly owned subsidiary, First Sourcing Logistics Pty Ltd.
41 The procurement internal audit assessed the appropriateness of COVID-19 related procurement activities undertaken by the department, in relation to PGPA Act requirements, and the principles and requirements of the CPRs, where relevant. The governance fundamentals internal audit reviewed the extent to which the arrangements for the establishment, oversight and ongoing management of the department’s COVID-19 taskforce activities are consistent with the fundamentals of sound governance.
42 Department of Finance, Commonwealth Procurement Rules, Finance, 20 April 2019, paragraph 9.7 states that the procurement threshold (including GST) is $80,000 for non-corporate Commonwealth entities, other than for procurements of construction services, and paragraph 9.8 states that open tender ‘involves publishing an open approach to market and inviting submissions.’ Open tender includes procurements conducted through panels that were established using open tender.
43 Department of Finance, COVID-19 Procurement Policy Note, [accessed from https://www.finance.gov.au/government/procurement/covid-19-procurement-policy-note on 13 August 2020].
44 Auditor-General Report No.27 2019–20 Australian Government Procurement Contract Reporting Update, Tables 3.3 and 3.4.
45 In addition, section 25 of the PGPA Act provides that the accountable authority of a non-corporate Commonwealth entity, such as a department of state, must govern the entity in accordance with paragraph 15(1)(a) in a way that is not inconsistent with the policies of the Australian Government.
46 ANAO Audit Report No.37 2019–20 Procurement of garrison support and welfare services, page 31.
47 Department of Defence, Defence Procurement Policy Manual, Version 1.5, Defence, 1 July 2019, pages 32 and 34 [Department of Defence, Procurement [Internet], available from https://www.defence.gov.au/ [accessed 24 October 2020].
48 Department of Health, Australian Health Sector Emergency Response Plan for Novel Coronavirus (COVID-19), Canberra, 2020.
49 Auditor-General Report No.53 2013–14 Management of the National Medical Stockpile, p. 84.
50 Health advised the ANAO that jIMMY is backed up regularly on portable hard drives held in separate locations.
51 Multiple users have access under a single identity. Actions in relation to the system, database or data cannot be attributed to an individual, and logs of actions undertaken cannot be maintained in a way that cannot be modified by these users.
52 An IRAP assessor assess the implementation, appropriateness and effectiveness of a system’s security controls [Australian Cyber Security Centre, What is an IRAP Assessment?, Australian Signals Directorate. Available from https://www.cyber.gov.au/acsc/view-all-content/programs/irap/what-is-ir… [accessed 17 September 2020]].
53 ANZICS is an advocacy organisation for intensive care related matters that conducts clinical research and analysis of critical care resources. Members include intensive care medical practitioners, allied health practitioners, nurses and trainees.
54 The Minister for Health announced the creation of CHRIS on 24 April, which was developed by Health, ANZICS and Ambulance Victoria. From 1 May the Commonwealth and state and territory health authorities were able to use CHRIS to access data on ICU capacity and utilisation.
55 A DISER ‘market investigation’ into COVID-19 testing supplies, provided to Health, reported that in a survey of the Public Health Laboratory Network 71 per cent of Australian public laboratories used this manufacturer’s system. The analysis did not include private laboratories.
56 The Medical Technology Association of Australia is a national association representing manufacturers and suppliers of medical technology used in the diagnosis, prevention, treatment and management of disease and disability.
57 The Public Health Laboratory Network is a group of laboratories in Australia and New Zealand with the role of providing leadership on public health microbiology and communicable disease control. It is a subcommittee of the Australian Health Protection Principal Committee and its membership includes representatives from the Communicable Diseases Network Australia, state and territory organisations and the World Health Organisation.
58 Global Trade Alert, University of St Gallen, Switzerland, Tackling COVID-19 together - the trade policy dimension (23 March 2020), p.2.
59 Department of Health, Coronavirus (COVID-19) in Australia – Pandemic Health Intelligence Plan, Canberra, 2020.
60 The demand models which meet these parameters in Appendix 3 are D1, D2, D3, D4, D5, D6, D7, D11, D17, D19 and D23.
61 Australian Government Department of Health, Budget 2020-21: Supporting our hospitals - COVID-19 National Medical Stockpile [Internet], Health, 6 October 2020, available from https://www.health.gov.au/resources/publications/budget-2020-21-support… [accessed 26 November 2020].
62 Procurement of pharmaceuticals, including of hydroxychloroquine, was not considered in this audit.
63 These totals exclude one aborted contract for an additional 500,000 point-of-care serology tests.