Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Management of the Manufacture and Supply of Domestic Fractionated Blood Plasma Products
Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- A reliable supply of blood and blood products is an integral component of Australia’s medical system.
- The National Fractionation Agreement for Australia (NaFAA) is the National Blood Authority’s (NBA) second largest contract, worth $3.4 billion over nine years.
- The NaFAA was established via a limited tender procurement in a sole provider market, and was exempt from some requirements of the Commonwealth Procurement Rules (CPRs).
Key facts
- The NaFAA is a contract from January 2018 to December 2026 between the NBA and CSL Behring (CSL) for the manufacture and supply of domestic fractionated blood plasma products.
- CSL is Australia’s sole domestic fractionator.
- Fractionation is a process that separates, purifies and concentrates different types of proteins found in blood plasma into therapeutic doses.
What did we find?
- The NBA has been largely effective at managing the manufacture and supply of domestic fractionated blood plasma products.
- The NBA conducted largely appropriate strategic procurement planning, including considering strategic supply objectives. Enterprise level risk management processes monitored and mitigated risks to the supply of blood products.
- The NaFAA procurement process largely supported the achievement of value for money, but the NBA did not demonstrate full compliance with the CPRs.
- Contract management arrangements including performance monitoring and risk management were largely effective.
- The NBA did not comply with various internal requirements throughout the procurement and contract management processes.
What did we recommend?
- The Auditor-General made three recommendations to the NBA. One recommendation was in relation to performance reporting, one related to contract risk management, and the third related to updating internal policies.
$811.9m
in total NaFAA expenditure from January 2018 to December 2020
$171.8m to $244.0m
is the range of potential savings expected from the NaFAA relative to the previous contract
4.738g
is the average amount of immunoglobulin produced by CSL per kg of starting plasma
Summary and recommendations
Background
1. A reliable supply of blood and blood products is an integral component of Australia’s medical system. Blood and blood products are critical in a wide range of medical uses, including for cancer patients, victims of traumatic accidents, people undergoing surgery, and those with blood disorders such as haemophilia. Governments in Australia spend over $1 billion a year on the supply of blood and blood products.
2. The National Blood Authority (NBA) was established in 2003 under the National Blood Authority Act 2003 following the signing of the National Blood Agreement by the Australian, state and territory governments. As the central purchasing agency for blood and blood products, the NBA establishes contracts with suppliers to meet the needs of patients, within the resources and policy parameters set by Australian, state and territory governments.
3. The National Fractionation Agreement for Australia (NaFAA) is a $3.4 billion nine-year contract with CSL Behring (CSL).1 Under the NaFAA, plasma donated by Australian donors is processed by CSL using a process called fractionation. CSL is the sole national plasma fractionator in Australia.
Rationale for undertaking the audit
4. The NaFAA was selected for audit because of the materiality of the services delivered under the contract, its relationship to the core functions and purpose of the NBA, and the limited market in which the services are procured. In addition, at $3.4 billion, the cost of this procurement is a significant use of public funds. A key challenge for the NBA is achieving value for money in a market with a sole provider, in accordance with the Australian Government procurement framework and in line with the policy objectives of the National Blood Agreement including to promote national self-sufficiency.
Audit objective and criteria
5. The audit objective was to assess the effectiveness of NBA’s management of the manufacture and supply of domestic fractionated blood plasma products. To form a conclusion against the objective, the following high-level criteria were adopted:
- Was there appropriate planning in place to support strategic procurement for the manufacture and supply of domestic fractionated blood plasma products?
- Did the procurement process support the achievement of value for money?
- Were effective contract management and monitoring arrangements established to ensure the delivery of the NaFAA?
Conclusion
6. The NBA has been largely effective at managing the manufacture and supply of domestic fractionated blood plasma products.
7. The NBA conducted largely appropriate planning that supported the strategic procurement for the manufacture and supply of domestic fractionated blood plasma products through the NaFAA. The NBA considered strategic supply objectives throughout the NaFAA procurement phase through preparation of the National Supply Plan and Budget, and enterprise- level risk management processes monitored and mitigated risks to the supply of blood products. The NBA did not document procurement planning in accordance with internal policies.
8. The processes undertaken for the procurement of the NaFAA largely supported the achievement of value for money. The NBA conducted benchmarking activities, modelling, and forecasting to assess CSL’s proposal for the NaFAA, and negotiated rates and conditions to support the achievement of value for money. The NBA did not comply with all mandatory requirements of the Commonwealth Procurement Rules (CPRs), including not fully implementing internal policies.
9. The NBA established contract management arrangements for the NaFAA that have been largely effective; however, they were not always implemented fully in accordance with the requirements of NBA internal policy.
Supporting findings
Planning to support strategic procurement
10. The NBA had an approved National Supply Plan and Budget in place at the time of planning for the NaFAA. The NaFAA costings and the 2018–19 National Supply Plan and Budget were developed concurrently, with both processes informing the proposed total contract price.
11. Strategic objectives were considered during the NaFAA procurement phase, through discussions and information provided by the NBA to the Jurisdictional Blood Committee (JBC) and the NBA Board. The NBA did not document procurement planning for the NaFAA in accordance with internal policies.
12. The NBA had in place organisational-level risk activities to monitor and mitigate risks to the supply of blood products, including for products delivered under the NaFAA.
Supporting the achievement of value for money
13. The NBA did not demonstrate that it complied with ethical behaviour requirements in the CPRs and relevant internal policies, as it did not document its consideration of probity risks nor develop a probity plan, and conflict of interest declarations were not completed by NBA staff covering the NaFAA procurement period. The NBA did not retain complete records of conversation for key negotiation meetings held with CSL, which would have provided additional transparency over the decision-making process.
14. NBA benchmarking activities, modelling, and forecasting formed a sound basis for demonstrating value for money in the NaFAA.
15. The NBA effectively negotiated proposed rates and conditions between the two proposals submitted by CSL by leveraging CSL’s preference for a longer contract period, negotiating a price reduction and the retention of indexation arrangements from the previous contract, and integrating conditions in the contract that provided greater flexibility with additional assurance over risk.
Contract management and monitoring arrangements
16. The NBA’s monitoring and management of CSL’s performance has been largely effective. The NBA monitors CSL’s performance against the NaFAA key performance indicators, and has enforced financial penalties and provided bonuses largely in accordance with the contract. Assurance processes for the NaFAA have been established but not consistently completed.
17. The NBA’s risk management for the NaFAA has been largely effective but has not been conducted fully in accordance with internal requirements. The NBA has focused primarily on managing supply and product risks. The NBA has been responsive to issues identified throughout the NaFAA and both parties have complied with key risk requirements under the contract.
18. The NBA’s approach to the design and management of the NaFAA was based on the previous contract with CSL. Review activities and proposals for improvement were undertaken although not through the established internal process.
Recommendations
Recommendation no. 1
Paragraph 4.22
The National Blood Authority review the reporting of CSL Behring’s performance against the NaFAA key performance indicators in the annual report, to ensure it accurately reflects the performance target and the result achieved. Results presented should provide the reader with a clear indication of performance against the targets.
National Blood Authority response: Agreed.
Recommendation no. 2
Paragraph 4.43
The National Blood Authority conduct risk management activities for the NaFAA in line with internal requirements, including:
- conducting an assessment of supplier and contract risks;
- establishing a NaFAA risk management plan to document the approach to risk management; and
- developing a timeline of risk management activities, including a review cycle for risk management activities.
National Blood Authority response: Agreed.
Recommendation no. 3
Paragraph 4.71
The National Blood Authority review and update its suite of internal policies and guidance for procurement and contract management. Internal policy should be consistent with current Australian Government legislative and policy requirements, be commensurate with business needs, and reflect the operating environment of the National Blood Authority. Procurement and contract management processes should be conducted in accordance with the updated internal policy requirements.
National Blood Authority response: Agreed.
Summary of National Blood Authority response
19. The NBA’s summary response to the report is provided below and its full response is at Appendix 1.
The National Blood Authority (NBA) thanks the ANAO for auditing the NBA’s performance in managing the manufacture and supply of domestic fractionated blood plasma products through the major contract negotiated and managed by the NBA with CSL Behring, and for the three recommendations made and agreed to improve the NBA’s future procurement and reporting processes.
The NBA notes that this and preceding contracts have delivered the effective and uninterrupted supply of a range of plasma derived products to Australian patients since 2003. The current contract is a long-term, high value contract of national significance that has delivered good outcomes for patients, governments, Australian industry and community. The audit findings that the NBA has been largely effective with its strategic procurement planning and contract management are welcomed as are the findings that the NBA’s benchmarking activities, modelling, and forecasting have formed a sound basis for demonstrating the contract’s value for money.
The NBA acknowledges that improvements can continue to be made to processes and it will respond to the audit recommendations accordingly.
Key messages from this audit for all Australian Government entities
Below is a summary of key messages, including instances of good practice, which have been identified in this audit and may be relevant for the operations of other Australian Government entities.
Procurement
Contract management
1. Background
Introduction
1.1 A reliable supply of blood and blood products is an integral component of Australia’s medical system. Blood and blood products are critical in a wide range of medical uses, including for cancer patients, victims of traumatic accidents, people undergoing surgery, and those with blood disorders such as haemophilia. Governments in Australia spend over $1 billion a year on the supply of blood and blood products. Blood products are provided free of charge to patients requiring treatment through health providers. Types of blood products are set out in Table 1.1.
Table 1.1: Blood product categories
|
Category |
Definition |
|
Fresh blood components |
Components of whole blood (red blood cells, platelets and fresh frozen plasma) are referred to as fresh blood components. The majority of these fresh components are collected by centrifuging the whole blood. The centrifugation process separates the whole blood into red blood cells, platelets and plasma. Platelets and plasma can also be collected by apheresis (a process where whole blood is removed from a donor and the required component(s) retained, while the remainder of the blood components are returned to the donor). |
|
Plasma-derived products |
These are products derived from plasma, using various techniques such as chromatography and Cohn cold-ethanol fractionation.a Proteins are isolated from the plasma and processed into a range of plasma-derived products, such as albumin, immunoglobulin, and coagulation factors (including factors VIII, IX and XI). |
|
Recombinant products |
Recombinant products are genetically engineered forms of plasma proteins and are not sourced from blood, but from host cells that contain an inserted copy of a human gene that produces the protein. |
Note a: Fractionation is a process in which different types of proteins found in blood plasma are separated, purified and concentrated into therapeutic doses. Further detail on fractionation is outlined from paragraph 1.12 below.
Source: National Blood Authority, National Blood Supply Contingency Plan July 2019, p.16.
1.2 The National Blood Authority (NBA) was established in 2003 under the National Blood Authority Act 2003 (NBA Act, or the Act) following the signing of the National Blood Agreement by the Australian, state and territory governments. The NBA is a non-corporate Commonwealth entity operating under the NBA Act, the Public Governance, Performance and Accountability Act 2013 (PGPA Act), and the Public Service Act 1999.
1.3 The National Blood Agreement lists Australian, state and territory governments’ policy objectives for the blood sector. The primary policy objectives are to:
- provide an adequate, safe, secure and affordable supply of blood products, blood related products and blood related services in Australia; and
- promote safe, high quality management and use of blood products, blood related products and blood related services in Australia.
1.4 These primary policy objectives are underpinned by secondary policy aims, including to promote national self-sufficiency.
1.5 As the central purchasing agency for blood and blood products, the NBA establishes contracts with suppliers to meet the needs of patients, within the resources and policy parameters set by Australian, state and territory governments.
Governance arrangements for the Australian blood sector
1.6 Governance arrangements for the Australian blood sector are set out in the National Blood Agreement and the NBA Act, and are depicted in Figure 1.1.
Figure 1.1: Governance arrangements for the Australian blood sector
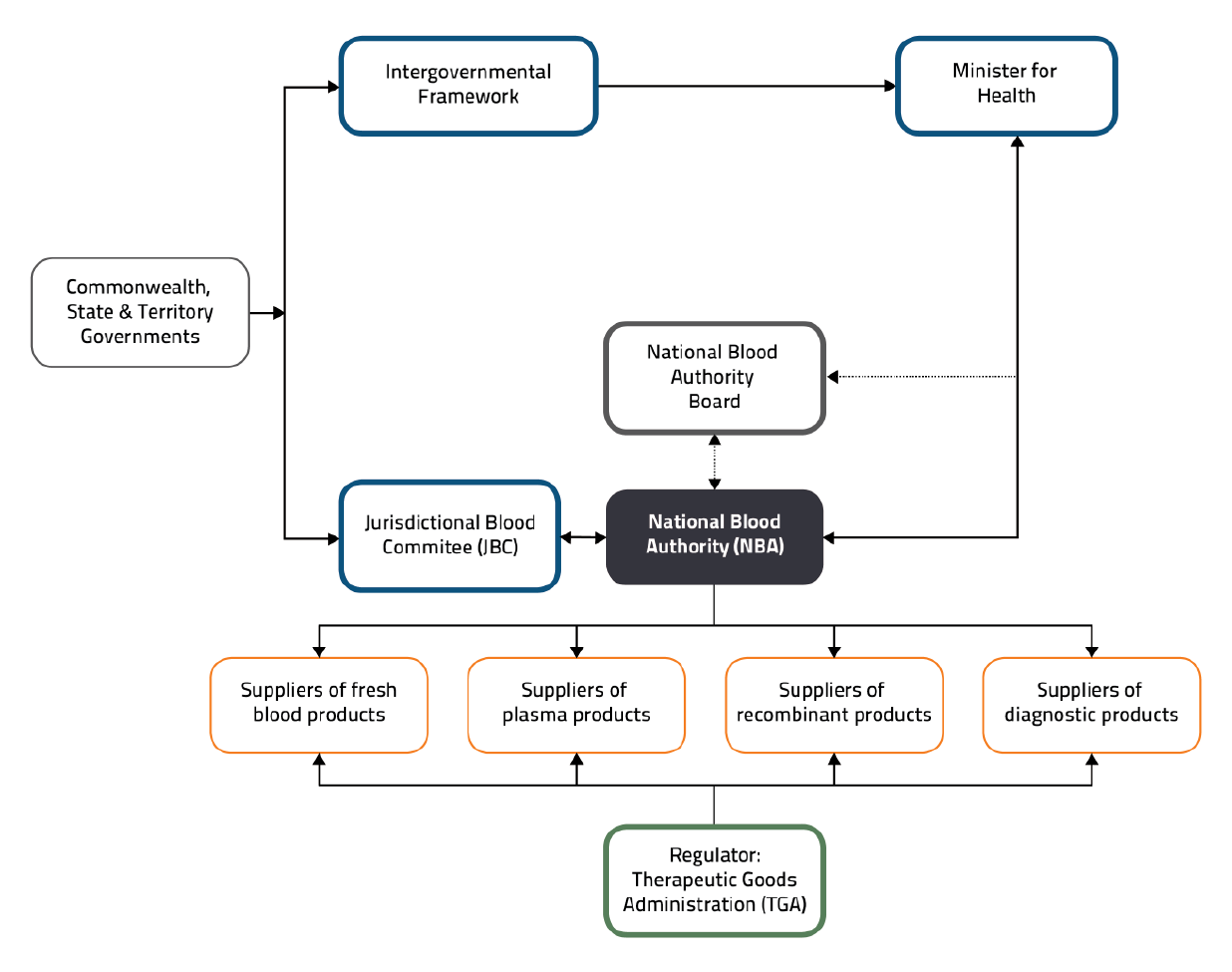
Source: National Blood Authority, Corporate Plan 2020–21 to 2023–24, p.7.
1.7 The Jurisdictional Blood Committee (JBC) — established in 2003 by the National Blood Agreement — is a committee of senior government officials who represent the Australian, state and territory governments. The JBC is responsible for all jurisdictional issues relating to the national blood supply, including: considering advice from, and providing advice to, the NBA on matters related to the national blood supply; and overseeing the NBA’s role in relation to the collection, production and distribution of products.2
1.8 The NBA Board was established under the NBA Act. The Board’s functions, set out in section 13 of the Act, include providing advice to the General Manager (now titled the Chief Executive) about the performance of the NBA’s functions.3 The Board is not a decision-making body but performs an advisory role, considering key strategic issues facing the NBA. The Chief Executive is the accountable authority of the NBA.
1.9 Under the National Blood Agreement, the Australian Government provides 63 per cent of the funding for the national blood supply and the NBA’s operating costs. State and territory governments collectively provide funding for the remaining 37 per cent on a price-volume basis according to products supplied to each state and territory. In 2019–20, total purchases of blood and blood products was $1.26 billion, with plasma products accounting for 48.2 per cent. Funding for 2020–21 is budgeted at $1.36 billion.
1.10 Domestic manufacturing of plasma and recombinant products in Australia is undertaken solely by CSL Behring (CSL) under a contract entered into with the NBA — the National Fractionation Agreement for Australia (NaFAA, or the contract).4
The National Fractionation Agreement for Australia
1.11 The NaFAA is a $3.4 billion nine-year contract with CSL in place from January 2018 to December 2026, subject to a review in 2022. The aim of the NaFAA is to ensure the manufacture and supply of domestic fractionated blood plasma products. The NaFAA aims to provide a safe, secure and affordable supply of plasma products while ensuring value for money for Australian, state and territory governments.
Figure 1.2: Purchaser-provider arrangements for the blood sector

Source: ANAO.
1.12 As illustrated in Figure 1.2, plasma donated by Australian donors and collected by Australian Red Cross Lifeblood is provided to CSL for fractionation under the NaFAA. Fractionation is a process that separates, purifies and concentrates different types of proteins found in blood plasma into therapeutic doses.5 Products manufactured through fractionation include:
- immunoglobulin (Ig) products — used to provide additional immunoglobulins to support patients’ immune systems and to support patients with auto-immune disorders;
- albumin — used to increase plasma volume and retain fluid in the blood stream; and
- clotting factors — used to treat patients with haemophilia and other bleeding disorders, including from surgery.
1.13 Specific blood plasma products manufactured by CSL under the NaFAA are set out in Appendix 2.
1.14 The NaFAA represents the NBA’s second largest contract in total value, with the NBA reporting expenditure of $273.3 million in 2019–20.6 A total of 802.6 tonnes of Australian plasma was pooled for fractionation under the NaFAA in 2019–20.
1.15 The NBA has reported that the NaFAA delivered savings of $7.4 million in 2018–19, and $4.26 million in 2019–20 for domestic Ig products.7 The NaFAA is expected to deliver savings of more than $200 million over the term of the contact, relative to the previous contract with CSL. These savings are partly a result of plans for improved manufacturing processes and efficiencies, referred to by CSL as ‘process migration’.
1.16 CSL is the sole fractionator for Australia and through the NaFAA, remains Australia’s national plasma fractionator — a role held since 1953. As set out in Figure 1.3, the Commonwealth has entered into three previous contracts with CSL for fractionation services since CSL was privatised in 1994.
Figure 1.3: Timeline of fractionation contracts between the Commonwealth and CSL

Source: ANAO analysis.
Government procurement
1.17 The Commonwealth Procurement Rules (CPRs), issued by the Finance Minister under subsection 105(b) of the Public Governance, Performance and Accountability Act 2013 (PGPA Act), set out the Australian Government’s procurement policy framework and establish principles that apply to all procurement processes.
1.18 In addition to the requirements of the CPRs, section 20A of the PGPA Act authorises accountable authorities to give instructions (Accountable Authority Instructions) to officials in their entities on any matter necessary or convenient for carrying out or giving effect to the PGPA Act. These are legally binding instructions relating to the financial administration of entities. Accountable Authority Instructions extend to entities’ procurement policies, which may be supplemented by more detailed guidance covering the various phases of the procurement cycle in the context of entities’ particular business environment.
1.19 The CPRs promote the use of sound and transparent procurement practices that seek to achieve value for money and encourage competition in government procurement.8 The CPRs include mandatory requirements that entities must follow. The extent to which all rules of the CPRs apply depends on the value of the procurement and is set out in the two divisions of the CPRs:
- Division 1 — rules applying to all procurements regardless of value. Officials must comply with the rules of Division 1 when conducting procurements; and
- Division 2 — additional rules that apply to all procurements valued at or above the relevant procurement thresholds, unless exempted under Appendix A.
1.20 Appendix A of the CPRs allows for the procurement of blood plasma products or plasma fractionation services to be exempt from Division 2, which sets out additional requirements for limited tender procurements.
1.21 In order to secure an adequate supply of blood products, the NBA manages a number of contracts for the supply of domestic and imported blood products.9 The NBA operates within a unique environment with a limited pool of companies manufacturing blood products.
Rationale for undertaking the audit
1.22 The NaFAA was selected for audit because of the materiality of the services delivered under the contract, its relationship to the core functions and purpose of the NBA, and the limited market in which the services are procured. In addition, at $3.4 billion, the cost of this procurement is a significant use of public funds. A key challenge for the NBA is achieving value for money in a market with a sole provider, in accordance with the Australian Government procurement framework and in line with the policy objectives of the National Blood Agreement including to promote national self-sufficiency.
Previous audit coverage
1.23 The ANAO conducted an audit of the NBA in 2011 (Auditor-General Report No.8 2011–12 The National Blood Authority’s Management of the National Blood Supply), focused primarily on the NBA’s Deed of Agreement with the then Australian Red Cross Blood Service (now Australian Red Cross Lifeblood). The audit scope included the NBA’s governance and administrative systems, contract management practices, and performance monitoring. The previous contract with CSL was excluded from the scope of the 2011 audit.
Audit approach
Audit objective, criteria and scope
1.24 The audit objective was to assess the effectiveness of NBA’s management of the manufacture and supply of domestic fractionated blood plasma products. To form a conclusion against the objective, the following high-level criteria were adopted:
- Was there appropriate planning in place to support strategic procurement for the manufacture and supply of domestic fractionated blood plasma products?
- Did the procurement process support the achievement of value for money?
- Were effective contract management and monitoring arrangements established to ensure the delivery of the NaFAA?
Audit methodology
1.25 The audit methodology included:
- examining and analysing entity documentation; and
- interviews with NBA staff.
1.26 The audit also sought public submissions via the ANAO website.
1.27 The audit was conducted in accordance with the ANAO Auditing Standards at a cost to the ANAO of approximately $393,000.
1.28 The team members for this audit were Freya Mathie, Hayley Pennock, Jennifer Zierk, Samuel Painting, and Peta Martyn.
2. Planning to support strategic procurement
Areas examined
This chapter examines whether the National Blood Authority (NBA) undertook appropriate planning for the National Fractionation Agreement of Australia (NaFAA) to support strategic procurement for the manufacture and supply of domestic fractionated blood plasma products.
Conclusion
The NBA conducted largely appropriate planning that supported the strategic procurement for the manufacture and supply of domestic fractionated blood plasma products through the NaFAA. The NBA considered strategic supply objectives throughout the NaFAA procurement phase through preparation of the National Supply Plan and Budget, and enterprise-level risk management processes monitored and mitigated risks to the supply of blood products. The NBA did not document procurement planning in accordance with internal policies.
2.1 As one of two major contracts managed by the NBA, it is important that the NaFAA aligns with the NBA’s broader strategic planning and budgeting process. To determine whether the NBA’s planning for the strategic procurement of the manufacture and supply of domestic blood plasma products was appropriate, the ANAO examined whether:
- the NBA’s annual supply planning and budgeting process informed the procurement for the manufacture and supply of domestic fractionated blood plasma products;
- the procurement planning supported the achievement of an affordable, safe, secure and adequate supply of domestic fractionated blood products; and
- the procurement planning addressed identified risks to the supply of domestic fractionated blood products.
2.2 The planning phase of the NaFAA commenced in April 2015 when the NBA first presented to the Board its plans for the next fractionation agreement, and continued throughout the procurement until the contract was signed in December 2017.
Did the National Supply Plan and Budget inform the procurement?
The NBA had an approved National Supply Plan and Budget in place at the time of planning for the NaFAA. The NaFAA costings and the 2018–19 National Supply Plan and Budget were developed concurrently, with both processes informing the proposed total contract price.
2.3 The NBA is required to undertake an annual supply planning and budgeting process under paragraph 25(b) of the National Blood Agreement and paragraph 8(1)(b) of the National Blood Authority Act 2003 (NBA Act, or the Act). The process results in the National Supply Plan and Budget, which forms a key part of the NBA’s management of the blood supply and achievement of its primary objectives.10 The National Supply Plan and Budget consists of a spreadsheet of forecasted volumes and costs of blood products over four years. It includes all fresh, plasma, recombinant and imported blood products, and any additional payments agreed by jurisdictions.
2.4 The National Supply Plan and Budget process begins in July each year for the following financial year. As set out in the NBA’s key business processes documentation, the process begins with modelling using the NBA’s contract management system, followed by consultation with states and territories (or jurisdictions), and suppliers, including CSL Behring (CSL) and the Australian Red Cross Lifeblood, to confirm volume estimates. Forecasts are then discussed with representatives from jurisdictions (often including key clinical representatives), with the refined forecasted volumes then used by the NBA to produce cost estimates based on contract values or estimated prices.
2.5 The spreadsheet outlining the forecasted volumes and costs of blood products is accompanied by a summary of the total NBA budget (administered and departmental) for each forecasted year, and the National Product Price List, which outlines products supplied under the National Blood Agreement.
2.6 Following a final review by jurisdictional representatives, the full National Supply Plan and Budget is provided to the Jurisdictional Blood Committee (JBC) for approval in December. The JBC approves the National Supply Plan and Budget for submission to the Clinical Principal Committee, Australian Health Ministers’ Advisory Council, and Health Council.
2.7 The NBA is responsible for the implementation and ongoing management and monitoring of blood product supply and demand against the approved National Supply Plan and Budget, including quarterly reporting to the JBC. The NBA conducts a mid-year review of the National Supply Plan and Budget to address emerging trends identified since the budget was approved, and adjustments are made if required. Jurisdictions are charged for the products they are issued during the year, not according to forecasted volumes, following a reconciliation process completed by NBA at the end of the financial year.
2.8 Throughout the procurement phase for the NaFAA, the NBA had three National Supply Plan and Budgets in place for 2016–17 to 2018–19, approved by the JBC in December 2015, December 2016 and December 2017 respectively. The 2018–19 National Supply Plan and Budget process ran concurrently with the finalisation of the NaFAA. As NaFAA negotiations reached their conclusion, proposed contract prices in the NaFAA informed the forecasted budget. The NBA used the proposed contract prices and National Supply Plan and Budget volume forecasts to estimate the total cost of the NaFAA, which was then used in the spending proposal minute approved by the NBA Chief Executive to finalise the NaFAA. This is consistent with the NBA process for the development of the National Supply Plan and Budget, which uses known contract prices where possible to produce a budget estimate.
2.9 The 2018–19 National Supply Plan and Budget was agreed in principle by the JBC on 1 December 2017, just prior to the NaFAA finalisation on 6 December 2017. The costings used to calculate the total figure for the NaFAA, as set out in the NBA’s spending proposal for the contract, were largely consistent with the 2018–19 National Supply Plan and Budget. Volumes set out under the NaFAA costing calculations matched the volumes set out in the 2018–19 National Supply Plan and Budget, and costings used for NaFAA calculations generally matched, with two exceptions that impacted on the NaFAA costings and the final amount included in the spending proposal:
- A formula error was identified in the NaFAA costings for three products.11 The formula relied on a calculation of product volume multiplied by product price, where the error resulted in the use of incorrect product prices. This resulted in the NaFAA calculations being $1.75 million lower than the National Supply Plan and Budget for these products over a five year period. The total spending proposal amount for the NaFAA was $2.02 million lower than if the correct prices had been used.
- An additional formula error was identified in the method used to split the NaFAA costings for the 2021–22 prices. Correct pricing was applied but the formula used resulted in only 11 months of the annual product volumes being counted. This error affected all products for 2021–22, and resulted in the spending proposal being $25.11 million lower than if the entire annual volume was counted.
2.10 The formula errors in the NaFAA costings resulted in the approved spending proposal being $27.13 million lower than if correct formulas were used, representing 0.9 per cent of the total NaFAA value.
2.11 In audits of other entities, the ANAO has previously noted the weaknesses of using spreadsheets as a primary tool for managing business information.12 Spreadsheets lack formalised change/version control and reporting, increasing the risk of error. This can make spreadsheets unreliable corporate data handling tools, as accidental or deliberate changes can be made to formulae and data, without there being a record of when, by whom, and what changes were made.
Did procurement planning support the achievement of strategic objectives?
Strategic objectives were considered during the NaFAA procurement phase, through discussions and information provided by the NBA to the JBC and the NBA Board. The NBA did not document procurement planning for the NaFAA in accordance with internal policies.
2.12 As set out in Chapter 1, one of the two primary policy objectives for the Australian blood sector is ‘to provide an adequate, safe, secure and affordable supply of blood products, blood related products and blood related services in Australia’.13 The NBA’s Outcome 1 is to provide:
Access to a secure supply of safe and affordable blood products, including through national supply arrangements and coordination of best practice standards within agreed funding policies under the national blood arrangements.14
2.13 The primary policy objectives are underpinned by secondary policy aims, including self-sufficiency. The JBC has noted the tensions between the primary policy objectives and the secondary policy aims of domestic self-sufficiency and reliance on voluntary, non-remunerated donations. The NBA is required to balance the policy of self-sufficiency with affordability and supply security.
2.14 The NBA’s website states:
The NBA relies on regulation by the Therapeutic Goods Administration to address the major policy aim of safety. The policy aim of affordability is addressed through various procurement mechanisms such as competitive tendering, price benchmarking and other specific negotiation approaches. The policy aims of adequacy and security of supply are addressed through a range of measures such as primary and contingency supply arrangements, inventory and inventory reserve requirements, and an integrated regime of supply and contract risk protections.15
2.15 In papers to the JBC, the NBA advised and sought approval from the committee in relation to: prices and costs; the negotiation approach for the NaFAA; and the supply arrangements through the National Supply Plan and Budget. The NaFAA sets out minimum inventory requirements linked to the key performance indicators (KPIs) for the contract, demonstrating NBA’s management mechanisms for the security of supply.
2.16 The NBA also stated on its website that preparations for tender processes include: gathering information on the global market; reviewing the current arrangements to identify where improvements might be gained (for example security of supply, safety and value for money); and using knowledge of normal supply chain and commercial arrangements to design arrangements that are achievable and sustainable.16 The NBA presented to the JBC and the Board ‘horizon scanning’ papers, which documented the NBA’s monitoring of international developments related to the management of blood and blood products.
2.17 In planning for the NaFAA procurement, in 2015 the NBA drafted a two-page procurement plan that sets out activities to be undertaken during the planning phase of the procurement. Activities outlined in the plan included: identifying issues with the then current contract with CSL — the CSL Australian Fractionation Agreement (CAFA); identifying issues that may impact on the term of the next contract; developing policy parameters for developing the next contract; seeking agreement from Government on the policy parameters; and developing project timeframes and resourcing requirements. The draft procurement plan was not finalised.
2.18 NBA internal procurement policy requires minimum documentation of a procurement strategy, project plan, risk management plan and project schedule. The NBA did not document procurement planning in accordance with internal policies. However, there was evidence of the NBA executing the activities identified in the draft procurement plan in papers provided by the NBA to the JBC and the NBA Board.
2.19 In addition to the JBC and Board papers, the NBA’s strategic goals, as set out in corporate plans, have consistently referenced the four primary policy objectives (affordable, safe, secure and adequate) over the planning period for the NaFAA. The final spending proposal for the NaFAA noted that ‘entering into this arrangement assists the NBA to achieve its purposes under the NBA Act particularly to carry out national blood arrangements to ensure that there is a safe, secure, sufficient and affordable supply of plasma blood products in all the States and covered Territories in Australia’.
Did procurement planning appropriately address identified risks to the supply of domestic fractionated blood plasma products?
The NBA had in place organisational-level risk activities to monitor and mitigate risks to the supply of blood products, including for products delivered under the NaFAA.
2.20 Under the National Blood Agreement and the NBA Act, the NBA is responsible for managing risks to the national blood supply, including risk mitigation and contingency measures.
2.21 Although a risk assessment and risk management plan was not completed for the NaFAA procurement (see from paragraph 3.28), the NBA conducted organisational-level risk activities that contributed to the monitoring and mitigation of blood supply risks. These included supply risks for products, including products supplied under the NaFAA. These risk processes were conducted on an annual basis and were in place at the time of planning for the NaFAA.
2.22 The Supply Risk and Risk Mitigation Update process, conducted annually, involves stakeholder consultation (including with suppliers) and review of international events that may impact on product supply. On completion of the process, a brief is submitted to the NBA’s Chief Executive accompanied by a product-level risk register, which outlines key risks and treatments for each blood product type, and a document outlining mitigation strategies and detailed information for each product.
2.23 Several risks were common across most product types: contamination of supply; unexpected increases in demand; and issues with distribution or supply leading to product losses. Treatments for common product supply risks in the product-level risk register often consist of contract mechanisms such as requirements for suppliers to maintain minimum levels of product stock and clauses outlining options for the supply of alternative products. These treatments stem from the NBA’s findings from the original Supply Risk and Risk Mitigation Project in 2004–05, which identified contract mechanisms to ensure national reserves and minimum product levels as some of the most effective in mitigating risk.
2.24 Another key organisational-level risk assessment activity conducted by the NBA in parallel with planning for the NaFAA was an activity the NBA terms ‘horizon scanning’. The National Blood Agreement requires the NBA to monitor the national and international environment in which the Australian Blood Sector operates for new technological, clinical, risk or other developments that may impact on the national blood supply and requires the NBA to maintain a systematic approach to identifying new developments. Horizon scanning reports were submitted to the JBC quarterly as a standing agenda item throughout the NaFAA procurement period from May 2015 to December 2017.
2.25 Common treatments cited in the Supply Risk and Risk Mitigation Update process from 2015–16 have been included in the NaFAA. These treatments were also included in the NBA’s previous fractionation agreement with CSL, the CAFA. The NaFAA includes requirements for CSL to hold a minimum level of starting plasma (which is plasma supplied to CSL by the NBA) and each manufactured product at all times, except for under scenarios outlined in the contract. CSL must also maintain a separate National Reserve of products under the NaFAA.
2.26 In addition, two of the five KPIs in the NaFAA reiterate these mechanisms and are stated to directly address supply risks by ensuring the maintenance of minimum inventory levels and the National Reserve, and that products in the National Reserve have sufficient shelf life remaining. KPIs are discussed further in Chapter 4. Although these clauses and KPIs were rolled over from the CAFA to form the basis of the NaFAA, risks were considered by the NBA during the planning phase of the NaFAA through entity level risk assessment processes outlined above.
3. Supporting the achievement of value for money
Areas examined
This chapter examines whether the National Blood Authority’s (NBA) procurement process for the National Fractionation Agreement for Australia (NaFAA) supported the achievement of value for money.
Conclusion
The processes undertaken for the procurement of the NaFAA largely supported the achievement of value for money. The NBA conducted benchmarking activities, modelling, and forecasting to assess CSL Behring’s (CSL) proposal for the NaFAA, and negotiated rates and conditions to support the achievement of value for money. The NBA did not comply with all mandatory requirements of the Commonwealth Procurement Rules (CPRs), including not fully implementing internal policies.
Areas for improvement
The ANAO made a suggestion that the NBA maintains records of negotiations of procurements to provide transparency over the decision-making process and ensure that information captured is accurate, complete, and corporate knowledge is retained.
3.1 The NaFAA was established following a limited tender process. Limited tender procurements generally reduce competition and therefore, under the CPRs, may only be undertaken in specific circumstances for high value procurements17; however, an entity must still demonstrate that the procurement achieves value for money. To determine whether the procurement undertaken by the NBA for the NaFAA supported the achievement of value for money, the ANAO examined whether:
- the procurement process was consistent with the July 2014 and March 2017 CPRs in force over the procurement period18;
- the basis for assessing the proposed rates as value for money was sound; and
- the proposed rates and conditions were effectively negotiated.
Was the procurement process consistent with the Commonwealth Procurement Rules?
The NBA did not demonstrate that it complied with ethical behaviour requirements in the CPRs and relevant internal policies, as it did not document its consideration of probity risks nor develop a probity plan, and conflict of interest declarations were not completed by NBA staff covering the NaFAA procurement period. The NBA did not retain complete records of conversation for key negotiation meetings held with CSL, which would have provided additional transparency over the decision-making process.
3.2 In undertaking procurements, officials from relevant entities are required to adhere to the CPRs.19 The CPRs establish procurement rules that apply to all procurement processes, and promote value for money as the core rule of the Australian Government’s procurement policy framework. Value for money is enhanced and complemented by other key rules—encouraging competition; efficient, effective, economical and ethical use of resources; and accountability and transparency in decision making.20 Value for money is achieved through proper procurement planning to support the selection of an appropriate procurement method that encourages fair and open competition commensurate with the size, scale and risk of the procurement.21
3.3 The NBA conducted the NaFAA as a limited tender, using exemption 12 under Appendix A of the March 2017 CPRs for the procurement of blood plasma products or plasma fractionation services.22 Using this exemption required the NBA to follow the rules outlined in Division 1 of the CPRs; however, the NBA was not required to comply with the additional Division 2 requirements for procurements above the relevant procurement threshold.23
3.4 The CPRs set out the mandatory requirements with which officials must comply when undertaking procurements. The CPRs have been designed to provide flexibility in developing and implementing procurement processes relevant to an entity’s needs that reflect the size, scope and risk of the procurement. More broadly, the CPRs also indicate good practice.24
3.5 The CPRs state that they provide a necessary framework for accountable authorities when issuing Accountable Authority Instructions and operational requirements in relation to procurement. The CPRs further set out that in the area of procurement, an accountable authority should provide a mechanism to:
- apply the principles and requirements of the resource management and procurement frameworks, focusing on the relevant entity’s operations; and
- provide primary operational instructions to relevant entity officials in carrying out their duties related to procurement, in a way that is tailored to a relevant entity’s particular circumstances and needs.25
3.6 Three NBA internal policies — or key business processes (KBP) — applied to the NaFAA procurement:
- KBP3 — Tendering and Contract Negotiation-Blood Products and Services, dated June 2015;
- KBP7 — Risk Management Framework, dated December 2013 and last updated in February 2016; and
- Management Instruction 17 — Disclosure and Management of Conflicts of Interest by NBA Staff, dated July 2008.
3.7 The NBA Accountable Authority Instructions specify that all KBPs must be followed by NBA employees. KBP3 outlines that procurement officers must follow the process, unless they receive clearance from the NBA Chief Executive or Deputy General Manager (now titled the Deputy Chief Executive).26 NBA staff working on the NaFAA procurement included the Chief Executive and Deputy Chief Executive, with the Chief Executive being the delegate to enter into the contract with CSL. The NBA advised that KBP3 was designed for an open tender process, and that some requirements did not apply for the NaFAA procurement. There was no evidence of a sign off by the Chief Executive or Deputy Chief Executive to vary internal policies or process for the NaFAA procurement.
3.8 The NBA’s NaFAA spending proposal provided advice to the Chief Executive that the NBA’s Accountable Authority Instructions and KBPs were followed during the procurement.
Achieving value for money
3.9 The core rule of the CPRs is achieving value for money. The CPRs state that ‘Officials responsible for a procurement must be satisfied, after reasonable enquires, that the procurement achieves a value for money outcome’.27 The CPRs also state that ‘an official must consider the relevant financial and non-financial costs and benefits of each submission.’ To meet the accountability and transparency requirements of the CPRs, it is important that entities select the best value for money proposals and document the reasons and process by which they arrived at their decision.
3.10 The NBA engaged KordaMentha Corporate (KordaMentha) to conduct assessment and modelling of potential NaFAA savings, and provide advice on the value of the contract and negotiation tactics.28 KordaMentha concluded that the final contract offer, against a baseline of the prior contract, could deliver savings between $171.8 and $244.0 million (dependent on the plasma growth assumptions and volumes) with the terms and conditions offering flexibility and additional protections. KordaMentha advised the NBA that the proposed terms negotiated with CSL over an extended period of time represented fair value. The terms and conditions negotiated to assist in delivering value for money from the NaFAA are discussed from paragraph 3.53 below.
3.11 The NBA Chief Executive approved a spending proposal minute on 6 December 2017 that documented the procurement strategy and approach for the NaFAA. The spending proposal justified value for money by setting out that CSL is the sole provider of fractionation services and that the previous contract was used as the baseline to assess savings achieved through the negotiation process. The spending proposal also noted that the NaFAA represented best value for money available within the current policy arrangements.
Ethical behaviour
3.12 The CPRs state that ‘Ethical relates to honesty, integrity, probity, diligence, fairness and consistency’.29 The CPRs further state that:
Officials undertaking procurement must act ethically throughout the procurement. Ethical behaviour includes:
a. recognising and dealing with actual, potential and perceived conflicts of interest;
b. dealing with potential suppliers, tenderers and suppliers equitably, including by
i. seeking appropriate internal or external advice when probity issues arise, and
ii. not accepting inappropriate gifts or hospitality;
c. carefully considering the use of public resources; and
d. complying with all directions, including relevant entity requirements, in relation to gifts or hospitality, the Australian Privacy Principles of the Privacy Act 1988 and the security provisions of the Crimes Act 1914.
3.13 In addition to the requirements of the CPRs, subsection 13(7) of the Code of Conduct contained in the Public Service Act 1999 requires an employee to take reasonable steps to avoid any conflict of interest (real or apparent) in connection with the employee’s employment, and disclose details of any material personal interest of the employee in connection with the employee’s employment. Agency heads and senior executive service employees of the Australian Public Service are required to declare in writing, at least annually, their own and their immediate family’s financial and other interests that could cause a real or apparent conflict of interest.30
3.14 Entity accountable authorities must promote the ethical management of public resources and establish and maintain appropriate systems relating to risk management, oversight and internal controls, including policies and procedures regarding the management of conflicts of interest.31
3.15 NBA Management Instruction 17 — Disclosure and Management of Conflicts of Interest by NBA Staff, sets out that the possible areas of conflict of interest that NBA staff members should be particularly aware of are in relation to current or potential future suppliers to the NBA, including both suppliers of blood and blood products. The management instruction requires that all NBA staff complete an annual conflict of interest disclosure and includes a declaration template. The management instruction also recognises that work on a project involving tendering or similar procurement activity is a common situation where it is appropriate to consider conflict of interest issues and states that staff may be asked to make a conflict of interest disclosure in these circumstances.
3.16 The NBA provided an annual conflict of interest disclosure for the Chief Executive that was completed in October 2016.32 A private interests declaration was completed by the Deputy Chief Executive in January 2016, when they were acting in the role of Chief Executive. These annual declarations were completed on a single occasion within the procurement period from April 2015 to December 2017, and no conflicts were declared. The NBA also provided annual conflict of interest declarations, with no conflicts of interest being declared, that were completed outside the NaFAA procurement period:
- Deputy Chief Executive: for January and December 2014; and
- Executive Director: for December 2013, and January and December 2014.
3.17 No conflict of interest disclosures were completed by NBA staff specifically for the purpose of working on the NaFAA procurement.
3.18 A probity plan provides the framework for ensuring that probity and transparency are maintained throughout a procurement process.33 In planning a procurement, the NBA’s KBP3 states that officials are required to apply and comply with probity principles and practices throughout all stages of every procurement. KBP3 also sets out that in addition to the minimum documentation required (procurement strategy, project plan, risk management plan and project schedule), officials should consider whether additional documentation, including a probity plan, is warranted.
3.19 Additional NBA guidance notes that the need for probity measures is typically as a result of a task or risk analysis indicating a need to obtain independent verification of the integrity of procurement or procurement related processes. This guidance outlines that probity measures typically include a probity plan and regular reporting to confirm compliance with probity requirements. There was no evidence of the NBA’s consideration of the need for a probity plan and it was therefore unclear how the NBA considered probity issues, either perceived, actual, or potential.
3.20 The NBA advised that probity risks were mitigated by generally ensuring that more than one NBA staff member was present at negotiation meetings held with CSL. The NBA provided evidence of 11 meetings between the NBA and CSL from 24 March 2017 to 27 November 2017. Multiple attendees were identified as attending 10 of the 11 meetings. One meeting was held between only the NBA Chief Executive and CSL General Manager. Talking points for the meeting were drafted with input from the Deputy Chief Executive and KordaMentha, and a summary of the content and result from the meeting was included in papers to the NBA Board in August 2017. KordaMentha also attended two meetings with the NBA and CSL.
3.21 Given that the NaFAA represents the NBA’s second largest contract in monetary value, and the close ongoing relationship between the NBA and CSL, there would have been merit in the NBA developing a probity plan for the NaFAA procurement.
3.22 Entities should also ensure that policies on conflict of interest management are consistent with policies relating to gifts and benefits.34 The NBA maintained a Gifts and Hospitality Register that did not include any declaration of gifts received by NBA officials from CSL during the procurement period for the NaFAA (April 2015 to December 2017).
Accountability and transparency
3.23 Mandatory requirements of the CPRs relevant to a sole source tender include:
- Officials must maintain for each procurement a level of documentation commensurate with the scale, scope and risk of the procurement.
- Relevant entities must have access to evidence of agreements with suppliers, in the form of one or a combination of the following documents: a written contract, a purchase order, an invoice or a receipt.
- In order to draw the market’s early attention to potential procurement opportunities, each relevant entity must maintain on AusTender a current procurement plan containing a short strategic procurement outlook.
- Relevant entities must report contracts and amendments on AusTender within 42 days of entering into (or amending) a contract if they are valued at or above the reporting threshold.35
3.24 Decisions regarding the contract and negotiation were documented in a summary form within papers that were provided to the NBA Board and the Jurisdictional Blood Committee (JBC). The NBA advised the ANAO that papers presented to the Board and the JBC were considered to be the records of the procurement.
3.25 The CPRs require a level of documentation to be kept which is commensurate with scale, scope and risk of the procurement. The NBA’s internal policy, KBP3, sets out that minutes of the negotiation process are key documents in conducting a procurement. The NBA did not maintain complete records of conversation from negotiation meetings with CSL. Maintaining complete records of discussions, including the attendees and agreed outcomes of negotiation meetings, would have provided additional transparency over the decision-making process and ensure that information captured is accurate, complete and corporate knowledge is retained to inform future procurements.
3.26 The NBA’s spending proposal minute documented financial approval from the Chief Executive. The NBA maintained records of agreements with CSL in the form of the executed contract and invoices.
3.27 The procurement was not included in the NBA’s annual procurement plan for 2016–17. The NBA reported the NaFAA on AusTender, within the required 42 days.
Procurement risk
3.28 The CPRs state that ‘Relevant entities must establish processes for the identification, analysis, allocation and treatment of risk when conducting a procurement’.36
3.29 The NBA’s risk management policy statement and internal risk management framework policy — KBP7 — requires each level of the organisation to undertake a five step process of risk management including identification, analysis, evaluation, treatment, monitoring and review. KBP7 includes a requirement for the use of a risk matrix relating to the consequence and likelihood of the risk occurring.
3.30 There was no evidence of the NBA conducting a risk process for the NaFAA procurement in line with its internal policies. The NBA’s 2015 draft procurement plan identified some procurement risks, including project timeliness, potential poor identification of issues, poorly developed terms of reference, and non-identification of potential consultancies. However, the plan was not used by the NBA as a basis for identifying, analysing, allocating and treating risk throughout the procurement.
3.31 In April 2015, the NBA provided a paper to the Board on the development of a strategy and approach for the CSL fractionation agreement. The paper identified key issues relating to the procurement, including that the environment in which CSL approaches the negotiations had changed since the contract preceding the NaFAA, and the need to explore potential risks of the new global production capacity at Broadmeadows.37
3.32 A paper that was presented to the JBC in April 2016, prior to NBA’s commencement of contract negotiations with CSL, demonstrated the NBA’s consideration of broader strategic issues, including CSL’s desire to make capital investment dependent on the next contract, related blood product transition implications and annual plasma volumes. These issues were raised in a letter to CSL in April 2016, and continued to be discussed throughout the negotiation. No mitigations or risk treatments were developed for these issues prior to negotiation.
Was the basis for assessing value for money sound?
NBA benchmarking activities, modelling, and forecasting formed a sound basis for demonstrating value for money in the NaFAA.
3.33 The market for fractionation services in Australia is a local monopoly, in a global industry that the NBA identifies as oligopolistic and having limited market transparency. In this type of market, value for money cannot be assessed through the usual evaluation of competitive tenders.38 Auditor-General Report No.48 2014–15 Limited Tender Procurement noted that it is generally more difficult for entities conducting a limited tender to demonstrate value for money, and identified activities that entities may undertake in addition to obtaining multiple quotes to increase the likelihood of achieving value for money, including:
- benchmarking costs for similar services procured previously; and
- negotiating strongly for discounted pricing or additional services rather than accepting initial quotes provided.39
Benchmarking from the previous contract
3.34 In May 2014, the NBA completed a mid-term review of the previous contract with CSL — the CSL Australian Fractionation Agreement (CAFA) — as required by the terms of the contract to determine the expiry date. The assessment was based on CSL’s achievement against key performance indicators (KPIs), the suitability of the range of products manufactured, and whether product prices remained competitive. Outcomes of the review determined whether the contract continued to the full term.
3.35 The CAFA set out a benchmarking methodology to be applied in the mid-term review to ensure that the prices under the contract remained competitive in comparison to other comparable markets, and relative or better in percentage terms to the pricing position as at 1 January 2010. The methodology considered whether prices remained competitive through a series of price benchmarking approaches:
- the NBA contingent intravenous immunoglobulin (IVIg) price (that is, the NBA price for imported IVIg);
- other plasma-derived product prices obtained by the NBA for comparable products (if any);
- pricing, trends and industry intelligence from publicly available data;
- price data for a comparable selection of countries from IMS (an international subscription service which collects and reports price/volume information)40; and
- published United States price data from the Centre for Medicare and Medicaid Services.
3.36 The methodology noted that prices considered must exclude all taxes, duties, wholesale margins, distribution costs and the cost of plasma. Pricing was affected by foreign exchange rates, with the CAFA determining that an accepted exchange rate to convert the average selling price into Australian dollars was the 12 month average historical exchange rate for the period immediately prior to the date of commencement of the review, for the relevant currency.
3.37 The CAFA required CSL to purchase additional commercial data, and conduct the benchmarking, which was completed in February 2014. In completing the benchmarking analysis, CSL made several assumptions and adjustments to the methodology. In the mid-term review report the NBA noted that CSL used:
- a plasma cost sourced from publicly quoted sources identifying small volume market trading in plasma, which were not representative of the efficiency of vertically integrated plasma collections undertaken by major global fractionation companies such as CSL itself;
- a mean reverting exchange rate rather than the 12 month average historical exchange rate in the CAFA41;
- prices from comparator countries weighted by volume to arrive at a price for each product in each of the reference countries; and
- average product prices derived from three different data sources, for comparator countries.
3.38 CSL’s analysis asserted that prices under the CAFA at the time of review:
- remained, on average, five per cent lower than comparator prices from the selection of reference countries;
- the average price of IVIg had remained flat in nominal terms since the commencement of the CAFA, and had fallen by eight per cent in real terms as a result of the reducing average price as volumes of IVIg supplied increased; and
- prices of all products supplied under the CAFA had declined in real terms consistent with the indexation provisions that allowed for unit price increases of one per cent less than the annual inflation rate.
3.39 The NBA re-performed CSL’s benchmarking analysis, with two differing key assumptions:
- plasma cost of US$111 per litre, which was a reported cost for CSL’s global collections, against a cost of US$122 used in the CSL analysis; and
- 12 month historical average exchange rate.
3.40 The NBA’s analysis noted that, dependant on the underlying assumptions, prices under the CAFA could be between 10 to 16 per cent higher than average. The NBA noted in the mid-term review report that the differences in the approach and findings:
…primarily serve to show that the process of relying directly on a figure derived from an international price benchmarking process is highly unreliable, given that this process relies on a methodology where the correct approach is debatable in a number of key respects, and source information which is not readily available or comparable on a basis which would represent the operation of a fully functioning and economically efficient marketplace.
3.41 The report noted scope for improvement of prices for a number of key blood products. The NBA decided against seeking revised prices from CSL on these products, on advice from the Board. Whilst the NBA did not pursue revised prices for these products at the time of review, NBA Board papers in February 2017 recorded that:
- in November 2014, at the request of the JBC, the NBA sought an efficiency dividend on the commercial pricing formula under the CAFA following the JBC’s endorsement of the Four Year Plasma Plan in 2014; and
- in April 2016, the NBA confirmed an efficiency mechanism with prices indexed by an amount of Consumer Price Index (CPI) minus three and a half per cent, rather than CPI minus one per cent.
3.42 The NBA’s CAFA mid-term review concluded that CSL had satisfactorily performed its obligations under the agreement, the range of products were generally equivalent to international products with a competitive yield for immunoglobulin (Ig), and product pricing was useful for budget management processes as the price of Ig products declined with volume and the prices of all products remained behind CPI increases.
Benchmarking for the NaFAA
3.43 The NBA did not re-perform international benchmarking activities in 2016–17 for the NaFAA procurement, with CSL’s first proposal for the NaFAA noting that re-performing the benchmarking methodology for the new agreement would cost approximately $150,000. Instead, the NBA engaged KordaMentha in June 2017 to provide financial modelling and assessment of CSL’s proposals, was provided a report commissioned by CSL from KPMG on value for money in the global fractionation market, and considered commercial intelligence analytics reports prepared by a financial services company.
3.44 In March 2017, CSL provided a first proposal for the new contract which referenced the benchmarking from the CAFA as the basis for value for money and included updated data from the KPMG commissioned report that evaluated price competitiveness.
3.45 The finalised KPMG report was provided to the NBA in June 2017 and did not follow the same benchmarking methodology set out in the CAFA. KPMG analysis found that when excluding the cost of plasma collection, the fractionation fee for plasma products supplied to Australia under the CAFA appeared to be in line with average commercial price benchmark against the USA, Germany, Japan, France, Italy, Canada, Sweden and Switzerland. KPMG caveated that price comparisons were sensitive to differences in plasma supply fractionation models, which were difficult to accurately adjust due to:
- the range of products supplied, which may impact cost recovery by allocating fixed costs across more or less products derived per litre of plasma;
- obligations and regulatory constraints under which suppliers operate, with costs typically increasing with an increase in obligations and constraints;
- differences in demand for products derived from the same litre of plasma; and
- length of contracts.
3.46 The NBA provided KordaMentha with a copy of the KPMG report in July 2017 and the commercial intelligence analytics reports on CSL prepared by a financial services company. The NBA advised that KordaMentha completed a reconciliation of KPMG’s report against its own modelling that had been based on assumptions and information agreed with the NBA. While the NBA considered that the KPMG report provided analysis and arguments that supported CSL’s negotiation position, the NBA advised that the matters presented in the report did not become the direct subject of negotiations.
3.47 KordaMentha conducted market analysis and investigation of CSL’s business, including assessments of return on equity, return on assets, and earnings. In an interim report to the NBA, KordaMentha confirmed that CSL was financially in line with competitors, and globally was not profiting more than its competitors. The report also noted that the imported blood product prices paid by the NBA were lower than CAFA prices, and that this may have been due to the contracted prices for imported Ig not being at market rates. In comparison to imported blood product prices, Ig costs under the CAFA and the first proposal were approximately 22 per cent higher inclusive of the yield bonus, block fee and national reserve fee, excluding the cost of plasma.42
3.48 As noted in paragraph 3.33, benchmarking costs for similar services procured previously may increase the likelihood of achieving value for money. The CAFA was used as the basis to demonstrate savings for the NaFAA as a comparison to previous supply costs. KordaMentha conducted financial modelling and analysis of CSL’s two proposals, which included:
- rolling forward the existing CAFA assuming all of the pricing and indexation formulas were in place for the duration of the new contract for each of the products as a baseline;
- using the four-year national blood plan to determine the volume of products to be provided, and average growth rate across the four years, to forecast the growth in forward years;
- adopting an inflation rate of two per cent from CSL’s first proposal. Modelling for the second proposal including an agreed indexation arrangement for Ig products (see discussion from paragraph 3.65); and
- scenario analysis for plasma growth.
3.49 KordaMentha modelled three scenarios for plasma growth for each proposal to determine the range of potential savings. This modelling is shown in Figure 3.1.
Figure 3.1: KordaMentha scenario analysis — NaFAA and CAFA cost comparisons

Source: NBA documentation.
Were proposed rates and conditions effectively negotiated?
The NBA effectively negotiated proposed rates and conditions between the two proposals submitted by CSL by leveraging CSL’s preference for a longer contract period, negotiating a price reduction and the retention of indexation arrangements from the previous contract, and integrating conditions in the contract that provided greater flexibility with additional assurance over risk.
3.50 On 15 February 2016, CSL wrote to the NBA to request a letter of intent to negotiate the next contract after the CAFA. CSL noted that the letter of intent would allow CSL to proceed with investing in the initial design of new facilities at its existing site in Broadmeadows, Victoria, to progress a manufacturing upgrade referred to as process migration.43 CSL presented information about the process migration project to the NBA Board in February 2016, with a subsequent presentation to the JBC in September 2016.
3.51 CSL’s letter to the NBA noted that potential for a longer term agreement would provide CSL with greater surety to move from the design phase into the construction phase for the process migration project. Efficiencies to be realised by process migration informed savings in CSL’s first proposal with the project expected to be fully realised by September 2021.
3.52 The NBA presented a paper to the JBC in April 2016 to agree to a response to CSL, resulting in a letter of intent. The letter of intent stated that the NBA anticipated the negotiation of a second agreement; however Australian, state and territory governments were still in the early stage of discussion on the contract term and conditions. The letter also sought clarification on outstanding issues regarding process migration, including:
- price benefits for key blood products, including products in the CAFA and potential new products with commercial KPIs or guarantees;
- yield, purity and quality improvements for IVIg and other key products; and
- clinical, supply planning, management or logistical implications of moving from current blood products to global blood products, which may not have been identified in the proposal.
3.53 The NBA and CSL held preliminary conversations in October and November 2016. NBA documentation following the meetings identified that issues of contract length would be critical for CSL, with a preference for an eight to ten year contract term. Substantive negotiations began on 24 March 2017. A timeline for the negotiation phase of the NaFAA procurement is set out at Figure 3.2. The NBA’s internal procurement policy KBP3 requires procurements to have an approved negotiation strategy. The NBA did not have an approved negotiation strategy for the NaFAA procurement, documentation of the negotiation approach was set out in papers to the NBA Board, and the JBC.
Figure 3.2: Timeline of contract negotiations

Source: ANAO analysis of NBA documentation.
3.54 Outcomes from the NBA’s initial negotiation meeting with CSL were reported to the JBC on 7 April 2017, noting that:
- the NBA would seek best possible value for money, with a preference for a shorter term agreement of two to four years that took into account uncertainties regarding process migration and potential government policy changes; and
- CSL indicated a continuing preference for a longer term agreement of preferably eight to ten years, and an interest in a potential opportunity for the commercial sale of surplus Australian albumin.
3.55 CSL’s first proposal identified approximately $110 million worth of savings over an eight year fixed term, compared to prices under the CAFA at 31 December 2017. This was based on 2017–18 plasma and product supply estimates and an annual CPI of two per cent. The proposal stated that savings would grow in excess of $150 million if the volume of plasma and Ig products were to grow by at least five per cent per year over the eight year period. CSL’s proposal stated that ‘the price offer is inextricably linked to the contract terms and should be considered a holistic package’.
3.56 The proposal also stated that the costs of process migration would be partly amortised by the contract. Savings were generated from a combination of:
- price reduction for Ig products of $2.20 per gram at commencement of the new contract, with all other products remaining at CAFA exit prices;
- an efficiency dividend through changing indexation to CPI minus 1 per cent for Ig products in any year where plasma and product volume growth of five per cent or greater is achieved;
- effective July 2019, all product prices and fees subject to annual indexation at CPI, removing indexation agreements made under the CAFA;
- from September 2021, a price reduction for Ig products by a further $2.00 per gram, with additional reductions for Albumin; and
- reduction of the block fee in 2021.
3.57 CSL’s offer included additional changes to CAFA contract conditions including:
- changes to manufacturing processes for five existing products and an addition of nine new products following process migration;
- maintaining the NBA’s step-in rights only until the introduction of process migration;
- establishing a Joint Operational Team to review and simplify relevant operational and administrative processes;
- minimum annual starting plasma volume of 650 tonnes;
- changes to KPI2 to express yield as the percentage of Ig recovered from starting plasma, as opposed to the grams of Ig produced per kilogram; and
- post-process migration, the replacement of one product with an imported alternative at a lower cost.44
3.58 On 29 June 2017, the NBA wrote to CSL advising that preliminary analysis of CSL’s proposal had been completed and had identified that the first proposal did not provide a significant improvement in value for money, when compared to the CAFA, to warrant a longer term contract. Key issues outlined by the NBA included:
- prices and fees across various elements of the contract;
- removal of discounts from indexation arrangements under the CAFA;
- production yield issues; and
- contractual performance and measurement.
3.59 As shown in in Figure 3.1, KordaMentha modelled several scenarios focused on plasma growth, measured against the costs of the CAFA, rolled forward eight years including indexation arrangements under the CAFA.
3.60 Initial modelling and analysis undertaken by KordaMentha was provided to the NBA in an interim report on 13 July 2017. The report suggested that the savings presented in CSL’s first proposal varied between $74 million and $119 million, with the majority of savings to be realised from September 2021, following expected process migration completion. The interim report sought additional clarification from CSL on price parameters and assumptions for modelling, process migration risks, justification for increased minimum plasma volume, and contract term length.
3.61 The NBA conducted five additional meetings with CSL until August 2017, when CSL submitted a second proposal, a ‘best and final offer’ on 9 August 2017. Meeting minutes were not maintained for these conversations — outcomes were primarily documented through papers to the JBC and NBA Board, some email exchanges, and draft internal NBA documents.
3.62 The NBA’s paper to the JBC in September 2017 reiterated that the first proposal was complex, with numerous interrelated elements for prices and fees. The NBA noted that the first proposal did not appear to provide a significant improvement on value for money from the CAFA and that the proposed term of eight years was inconsistent with the NBA’s preference for a two to four year contract. The analysis also identified that the potential implications and benefits of process migration were unclear.
3.63 Variations between the first and second proposals from CSL indicate the NBA’s ability to negotiate against issues identified during the procurement. Modelling undertaken by KordaMentha estimated final savings against the CAFA in the range of $171.8 to $244.0 million over nine years. These savings demonstrated a $38.6 million improvement over the first proposal45, more evenly spread across the agreement, rather than back-loading savings post process migration. The second offer included changes in a number of areas of the agreement, as outlined in the sections below.
Pricing and indexation
3.64 CSL’s second offer included a further reduction in the price of Ig and the block fee, with differing indexation arrangements. Price reductions in 2021 were the result of the expected implementation of process migration, with the reductions occurring regardless of whether the process migration project was completed.
3.65 The CAFA contained a general price indexation mechanism of CPI minus one per cent for some products that was later reduced to CPI minus three and a half per cent in April 2016 (as described in paragraph 3.41). This indexation mechanism was not included in CSL’s first proposal. The NBA negotiated the retention of the indexation arrangement to assist in generating greater potential savings in the NaFAA from discounts achieved through indexation. The agreed indexation was CPI minus one per cent for some products.
3.66 For Ig products, CSL’s second proposal incorporated a price reduction of $2.70 per gram from the commencement of the contract until process migration, when it would reduce by a further $1.50 per gram.
3.67 CSL’s second proposal also weighted IVIg price to the block fee, declining at an average of 1.1 per cent annually across nine years. CSL assumed annual growth in plasma and Ig products of five per cent, with an annual CPI of two per cent, setting out that this mechanism would deliver an annual efficiency dividend of three per cent.
3.68 The second proposal included the continuation of the block fee, at $32 million per year with no indexation until 30 June 2020, and indexed at CPI minus one per cent thereafter. This represented a reduction of approximately $1.66 million on the block fee at the end of the CAFA following indexation agreements. The block fee would be reduced by a further $1 million at September 2021.
Plasma volume and growth
3.69 CSL’s second proposal was made on the basis of compound annual growth for both plasma for fractionation and Ig products of five per cent determined on the CAFA exit plasma volume of 662 tonnes. The final minimum annual starting plasma volume negotiated in the NaFAA is 496 tonnes, approximately 75 per cent of the 662 tonnes proposed by CSL in the second proposal.
3.70 Plasma growth was based on the NBA’s agreement with Lifeblood and a Plasma Strategy agreed with the JBC in 2014; however, this was not included in the contract as a target. Scenario modelling conducted by KordaMentha (see Figure 3.1) indicated that the NBA would still achieve savings from the NaFAA when compared to the CAFA, although this would be at a reduced rate without the planned plasma growth.
Contract length and review
3.71 The NBA’s letter to CSL on 29 June 2016 noted that the NBA’s preference would be for a shorter term contract of two to four years (as opposed to CSL’s proposed eight year term) taking into account uncertainties regarding process migration and potential government policy changes. The letter advised that a longer term proposal would not be ruled out; however a proposal for a longer term contract would need to be ‘compelling’ in terms of value for money.
3.72 Noting CSL’s preference for a longer term contract, the NBA requested a five year agreement with two options to extend out to a total of nine years. CSL’s second proposal included an initial core period of five years, with the option to continue for an additional four years.
3.73 A review in year five determines the continuation of the contract dependent on: satisfactory achievement of KPIs and default obligations; satisfactory supply of products that meet clinical needs of patients; satisfactory implementation of process migration; and any material policy changes by Australian, state and territory governments.
3.74 The NBA advised the JBC that these review parameters were broader than those set out in the CAFA and provided necessary flexibility for the NBA noting that contract duration can be adjusted if governments are not comfortable with continuing the contract beyond the core period. A longer contract provided CSL with surety to invest in process migration.
Process Migration Governance Committee
3.75 In an interim report to the NBA provided on 13 July 2017, KordaMentha noted that process migration might contain a level of risk for the NBA, and encouraged discussions on mitigation and due diligence processes. An initial assessment by the NBA noted the exclusion of step-in rights after process migration that prompted questions to CSL regarding production access and priority of Australian requirements.
3.76 CSL’s second proposal included a Process Migration Governance Committee, with terms of reference to be agreed by formal notice at a later date. The joint governance arrangement between the NBA and CSL was to oversee improvements to the manufacturing processes to be implemented in process migration and ensure that associated efficiencies are realised.
Power of direction
3.77 Under the CAFA, the NBA could provide a step-in notice to CSL in the event of material non-performance by CSL of its obligations under the contract, gross mismanagement, or other events that give rise to a material risk of disruption to the supply of products. Step-in rights required CSL to commence remediation activities within five working days and entitled the NBA to assume temporary or partial possession, management and operation of relevant parts of CSL to remedy the issue.
3.78 CSL’s first proposal maintained step-in rights only until the implementation of process migration. A power of direction clause was negotiated as an alternative, where the NBA can issue a direction to CSL in the event of material non-performance or gross mismanagement of CSL, or other event that gives rise to material risk to disruption of supply. Actions available to NBA include requiring the provision of information by CSL to the NBA, deployment of CSL’s resources in relation to obligations relating to the supply of products and services, monitoring, maintenance and operation of CSL assets, and the provision of an action plan. CSL’s second proposal included step-in rights until process migration with the power of direction beginning at the commencement of process migration until the end of the contract.
Home delivery
3.79 The NBA advised that home delivery was originally developed as an extra-contractual offering for imported clotting factors under previous contracts. The NaFAA includes provisions for home delivery on relevant products, with prior clauses and clinical protocols used as the basis for contract terms drafting and negotiation.46
Finalising the contract
3.80 CSL’s second proposal was used as the basis for ongoing negotiations from August 2017 to finalisation of the contract in December 2017. These negotiations focused on defining changes to relevant clauses in the contract. Negotiations focused primarily on the proposed Ig yield calculations for KPIs 1 and 2.
3.81 KordaMentha provided the NBA with a final evaluation of both of CSL’s proposals in November 2017, with scenario modelling conducted from three different plasma growth scenarios. The report estimated that savings against the previous contract were between approximately $171.8 million and $244.0 million.
3.82 The NBA Chief Executive approved the spending proposal for the NaFAA on 6 December 2017, with the final agreement between NBA and CSL signed on 6 December 2017, following endorsement from state and territory governments and the Australian Government by 4 December 2017.
4. Contract management and monitoring arrangements
Areas examined
This chapter examines whether the National Blood Authority (NBA) established effective contract management and monitoring arrangements to ensure the delivery of the National Fractionation Agreement for Australia (NaFAA or the contract).
Conclusion
The NBA established contract management arrangements for the NaFAA which have been largely effective; however, they were not always implemented fully in accordance with requirements of NBA internal policy.
Areas for improvement
The ANAO made three recommendations to the NBA aimed at:
- reviewing the reporting of CSL’s performance against the NaFAA KPIs;
- aligning NaFAA risk management with internal policy requirements; and
- updating internal policies to support business needs and reflect the NBA’s operating environment.
The ANAO identified opportunities for improvement regarding:
- reviewing and updating the NaFAA contract management plan in accordance with internal policy;
- improving consistency of issues management; and
- capturing lessons learned through the established internal processes.
4.1 It is important that the NBA effectively manages the NaFAA in order to ensure the delivery of services and the ongoing achievement of value for money. To assess whether effective contract management and monitoring arrangements had been established, the ANAO examined:
- whether the NBA effectively monitored CSL’s performance against deliverables and key performance indicators (KPIs);
- whether the NBA managed risk in accordance with internal guidance and contract requirements; and
- whether the NBA’s contract management approach for the NaFAA was informed by previous experience and lessons learned.
Does the NBA effectively monitor and manage the service provider’s performance under the NaFAA?
The NBA’s monitoring and management of CSL’s performance has been largely effective. The NBA monitored CSL’s performance against the NaFAA KPIs, and enforced financial penalties and provided bonuses largely in accordance with the contract. Assurance processes for the NaFAA have been established but not consistently completed.
Contract deliverables and payments
4.2 There are four regular payments that are payable by NBA to CSL under the NaFAA47:
- per-unit payments for products, which are made monthly for both completed deliveries and units placed into the National CSL Reserve48;
- a monthly block fee for production and supply of the immunoglobulin (Ig) products intravenous immunoglobulin (IVIg) and subcutaneous immunoglobulin (SCIg);
- a monthly fee for management of the National CSL Reserve; and
- per-unit payments for products that expire in CSL inventory or National CSL Reserve, where CSL has met all of their relevant obligations.
4.3 The NBA monitored product movements via the integrated data management system (IDMS). Data provided by CSL on production and supply of products is imported into IDMS and used by the NBA to validate and pay invoices. Total expenditure under the NaFAA from 1 January 2018 to 31 December 2020 was $811.9 million (see Table 4.1).
Table 4.1: Total NaFAA expenditure
|
Year |
Payments for products |
CSL reserve management fee |
Block fee |
Payments for expired products |
Total |
|
2017–18 (from 1 January 2018) |
$115,428,926 |
$136,340 |
$16,000,000 |
$45,209 |
$131,610,474 |
|
2018–19 |
$230,746,764 |
$272,679 |
$32,000,000 |
$559,472 |
$263,578,915 |
|
2019–20 |
$236,689,305 |
$276,224 |
$32,096,000 |
$27,991 |
$269,089,520 |
|
2020–21 (up to December 2020) |
$131,209,113 |
$141,151 |
$16,240,576 |
– |
$147,590,839 |
|
Totala |
$714,074,107 |
$826,393 |
$96,336,576 |
$632,672 |
$811,869,748 |
Note a: All prices are excluding GST.
Source: ANAO presentation of NBA data.
Contract management processes
Internal requirements
4.4 The NBA had established a suite of internal guidelines for key business processes (KBP). Guidance for contract management — KBP4 — was available on the NBA’s intranet and dated 2008.
4.5 KPB4 requires the development and approval of a contract management plan. The NBA established a contract management plan for the NaFAA that complied with requirements of KBP4, and included content consistent with the Australian Government Contract Management Guide. The NBA could not provide evidence of the plan being approved.
4.6 Review of the contract management plan was listed on the NBA’s activity schedule49, but the plan has not been reviewed or updated. Annual review and updating of contract management plans is recommended by KBP4, particularly for multi–year contracts. To be consistent with internal guidance, the NBA should review and update the NaFAA contract management plan to ensure it remains consistent with the approach being used.
4.7 The NBA established additional supporting documentation for contract management processes. The NBA developed standard operating procedures (SOP) specific to the NaFAA for KPI calculation, KPI quality management, and annual stocktake. Instructions in the SOP for KPI calculation were consistent with the calculation methods specified in the NaFAA. Other SOPs were established for processes relevant to all NBA contracts, including the NaFAA. These SOPs cover goods ordering and receipt verification; and invoice processing.
Contract requirements
4.8 The NaFAA outlines a contract management process which requires the following meetings to be held:
- annual risk management workshops;
- bi-annual Chief Executive update and planning meetings; and
- quarterly contract management meetings.
4.9 Under the NaFAA, the parties have also agreed to maintain continuous dialogue and hold additional meetings as required.
4.10 Meetings were mostly held as required, with the exception of one Chief Executive meeting and one contract management meeting scheduled for June and July 2020, respectively, which were cancelled at NBA’s request. The NBA advised that the meetings were not held due to issues being managed by both parties arising from the COVID-19 pandemic. Risk management workshops have been held annually. Risk management aspects of contract management are discussed further from paragraph 4.41 onwards.
4.11 Under the NaFAA, CSL is required to provide a total of 23 different types of regular reports, and two types of reports upon request. Regular reports are to be provided to the NBA on a monthly, quarterly, bi-annual, or annual basis, depending on the report. The NBA monitored the receipt of the CSL reports with a spreadsheet, and reports had been received and stored in the NBA’s record management system.
Performance monitoring
Key performance indicators
4.12 The NaFAA includes five KPIs to monitor CSL’s performance. KPIs are aligned with contract deliverables and impose a financial penalty where requirements are not met, and in one case a bonus where CSL exceeds the KPI target. A summary of KPI requirements and financial consequences can be seen at Table 4.2.
Table 4.2: ANAO summary of NaFAA KPIs
|
KPI title |
ANAO summary of requirements |
ANAO summary of financial consequence |
|
KPI1 – Plasma Stewardship |
Loss of starting plasma must be within tolerated levels. Target Less than 2000 kg for starting plasma for Ig products Less than 200 kg for starting plasma for hyperimmune products 0 kg for starting plasma lost from: failed production batches; low yielding production batches; and loss or expiry of products |
If loss of starting plasma is higher than tolerated levels, a rebate is payable by CSL to NBA. |
|
KPI2 – Production Yield |
Production yield for IVIg and SCIg products must meet annual yield target levels. Target Between 4.7 g and 4.9 g of Ig per kg of starting plasma for IVIg (revised annually) Between 4.4 g and 4.6 g of Ig per kg of starting plasma for SCIg (revised annually) |
If yield exceeds target range, CSL receives a bonus. If yield falls short of target range, NBA reduces monthly block payment to CSL. |
|
KPI3 – Management of Minimum Inventory and National CSL Reserve levels |
Inventory must be maintained at required levels for: starting plasma; products in CSL Inventory; and products in National CSL Reserve. Target 100 per cent |
If any levels are identified below minimum, a rebate is payable from CSL to NBA. |
|
KPI4 – Fulfilment of Orders |
Product orders must be completed in accordance with contract requirements. Target Order accuracy of 98 per cent per month |
Where order accuracy is less than 98 per cent for the month, a rebate is payable from CSL to NBA. |
|
KPI5 – Shelf Life of Products in National CSL Reserve |
Shelf life for products in the National CSL Reserve must match the required minimum shelf life specified in the contract. Target 100 per cent |
If any product in the National CSL Reserve has less than the required minimum shelf life then a rebate is payable from CSL to NBA. |
Source: ANAO analysis of NaFAA requirements.
4.13 The established KPI calculation and reconciliation process is outlined in Figure 4.1. KPI results are to be assessed monthly and financial penalties or bonuses finalised annually. The ANAO observed the NBA assess KPI results for the month of September 2020. The assessment was conducted in accordance with the calculation SOP using information from the relevant CSL reports. Formulas used in the KPI spreadsheet were reviewed and no issues were identified.
Figure 4.1: NaFAA KPI reconciliation process

Source: ANAO summary of NBA documentation.
4.14 Monthly reconciliations were completed for 28 of the 34 months examined, with discrepancies being identified by the NBA in 12 of 28 (43 per cent) completed reconciliations. The NBA discussed and resolved discrepancies with CSL via e-mail. The remaining six months of reconciliations were not completed in the regular timeframe. The NBA provided evidence of the results for these six months being reconciled at a later date.
4.15 Quarterly discussion of results has occurred at all contract management meetings since the NaFAA commenced. The NBA finalised KPI results for the NaFAA for 2017–18 and 2018–19 and complied with internal requirements for approval and invoicing. As of March 2021, KPI results and financial consequences for 2019–20 had not been finalised internally, but draft results were included in the 2019–20 NBA annual report.
Performance results
4.16 Between January 2018 (when the NaFAA commenced) and 30 June 2020, CSL has paid a total of $522,983 in KPI penalties and earned $101,814 in bonus payments. The NBA has effectively monitored performance against most KPIs, enforced penalties, and awarded bonuses in accordance with the NaFAA. The NBA advised that it considers CSL to have performed well under the contract.
4.17 The NBA has not monitored against one aspect of KPI3. The NaFAA states that CSL must hold a minimum inventory of starting plasma that is no more or less than 30,000 kg and under KPI3 a penalty applies where inventory is not maintained at the required level. The NBA advised that the requirement for CSL to hold a minimum plasma inventory has not been enforced as all plasma collected since the NaFAA commenced has been used to meet product demand. NBA further advised that a starting plasma inventory will be established during 2020–21.
Table 4.3: NaFAA KPI results
|
KPI and target description |
2017–18 (from January 2018) |
2018–19 |
2019–20 |
|
|
KPI |
Target |
Result |
||
|
KPI1 –Plasma Stewardship
|
Starting plasma loss for Ig products: ≤2000 kg |
0.273 kg |
0 kg |
0 kg |
|
Starting plasma loss for hyperimmune products: ≤200 kg |
0.309 kg |
0 kg |
0 kg |
|
|
Plasma loss from failed production: 0 kg |
0 kg |
0 kg |
0 kg |
|
|
Plasma loss from low yielding batch: 0 kg |
2254.2 kg |
121.1 kg |
0 kg |
|
|
Plasma loss from product loss or expiry: 0 kg |
307.0 kg |
1550.1 kg |
124.4 kg |
|
|
KPI1 Penalty |
($386,484) |
($22,282) |
($37,805) |
|
|
KPI2 – Production Yield |
Yield target ranges for IVIg and SCIg are revised annually. |
IVIg Target: 4.644 –4.842 g/kg Result: 4.682 g/kg (within target) |
IVIg Target: 4.706 – 4.906 g/kg Result: 4.846 g/kg (within target) |
IVIg: Target: 4.780 – 4.983 g/kg Result: 5.016 g/kg (above target) |
|
SCIg Target: 4.348 – 4.545 g/kg Result: 4.658 g/kg (above target) |
SCIg Target: 4.406 –4.606 g/kg Result: 4.542 g/kg (within target) |
SCIg: Target: 4.474 –4.678 g/kg Result: 4.681 g/kg (above target) |
||
|
KPI2 Penalty/Bonus |
$5,424 |
$0.00 |
$96,390 |
|
|
KPI3 –Management of Minimum Inventory and National CSL Reserve levels |
Starting plasma inventory: 100% |
N/A |
N/A |
N/A |
|
Minimum product inventory: 100% |
100% |
100% |
100% |
|
|
National CSL Reserve: 100% |
100% |
100% |
100% |
|
|
KPI3 Penalty |
$0.00 |
$0.00 |
$0.00 |
|
|
KPI4 –Fulfilment of Ordersa |
Order accuracy of 98% or above per month |
Target achieved 3 out of 6 months Annual order compliance: 97.9% |
Target achieved 10 out of 12 months Annual order compliance: 99.8% |
Target achieved 9 out of 12 months Annual order compliance: 99.7% |
|
KPI4 Penalty |
($31,643) |
($39,523) |
($5,246) |
|
|
KPI5 – Shelf Life of Products in National CSL Reserve |
100% |
100% |
100% |
100% |
|
KPI5 Penalty |
$0.00 |
$0.00 |
$0.00 |
|
|
Total penalty/bonus |
($412,703) |
($61,805) |
$53,339 |
|
Note a: Financial penalties for KPI4 do not directly correlate to overall compliance. Penalties apply for missed deliveries which are greater than 2 per cent of the total number of orders for a given month. Penalties are calculated using a percentage of the value of the surplus missed orders. The compliance measure indicates the rate at which 98 per cent or greater order accuracy was achieved for each month as a percentage of total orders.
Source: ANAO analysis of NBA documentation.
Reporting
4.18 The NBA included a table of CSL’s performance against the NaFAA KPIs in the NBA annual reports for 2017–18, 2018–19, and 2019–20. The results presented are simplified and do not include financial penalties.
4.19 For KPI1, the NBA reports the result as either achieved or not achieved and does not report the amount of plasma lost. The report states that a result of achieved is equivalent to a result of 90 per cent or above. The NBA has reported KPI1 as achieved for all years, despite plasma losses being recorded in categories with a 0 kilogram tolerance, as outlined in Table 4.3.
4.20 The NBA was not able to provide an explanation for how the result of achieved has been determined for KPI1. The NBA’s response stated:
The approach taken to representing commercial supplier performance against KPIs in recent NBA annual reports has been not to specifically report on the detailed KPI achievement as measured by the various provisions of NBA contracts, including the NaFAA. Rather, a more generalised functional approach has been taken in representing performance outcomes, for a general audience.
4.21 For KPI2, the NBA reports CSL’s achieved yield for each quarter and the average for the year, for example 4.682 grams per kilogram for IVIg in 2017–18. The target yield range, which is revised annually, is not included. The achieved yield alone, as reported by NBA, does not indicate whether the KPI2 result is within, above, or below the target range.
Recommendation no.1
4.22 The National Blood Authority review the reporting of CSL Behring’s performance against the NaFAA key performance indicators in the annual report, to ensure it accurately reflects the performance target and the result achieved. Results presented should provide the reader with a clear indication of performance against the targets.
National Blood Authority response: Agreed.
4.23 As indicated in the audit report, the NBA monitors the performance of CSL Behring against the key performance indicators of the NaFAA. The NBA will review the reporting of this performance in the NBA Annual Report, together with that of other commercial suppliers, to ensure that performance information is publicly reported in the most appropriate way.
Waiving of penalties
4.24 The NaFAA allows for KPI penalties to not be enforced under certain circumstances. These circumstances are listed below.
- Force majeure events (events out of the reasonable control of CSL) will be ignored and excluded from the measurement and determination of a KPI consequence.
- Starting plasma approved for special use, such as research and development, will be excluded from plasma loss under KPI1.
- Where minimum product inventory or National CSL Reserve is accessed with approval from the NBA, this will not be recorded as a breach of requirements under KPI3.
- For home delivery orders, where requirements are not met due to an event outside of CSL’s control (such as a recipient not being home) this will not be recorded as a missed delivery under KPI4.
4.25 The NBA has waived a total of $374,679 worth of KPI penalties. There were three specific instances where KPI penalties were waived, outlined in Table 4.4. In two of the three instances, penalties were waived in accordance with the NaFAA. In one instance, the NBA’s basis for waiving the penalty was not clear. The internal minute approving the waiver did not reference any section of the NaFAA which allowed the KPI to be waived in this instance.
Table 4.4: KPI waivers issued by NBA
|
Issue |
KPI penalty |
Waived in accordance with contract requirements? |
|
Higher than estimated demand, access to minimum product inventory required to fulfil orders |
KPI3 –$137,948 |
Yes – NBA provided approval for CSL to access inventory under clause 16.7.1. Between January 2018 and March 2019, inventory was accessed in 9 out of 15 months in relation to this issue and penalties were waived on a month by month basis. |
|
Product written-off as a result of traffic accident |
KPI1 – $231,597 |
Yes – Formal notice of force majeure event provided by CSL, as required under clause 43.3. NBA reviewed details and agreed that the incident was a force majeure event under clause 43. |
|
Late delivery due to re-scheduled flight (occurring in May 2020, during COVID-19 pandemic) |
KPI4 –$5,134 |
No – CSL notified NBA via e-mail, no formal notice provided. NBA agreed event was out of CSL’s control, but approval minute does not refer to force majeure requirements and no other source of authority to waive penalty is cited. |
Source: ANAO analysis.
CSL process migration and achievement of savings
4.26 As set out in Chapter 3, CSL is planning to implement process migration for some of the plasma products supplied under the NaFAA, including the two major Ig products, IVIg and SCIg. The NaFAA requires that a Process Migration Governance Committee (the Committee) be established to oversee the implementation of CSL’s process migration changes. The NaFAA states that the purpose of the Committee is to allow the NBA, on behalf of Australian, state and territory governments, to:
- be provided with information a timely manner;
- provide input and advice on matters of interest or concern;
- undertake any confirmatory checks or obtain any appropriate expert advice; and
- make any necessary or appropriate decisions, including to issue any formal notice or to vary the contract.
4.27 The Committee and a terms of reference were established in accordance with the NaFAA requirements, and meetings have mostly occurred on a quarterly basis, as agreed by both parties. Minutes have been kept for meetings, and an action item register has been established. One scheduled meeting was cancelled in July 2020, at the request of NBA, due to resourcing constraints for a concurrent procurement.
4.28 The NBA has fulfilled its intended role in overseeing elements of CSL process migration. The NBA has: received copies of CSL’s project plan, regulatory strategy, and transition and communication strategy; conducted analysis of these documents; and provided feedback to CSL. Expert advice was also sought on potential clinical impacts of product changes.
4.29 The NBA has identified and discussed potential issues with process migration. One ongoing matter was in relation to the manufacturing process for a new human prothrombin complex product, which will not be made entirely from Australian plasma and will be partly manufactured by CSL in Marburg, Germany. The NBA requested and received a risk assessment and mitigation plan from CSL for the new product and has conducted analysis on the impacts of this potential change.
4.30 The NBA advised the Jurisdictional Blood Committee (JBC) of the details of the proposed product changes on 14 March 2019. The JBC provided in-principle agreement to the process migration product changes, provided that certain conditions are met and that the arrangements are consistent with relevant Commonwealth legislation.
4.31 As of March 2021, the NBA continues to work through matters with CSL in relation to process migration. Manufacturing of process migrated products is expected to commence in May 2022, provided that final products are approved by the Therapeutic Goods Administration.50
4.32 The achievement of savings from the NaFAA is reliant on updated pricing which was agreed partly as a result of the process migration changes. Under the product pricing schedule in the NaFAA, reduced prices for some existing products will come into effect from September 2021, regardless of whether process migration has been completed.
Table 4.5: Domestic Ig savings from the NaFAA
|
2017–18 (from Jan 2018) |
2018–19 |
2019–20a |
Total |
|
– |
$7,400,000 |
$4,260,000 |
$11,660,000 |
Note a: The NBA reported annual savings of $4.26 million in the 2019–20 annual report. The NBA was unable to provide the original calculations for this, and upon recalculation arrived at a higher figure of $7.16 million.
Source: NBA annual reports.
4.33 The NBA’s reported savings of $11.7 million are for domestic Ig, which includes only IVIg and SCIg supplied under the NaFAA. The total expected savings of up to $244.0 million (discussed in Chapter 3) were based on the total contract expenditure over nine years, given certain assumptions on the volume of plasma collected and amount of Ig produced. The reported savings amount is not directly comparable to the expected savings amount. The NBA does not report on savings achieved for total contract expenditure.
Assurance
4.34 Performance monitoring for the NaFAA is reliant on accurate reporting by CSL. It is important that the NBA has assurance that information used to assess performance and make payments is accurate. The NBA has two mechanisms for gaining assurance over the information provided by CSL, an annual stocktake, and the goods ordering receipt verification (GORV) audit process.
4.35 The NBA undertakes an annual stocktake of the blood products in the National CSL Reserve at the end of each financial year. This allows the NBA to physically compare quantities of products to the amounts displayed on CSL’s system reports.
4.36 The NBA completed stocktakes for 2017–18 and 2018–19. No material discrepancies were identified by the NBA for these years. For 2019–20, the NBA could not complete a physical stocktake due to COVID-19 restrictions, but completed other procedures including receipt of written confirmation from CSL of the accuracy of inventory reports. Work undertaken by the ANAO for the 2019–20 financial statement audit did not identify any audit issues relating to the existence and valuation of administered inventory (including products under the NaFAA).
4.37 The GORV audit process is to be completed quarterly and involves the NBA obtaining documentary evidence for a random sample of product orders. The process is important for the NBA to gain assurance over the accuracy of payments. The ANAO 2019–20 financial statement audit assessed the GORV audit process as an effective control for verifying transactions.
4.38 The NBA’s internal GORV work program has included quarterly GORV audits for the NaFAA for all financial years since commencement. As of January 2021, the NBA had completed five GORV audits for the NaFAA and cancelled four (see Table 4.6). Internal approval was provided for all cancelled audits and e-mail records cited resourcing constraints as the reason for cancellation in all cases.
Table 4.6: NaFAA GORV audits completed between January 2018 and April 2021
|
Audit period |
2017–18 |
2018–19 |
2019–20 |
2020–21 |
|
Quarter 1 |
N/A |
✔ |
✘ |
– |
|
Quarter 2 |
N/A |
✘ |
✔ |
– |
|
Quarter 3 |
✔ |
✔ |
✔ |
– |
|
Quarter 4 |
✘ |
✘ |
– |
– |
|
Total completed/planned |
1/2 |
2/4 |
2/4 |
0/4 |
✔ Indicates that an audit was completed.
✘ Indicates that an audit was cancelled.
– Indicates that an audit has not yet been completed and no evidence of cancellation has been seen.
Source: ANAO analysis.
4.39 The NBA has noted some exceptions but no material findings from completed GORV audits of NaFAA transactions. The NBA advised that delays in completing GORV audits during 2020 were due to staff movements, staff training, and the COVID-19 pandemic. The NBA is aiming to complete GORV audits for transactions from Q4 2019–20 and Q1 2020–21 by 30 June 2021.
4.40 The NaFAA includes a clause stating that the NBA may establish a process to audit CSL’s measurement of the Ig yield of starting plasma, which is used to determine targets for KPI1 and KPI2. Targets are currently set based on information reported by CSL. Establishing this process would provide the NBA with further assurance over the accuracy of information provided by CSL.
Has the NBA effectively managed risks to the delivery of the NaFAA?
The NBA’s risk management for the NaFAA has been largely effective but has not been conducted fully in accordance with internal requirements. The NBA has focused primarily on managing supply and product risks. The NBA has been responsive to issues identified throughout the NaFAA and both parties have complied with key risk requirements under the contract.
Internal risk management processes
4.41 The NBA’s KBP4 guidance requires that a risk assessment be conducted for all contracts for both supplier and contract risks. It also requires that a contract risk management plan and a timeline of risk management activities are developed and regularly reviewed.
4.42 The NBA had not established a risk management plan for the NaFAA and had not conducted a formal risk assessment. The NBA completed other risk management processes (discussed below) which have been effective at managing certain risks, but do not address all supplier and contract risks present under the NaFAA. A previous ANAO performance audit of the NBA identified opportunities for improvement in NBA’s contract management through identification of risks and implementation of risk plans for contracts.51
Recommendation no.2
4.43 The National Blood Authority conduct risk management activities for the NaFAA in line with internal requirements, including:
- conducting an assessment of supplier and contract risks;
- establishing a NaFAA risk management plan to document the approach to risk management; and
- developing a timeline of risk management activities, including a review cycle for risk management activities.
National Blood Authority response: Agreed.
4.44 As noted in the audit report, the NBA undertakes enterprise level risk management processes that monitor and mitigate risks to the supply of blood products, including those supplied by CSL Behring. The NBA will continue to review and revise internal documentation for procurement and contract management processes, including specific additional risk management activities, and their application to individual commercial suppliers as appropriate, including CSL Behring.
Issues log
4.45 The NBA established an issues log as a register for specific issues which have arisen throughout management of the NaFAA. Each issue includes a description, an impact rating, and a list of planned and completed actions. Examples of issues recorded by the NBA include:
- instances of KPI breaches or contract non-compliance, such as: products out of temperature specification; late deliveries to home delivery recipients; and short shelf life on delivery; and
- specific events with potential supply or safety impacts, such as: a product quality investigation; a power outage at Broadmeadows; and a cyber-security incident involving a subcontractor.
4.46 Since contract commencement in January 2018, the NBA recorded a total of 42 issues with 10 issues being assigned a high impact rating. Documentation was reviewed for all 10 high impact entries which showed regular correspondence with CSL and internal escalation generally occurring. Actions taken by both parties had also been recorded, except in one case.
4.47 The issues management process has not been consistently completed since March 2020. The one high impact exception was from the 2019–20 log and had no actions recorded against it, but other documentation showed that actions had been taken. As of January 2021, 13 out of 20 issues remained ‘open’ on the 2019–20 issues log, with the last entry dated 16 March 2020. No issues had been recorded or carried over onto the 2020–21 issues log.
4.48 Some inconsistencies were observed in the NBA’s use of the issues log. Similar issues had been assigned different impact ratings without clear justification. Other information for these issues indicated that the management approach used did not differ despite the different impact ratings.
4.49 There is no requirement for use of the issues log, nor guidance on assigning impact ratings or managing issues of different impacts. For issues up until March 2020, the NBA had been responsive and demonstrated a proactive approach to managing identified issues. A documented approach to impact assessment and guidance on the management of issues could provide a more consistent approach, provided that the NBA continues its use for the NaFAA.
4.50 During 2020, a COVID-19 issues log was also established to monitor issues reported by suppliers for all contracts, including the NaFAA. As of January 2021, there were 19 issues recorded across all contracts, all of which were listed as ‘open’. Issues included demand fluctuations, over-ordering and stockpiling of products by providers, and impacts to CSL’s production and distribution arrangements from pandemic response restrictions in Victoria.
Annual Supply Risk and Risk Mitigation Update
4.51 As discussed in Chapter 2, the annual Supply Risk and Risk Mitigation Update includes all plasma and recombinant products which are supplied under various contracts, including the NaFAA. Its purpose is to manage high-level product and supply risks, rather than specific contract risks.
4.52 Each product in the plan has been assigned a risk rating and includes mitigation strategies. Risk mitigations include the minimum product inventory and National CSL Reserve levels from the NaFAA itself. The 2017–18 update resulted in NaFAA minimum product inventory levels being increased for three products. This was done as a variation to the contract, in accordance with NaFAA requirements.
4.53 On the 2018–19 update, all NaFAA products were assigned a low residual risk rating after risk mitigation; however some products were noted as requiring further review. The Supply Risk and Risk Mitigation Update was not completed for 2019–20.
4.54 During 2020, the NBA managed risks which arose from the COVID-19 pandemic by establishing a COVID-19 risk register. The register lists risks, controls, and control effectiveness ratings, and assigns an overall risk rating based on consequence and likelihood.
4.55 The NBA conducted other supply management activities in response to COVID-19. In March 2020, it was identified that as a result of COVID-19 safety measures, a number of patients in Australia began switching from IVIg, which is administered in hospital, to SCIg, which can be administered at home. This resulted in an unexpected increase in demand for domestic SCIg. There were no minimum product inventory or national reserve levels established under the NaFAA for SCIg. The NBA noted this as a risk on the 2017–18 and 2018–19 Supply Risk and Risk Mitigation Updates but considered it to be largely mitigated due to the availability of imported SCIg.
4.56 In response to the SCIg issue, the NBA introduced intensive product management under the contract for all Ig products, liaised with CSL, and completed additional supply planning. In August 2020, the NBA formally advised CSL of its intention to establish a minimum product inventory for SCIg. The NBA is required to issue a formal notice of specific required levels and vary the NaFAA accordingly to implement these arrangements.
Compliance with NaFAA risk requirements
4.57 The NaFAA contains requirements for risk management for both the NBA and CSL. Requirements include:
- an annual risk management workshop to be conducted with both parties;
- CSL providing a risk management plan and business continuity plan annually, and other relevant reports at varying intervals52;
- CSL providing notices of notifiable events, and having a general obligation to keep the NBA informed;
- alternative supply arrangements being established through performance guarantees with CSL’s overseas affiliates;
- CSL developing product support plans for approved home delivery products; and
- CSL providing a financial undertaking worth $3 million, which the NBA can draw upon to recover any costs related to damages or losses suffered.
4.58 The NBA and CSL have conducted annual risk management workshops, and CSL has provided a risk management plan and business continuity plan to the NBA annually. Other regular reports relevant to risk management have been provided. The NBA monitored the receipt of reports and some evidence has been seen of report content being reviewed. However, in the absence of a documented risk assessment or risk management plan for the NaFAA, it is not clear how information obtained through risk workshops and CSL reports informs the NBA’s approach to risk management.
4.59 CSL has issued four notices of notifiable events to the NBA. These were recorded on the NaFAA issues log. Evidence was also seen of issues being discussed at quarterly contract management meetings.
4.60 The NBA established alternative supply arrangements via performance guarantees with CSL’s overseas affiliates for the production and supply of equivalent products. NBA also received and reviewed product support material for home delivery products.
4.61 At commencement of the NaFAA, CSL requested to reuse the financial undertaking from the previous contract — the CSL Australian Fractionation Agreement (CAFA) — rather than establish a new undertaking for the NaFAA. The NBA agreed to this despite not being able to locate the signed copy of the original financial undertaking. The NBA’s internal legal advice noted that not having the original signed financial undertaking could impede the NBA’s ability to draw upon the undertaking if necessary. The NBA was unable to provide the original undertaking from the CAFA for the audit.
Is the NBA’s approach to contract management informed by learnings from earlier contracts?
The NBA’s approach to the design and management of the NaFAA was based on the previous contract with CSL. Review activities and proposals for improvement were undertaken although not through the established internal process.
4.62 The NBA’s KBP4 guidance outlines requirements for the contract completion stage. KBP4 states that at the completion of a contract, a report must be prepared for the NBA Chief Executive on the overall operation of the contract. A process is also outlined for identified contract improvements to be submitted and reviewed by the NBA Executive Management Committee as performance improvement proposals.
4.63 The previous contract with CSL, the CAFA, was in operation from 1 January 2010 until 31 December 2017. The CAFA contract management plan stated that, in addition to the required mid-term review report, a contract completion report would be completed by 31 March 2018, which would include review of contract management activities, issues and risks, and identify any lessons learned for future contracts.
4.64 A CAFA completion report was not completed and no other report on the overall operation of the contract was provided to the NBA Chief Executive after completion, as required by KBP4. While other documentation, outlined below, shows that contract improvements were identified, no evidence was provided of these being submitted to the Executive Management Committee.
4.65 As discussed in Chapter 3, the NBA completed the CAFA mid-term review, as required under the CAFA, on 7 May 2014. The report identified positive aspects to date and concluded that CSL had satisfactorily performed its obligations under the CAFA. The NBA engaged an independent expert to review the product range supplied under the CAFA, and some opportunities for improvement were also identified around product manufacturing and pricing. The report also identified contract management issues regarding the current KPIs.
4.66 Formal variations had been made to different sections of the CAFA. The NBA used the amended version of the CAFA as a basis for the design of the NaFAA. As a result, much of the NaFAA design was a continuation of existing arrangements. Negotiations for the NaFAA commenced while the CAFA was still in operation.
4.67 During the negotiation stage, the NBA reviewed various aspects of the existing arrangements which informed the design of the NaFAA. This included changes to KPI details, review of reporting requirements, and review of contract schedules and templates. The NBA also completed a general review of contract clauses and requirements and discussed these with CSL during negotiations.
4.68 While evidence shows that the NBA’s approach to the NaFAA has been informed by its prior experiences, the NBA could improve its approach to capturing lessons learned by ensuring contract improvements are documented and approved through the established formal process.
4.69 The NaFAA term review is to be completed by 30 June 2022. This will provide the NBA an opportunity to evaluate the success of current arrangements in achieving value for money and identify further improvements for contract management.
4.70 As noted throughout this report, the NBA has consistently not complied with internal policy requirements throughout both the procurement and contract management phases of the NaFAA. The NaFAA is the second largest contract administered by the NBA and represents a key part of the NBA’s core business.
Recommendation no.3
4.71 The National Blood Authority review and update its suite of internal policies and guidance for procurement and contract management. Internal policy should be consistent with current Australian Government legislative and policy requirements, be commensurate with business needs, and reflect the operating environment of the National Blood Authority. Procurement and contract management processes should be conducted in accordance with the updated internal policy requirements.
National Blood Authority response: Agreed.
4.72 The NBA maintains an ongoing program of review and updating of internal policies, procedures and guidance which is overseen and prioritised through the corporate governance arrangements of the NBA Business Committee. Documentation relating to procurement and contract management will be reviewed in this context and to ensure procurement and contract management processes are undertaken in accordance with internal policy requirements.
Appendices
Appendix 1 National Blood Authority response



Appendix 2 Products manufactured under the National Fractionation Agreement for Australia
Products manufactured under the National Fractionation Agreement for Australia
|
Product type |
Product name |
Presentation |
Price per unit (excluding GST) at 1 July 2020 |
Clinical use |
|
Albumin |
Albumex 20% |
10mL |
$16.05 |
Used in critical care scenarios. 20% albumin solutions are used in shock, burns, respiratory distress syndrome, low blood albumin and plasma exchange. 4% albumin solutions are used to replace lost blood volume, and in plasma exchange. |
|
100mL |
$70.01 |
|||
|
Albumex 4% |
50mL |
$16.05 |
||
|
500mL |
$70.01 |
|||
|
Intravenous immunoglobulin (IVIg) |
Intragam Pa |
50mL |
N/A |
Antibody replacement and treatment of some immune disorders in the hospital setting. |
|
200mL |
N/A |
|||
|
Intragam 10 |
25mL |
$145.50 |
||
|
100mL |
$581.99 |
|||
|
200mL |
$1,163.98 |
|||
|
Subcutaneous immunoglobulin (SCIg) |
Evogam |
5mL |
$48.56 |
Antibody replacement and treatment of some immune disorders in the home setting. |
|
20mL |
$186.24 |
|||
|
Factor VIII |
Biostate |
250IU |
$225.84 |
Treatment and prevention of bleeding in Haemophilia A and treatment of bleeding in von Willebrand disease. |
|
500IU |
$451.68 |
|||
|
100IU |
$903.36 |
|||
|
Factor IX |
MonoFIX-VF |
1000IU |
$903.36 |
Treatment and prevention of bleeding in haemophilia B. |
|
Human prothrombin complex |
Prothrombinex-VF |
500IU |
$287.96 |
Treatment of bleeding due to deficiency of coagulation factors II, IX or X, and to reverse warfarin. |
|
Antithrombin III concentrate |
Thrombotrol-VF |
1000IU |
$1,456.67 |
Treatment of Antithrombin III deficiency. |
|
Cytomegalovirus immunoglobulin |
CMV immunoglobulin-VF |
1.5MILL U |
$1,245.58 |
Helps protect against Cytomegalovirus. |
|
Hepatitis B immunoglobulin |
Hepatitis B immunoglobulin-VF |
100IU |
$45.53 |
Helps protect against Hepatitis B. |
|
400IU |
$104.24 |
|||
|
Normal immunoglobulin |
Normal immunoglobulin-VF |
2mL |
$32.77 |
Helps protect against Hepatitis A.
|
|
5mL |
$53.72 |
|||
|
RhD immunoglobulin (Glycine Formulation) |
Rh (D) immunoglobulin-VF |
250IU |
$30.84 |
Helps prevent haemolytic disease of the newborn. |
|
625IU |
$77.07 |
|||
|
Tetanus immunoglobulin |
Tetanus immunoglobulin-VF |
250IU |
$45.01 |
Helps protect against Tetanus. |
|
4000IU |
$720.02 |
|||
|
Zoster immunoglobulin |
Zoster immunoglobulin-VF |
200IU |
$285.48 |
Helps protect against Zoster (the virus that causes Chicken pox and Shingles). |
Note a: Intragam P was transitioned to Intragam 10 in March 2017. The product has not been supplied since then.
Source: NBA documentation.
Footnotes
1 The NaFAA is for a nine-year term with an expiry date of 31 December 2026. An earlier expiry date of 31 December 2022 can be determined depending on the findings of the contract term review due to be completed by 30 June 2022.
2 Specific roles of the JBC relating to the national blood supply are set out in Clause 24 of the National Blood Agreement. The JBC arrangements are potentially subject to change following the Review of Council of Australian Governments (COAG) Councils and Ministerial Forums completed in October 2020.
3 The title of ‘Chief Executive’ is used throughout the audit report as this is the terminology currently used by the NBA for the position of the General Manager established by the NBA Act.
4 Formerly the Commonwealth Serum Laboratories, CSL Limited was incorporated in 1991 and listed on the Australian Securities Exchange in 1994. CSL Limited is based in Melbourne, Victoria, and is the parent company of CSL Behring. CSL’s Australian-based fractionation activities are undertaken by CSL Behring (Australia) Pty Ltd, which manufactures plasma products from its facility in Broadmeadows, Victoria.
5 National Blood Authority, Plasma and Recombinant Products [Internet], NBA, available from https://www.blood.gov.au/plasma-and-recombinant-products [accessed 20 January 2021].
6 The NBA’s largest contract is with Australian Red Cross Lifeblood, worth $8.8 billion.
7 National Blood Authority, Annual Report 2018–19, NBA, 2019, p.43; and National Blood Authority, Annual Report 2019–20, NBA, 2020, p.43.
8 Department of Finance, Commonwealth Procurement Rules [Internet], Finance, 2019, available from https://www.finance.gov.au/sites/default/files/2019-11/CPRs-20-April-2019_1.pdf [accessed 28 August 2020].
9 The NBA has contracts with national suppliers for the importation of selected plasma derived and recombinant blood products to augment domestic supply where these products are not produced in Australia or domestic production cannot meet demand. In 2019-20, NBA expenditure on plasma derived and recombinant blood products totalled $460.48 million, with expenditure under the NaFAA representing 59 per cent of the total, and 41 per cent of total expenditure was on the importation of selected plasma derived and recombinant blood products to augment domestic supply.
10 National Blood Authority, Supply Planning and Management [Internet], NBA, available from https://www.blood.gov.au/supply-planning [accessed January 2021].
11 The three products were: Tetanus Immunoglobulin 2VI 250IU; Tetanus Immunoglobulin 2VI 4000IU; and Zoster Immunoglobulin 2VI - 200IU.
12 Auditor-General Report No.49 2018–19 Management of Commonwealth National Parks, p.26; Auditor-General Report No.20 2018–19 2017–18 Major Projects Report, p. 30; Auditor-General Report No.33 2017–18 Implementation of the Annual Performance Statements Requirements 2016–17, p. 64; Auditor-General Report No.17 2018–19 Implementation of the Annual Performance Statements Requirements 2017–18, p. 67.
13 National Blood Agreement, NBA, 2003, Paragraph 1(a), p.2.
14 Australian Government, Portfolio Budget Statements 2020–21: Budget Related Paper No.1.7 Health Portfolio, Commonwealth of Australia, Canberra, 2020, p.308.
15 National Blood Authority, Contracts with Suppliers [Internet], NBA, available from https://www.blood.gov.au/contracts-suppliers [accessed January 2021].
16 ibid.
17 Department of Finance (Finance), Commonwealth Procurement Rules, March 2017, available from https://www.legislation.gov.au/Details/F2017L00136 [accessed 28 August 2020].
A limited tender process involves a relevant entity approaching one or more potential suppliers to make submissions, when the process does not meet the rules for open tender. For procurements at or above the relevant procurement threshold, limited tender can only be conducted in accordance with paragraph 9.7 of the March 2017 CPRs, or when a procurement is exempt as detailed in Appendix A of the CPRs.
18 The March 2017 revisions to the CPRs made changes to Division 2, meaning they only applied to covered procurements, and not to procurements that are exempt from Division 2 of the CPRs. The March 2017 version of the CPRs applied during the majority of the procurement period and for this reason is the version referred to in the report.
19 Finance, Commonwealth Procurement Rules, March 2017, paragraph 2.2.
Relevant entities are non-corporate Commonwealth entities and prescribed corporate Commonwealth entities listed in section 30 of the Public Governance, Performance and Accountability Rule 2014.
20 ibid., paragraph 4.4.
21 Auditor-General Report No.48 2014–15 Limited Tender Procurement, June 2015, page 13, paragraph 2.
22 ‘The procurement of blood plasma or plasma fractionation products’ was listed as exemption 12 in Appendix A of the CPRs dated March 2017. The CPRs were updated in December 2020 and this exemption is listed as exemption 11 in Appendix A.
23 Finance, Commonwealth Procurement Rules, March 2017, paragraph 9.7.
For non-corporate Commonwealth entities, other than for procurements of construction services, the procurement threshold is $80,000.
24 ibid., Foreword, and paragraph 2.3.
25 ibid., paragraph 2.12.
26 The title of ‘Deputy Chief Executive’ is used throughout the audit report as this is the terminology currently used by the NBA for the position of the Deputy General Manager.
27 Finance, Commonwealth Procurement Rules, March 2017, paragraph 4.4.
28 KordaMentha specialise in forensic accounting, restructuring, corporate consulting and corporate finance.
29 Finance, Commonwealth Procurement Rules, March 2017, paragraph 6.5.
30 Australian Public Service Commission, Declaration of interests [Internet], APSC, available from https://www.apsc.gov.au/working-aps/integrity/declaration-interests [accessed March 2021].
31 Australian National Audit Office, Audit Insights: Management of Conflicts of Interest in Procurement Activity and Grants Programs [Internet], ANAO, available from https://www.anao.gov.au/work/audit-insights/management-of-conflicts-of-interest-in-procurement-activity-and-grants-programs [accessed January 2021].
32 The Chief Executive commenced in the role on 4 October 2016.
33 Auditor-General Report No.4 2020–21, Establishment and Use of ICT Related Procurement Panels and Arrangements, p.31, footnote 51.
34 Australian National Audit Office, Audit Insights: Management of Conflicts of Interest in Procurement Activity and Grants Programs [Internet], ANAO, available from https://www.anao.gov.au/work/audit-insights/management-of-conflicts-of-interest-in-procurement-activity-and-grants-programs [accessed January 2021].
35 Finance, Commonwealth Procurement Rules, March 2017, paragraphs 7.2, 7.3, 7.7, 716 and 7.17.
36 ibid., paragraph 8.2.
37 The paper outlined that CSL had undergone a significant global restructure that had diminished Australian based management and resulted in the Australian fractionation contract representing a smaller part of their business.
38 Auditor-General Audit Report No.8 2011–12, The National Blood Authority’s Management of the National Blood Supply, paragraph 3.42.
39 Auditor-General Report No.48 2014–15, Limited Tender Procurement, p.14, paragraph 4 and pp.45–46, paragraph 2.32.
40 Dependant on data available at the point of review, a collection of ten countries was used to benchmark including United States of America, Canada, United Kingdom, France, Spain, Italy, Germany, Sweden, Switzerland, and Japan.
41 A mean reverting exchange rate (or long run average) assumes that the exchange rate will revert to its average value in a linear fashion over the course of a forward estimates period. CSL considered that using a 12 month historical average would not give a robust indication of whether CAFA prices remained competitive due to volatility in the exchange rate. CSL referenced a 2009 Government of Western Australia paper to support the use of the mean reverting exchange rate methodology (available: https://www.wa.gov.au/sites/default/files/2020-01/economic-research-papers-exchange-rate-forecasting-review.pdf).
42 A monthly block fee is charged for the production and supply of immunoglobulin products.
43 CSL considered that the process migration project would bring Australia’s fractionation facility in line with CSL’s international manufacturing facilities, providing greater yield, purity improvements, potential for new plasma products, and increased efficiencies passed on to the NBA through savings on the previous contract. Process migration alters the base product of fractionated plasma, prior to this being further manufactured into a range of plasma products.
44 The Factor IX product supplied under the NaFAA, MonoFIX-VF, was to be replaced with imported Mononine.
45 In comparing the first and second proposal, KordaMentha projected the prices and conditions from the first proposal forward one year, to cover the nine year period in CSL’s second proposal.
46 CSL delivers relevant blood products directly to the homes of patients who can appropriately self-administer treatment at home within a defined set of eligibility criteria.
47 Additional payments that the NBA may be required to pay include: payment following termination of the contract; payments for alternative products supplied by CSL; and a plasma volume payment where delivered annual starting plasma is below the minimum annual starting plasma volume as a result of a decision by Australian, state and territory governments.
48 The NaFAA requires that CSL establish and hold the National CSL Reserve as a separately identified and managed inventory of products. The National CSL Reserve is dispersed across multiple states and territories.
49 The activity schedule is an internal work plan for the NBA Commercial Blood Products Team which sets out planned activities for the coming year.
50 Manufacturing of process migrated products was originally planned to commence in September 2021.
51 Auditor-General Report No.8 2011–12 The National Blood Authority’s Management of the National Blood Supply, pp.114–115.
52 This includes a quality report, subcontractors report, list of insurances held, business activities and horizon scanning report, and product research and development report.