Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
National Cervical Screening Program-Follow-up
The objective of this audit was to assess the progress made by DoHA and Medicare Australia (recommendation 3) in addressing the four recommendations from ANAO Audit Report No.50, 2000–01 designed to improve the administration and performance of NCSP.
Summary
Overview
In 2001, the Australian National Audit Office (ANAO) completed an audit of the Australian Government Department of Health and Aged Care's (now the Department of Health and Ageing—DoHA) administration of the National Cervical Screening Program (NCSP). The audit report included four recommendations.1 Of these, three were directed to DoHA and one recommendation was jointly directed to DoHA and to the Health Insurance Commission (now Medicare Australia).
Background
Cervical cancer, like other cancers, is a disease where normal cells change, begin to multiply, and form a growth or tumour.2 The Cancer Council of Australia has reported that the risk of Australian women developing cervical cancer before the age of 75 years is one in 183, with cervical cancer the eighteenth most common cause of cancer death in Australian women.3
The incidence of, and mortality from, cervical cancer in Australia has decreased significantly over the last two decades. A major factor contributing to improved cervical cancer health outcomes for Australian women has been the introduction of a coordinated population screening program that aims to detect pre-cancerous abnormalities and reduce the number of abnormalities that develop into cervical cancer.
Cervical screening has been available for Australian women since the 1960s, but it was largely opportunistic. Cervical screening became a more structured program in 1991. In 1995, the program became known as the National Cervical Screening Program. NCSP aims to reduce morbidity and deaths from cervical cancer, in a cost-effective manner through an organised approach to cervical screening.
Under the auspices of NCSP, nearly three and a half million women in Australia, aged 20 and over, had Pap smears in 2003–04.4
Audit Objective
The objective of this audit was to assess the progress made by DoHA and Medicare Australia (recommendation 3) in addressing the four recommendations from ANAO Audit Report No.50, 2000–01 designed to improve the administration and performance of NCSP.
Key findings
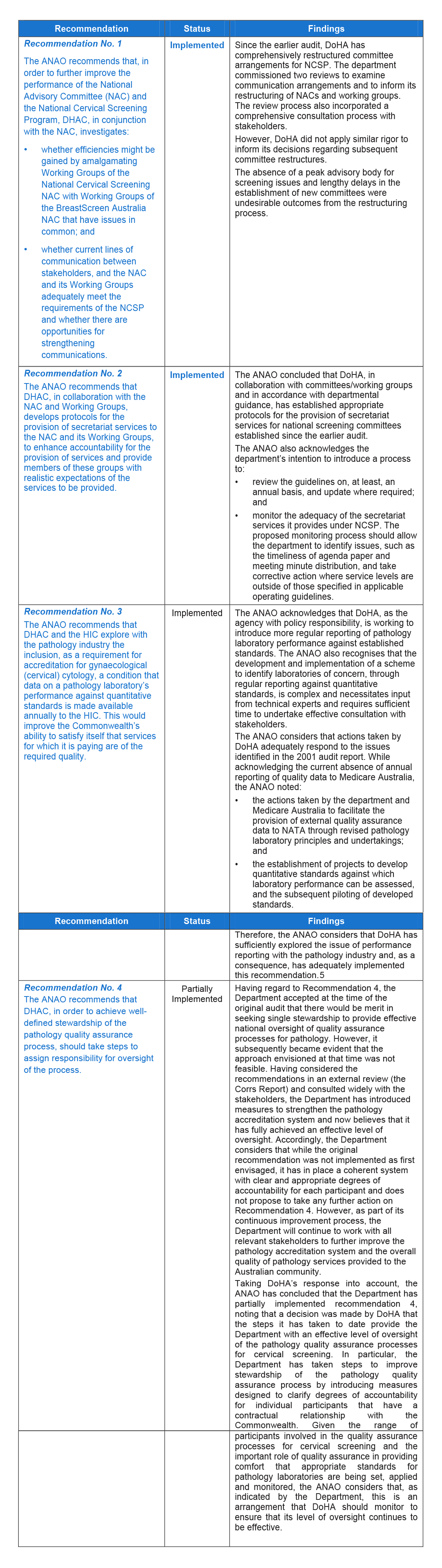
Table 1 summarises the ANAO's key findings against each of the recommendations from the earlier audit.
Table 1: Key findings
Overall conclusion
The ANAO concluded that DoHA has made progress against the recommendations of Audit Report No.50, 2000–01 directed to improvements in the administration of NCSP, with three recommendations implemented6 and for one recommendation (recommendation 4), partially implemented.
Notwithstanding, the ANAO considers that DoHA's implementation of all the recommendations from the earlier audit would have benefited from a more structured approach to planning and greater consideration of the risks to timely implementation. The ANAO also noted weaknesses in DoHA's monitoring of implementation activities, which impacted upon the department's ability to make informed decisions regarding the actions required to successfully implement the recommendations.
The ANAO makes no further recommendations in this report.
Agency responses
DoHA's response
DoHA provided the following overall comment on the follow-up audit:
The Department notes the ANAO conclusion that Recommendation 4 from Audit Report No. 50 of 2000–2001 has been partially implemented.
At the time of the original audit, the Department accepted that there would be merit in seeking single stewardship to provide effective national oversight of quality assurance processes for pathology. However, it subsequently became evident that the approach envisioned at that time was not feasible. Having considered the recommendations in an external review (the Corrs Report) and consulted widely with the stakeholders, the Department has introduced measures to strengthen the pathology accreditation system and now believes that it has fully achieved an effective level of oversight. Accordingly, the Department considers that while the original recommendation was not implemented as first envisaged, it has in place a coherent system with clear and appropriate degrees of accountability for each participant and does not propose to take any further action on Recommendation 4. However, as part of its continuous improvement process, the Department will continue to work with all relevant stakeholders to further improve the pathology accreditation system and the overall quality of pathology services provided to the Australian community.
Medicare Australia's response
Medicare Australia provided the following overall comment on the follow-up audit.
Medicare Australia agrees with the ANAO that recommendation 3 has been implemented and supports the findings identified by ANAO in relation to that recommendation.
Footnotes
1 Australian National Audit Office Audit Report No.50, 2000–01, The National Cervical Screening Program, Canberra.
2 See Appendix 1 for further information on cervical cancer.
3 The Cancer Council of Australia, Position Statement: Cervical Cancer Screening, [Internet] Sydney, 2006, p.1, available from <http://www.cancer.org.au/documents/Cervical_cancer_screening_pos_statem…;
[accessed 23 January 2007].
4 Australian Institute of Health and Welfare, Media Release: Early detection curbing cervical cancer rates [Internet], Canberra, 2006, available from <http://www.aihw.gov.au/mediacentre/2006/mr20060822.cfm> [accessed 30 January 2007].
5 It should be noted that the ANAO has not formed an opinion on the adequacy or appropriateness of the key performance indicators developed and piloted under the KPI development and implementation projects.
6 This includes the joint recommendation with Medicare Australia.