Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Diagnostic Imaging Reforms

Please direct enquiries relating to reports through our contact page.
The audit objective was to assess the effectiveness of Health's implementation of the Diagnostic Imaging Review Reform Package, some three years into the five year reform period.
Summary
Introduction
1. Diagnostic imaging supports the diagnosis of a wide range of medical conditions, from musculoskeletal injuries to cancer. It requires specialised machinery such as ultrasound, X-ray, computed tomography (CT), magnetic resonance imaging (MRI) and gamma cameras, and the professional input of a radiologist or other medical specialist to interpret the generated images. Some 15 per cent of all Medicare1 outlays in 2014–15, approximately $3.1 billion, are related to diagnostic imaging services.
2. Expenses for Medicare benefits, including diagnostic imaging services, continue to increase in real terms driven by higher demand for increasingly expensive health services and a growing and ageing population. Medicare outlays on diagnostic imaging services have grown at an annual average rate of nine per cent between 1984–85 and 2013–14, increasing from $225 million to $2.9 billion during this time.2 Against this background, successive governments have sought to maintain the sustainability of Medicare in the face of rising costs and demand for medical services.3
3. The Diagnostic Imaging Review Reform Package (the reform package), a 2011–12 Budget measure, was introduced to help improve the overall sustainability of Medicare by reforming key aspects of government-funded diagnostic imaging following two detailed funding reviews. The five year reform package, to be implemented between 1 July 2011 to 30 June 2016, focussed on achieving six objectives for diagnostic imaging services funded through the Medicare Benefits Schedule (MBS)4:
- patients’ access to affordable and convenient diagnostic imaging services be maintained;
- patients in rural and remote areas have continued access to quality diagnostic imaging services;
- requesting practitioners and imaging services communicate effectively to ensure that patients receive appropriate imaging;
- each diagnostic imaging service reflects best clinical practice, is performed by an appropriately qualified practitioner and is provided within a facility which meets all necessary accreditation standards, minimising exposure to unnecessary radiation;
- ongoing Government expenditure for diagnostic imaging services is sustainable; and
- the diagnostic imaging sector is sustainable.5
4. The key elements of the reform package are to:
- expand patient access to MRI through a number of measures, including an increase in the number of MBS-eligible MRI machines;
- improve the requesting practices of medical practitioners of diagnostic imaging services – known as ‘appropriate requesting’;
- address fee relativities and incentives by restructuring the Diagnostic Imaging Services Table (DIST)6 to align with current clinical practice and review MBS fees for diagnostic imaging services so that they are appropriate and do not provide unintended incentives; and
- enhance the Diagnostic Imaging Accreditation Scheme (DIAS)7 by introducing more stringent quality and safety standards.
5. The reform package included additional funding of $104.4 million over four years from 2011–12 to improve and expand the provision of diagnostic imaging services, including $94.5 million in Medicare funding for MRI expansion initiatives. Initiatives such as ‘appropriate requesting’ were intended to help offset the cost to Medicare of expanding MRI services, following studies showing that some 20 to 50 per cent of diagnostic imaging services are potentially redundant or unnecessary.8 Further, as the cost to Medicare of different types of diagnostic imaging services can vary significantly – an MRI scan is approximately seven times more expensive than an X-ray and 20 per cent more expensive than a CT scan9 – the requesting practices of health professionals can directly affect the cost-effectiveness of patient care.
6. The Department of Health (Health), which is responsible for health policy and program implementation, has primary responsibility within government for implementing the reform package, which also relies for its success on effective consultation with the diagnostic imaging service industry and medical profession, and the take-up of key measures by them.
Audit objective, criteria and scope
7. The audit objective was to assess the effectiveness of Health’s implementation of the Diagnostic Imaging Review Reform Package, some three years into the five year reform period.
8. To conclude on this objective, the ANAO examined the progress and impact of the reform initiatives to date and whether Health has:
- worked effectively with stakeholders, planned for implementation of the reform measures, and monitors and reports on the progress and achievement of the reform package;
- effectively progressed initiatives intended to improve the financial sustainability of Medicare related to diagnostic imaging, in particular improving ‘appropriate requesting’ of imaging services and addressing fee relativities and incentives;
- effectively implemented reforms to enable patient access to affordable and convenient MRI services; and
- enabled diagnostic imaging service quality and safety enhancements by introducing relevant regulations and guidance in a timely manner.
9. The ANAO interviewed Health staff involved in the implementation of the reform package and key stakeholders, considered submissions from stakeholders received via the ANAO’s citizen input portal, and reviewed key reform implementation materials and documents. The ANAO also examined a sample of approved applications for MBS-eligibility of MRI machines and analysed key MBS service and expenditure data related to the diagnostic imaging reform initiatives.
10. The audit did not examine: the administration of related grant programs; the Department of Human Services’ (DHS’) roles and responsibilities in relation to Medicare payment processing and compliance, Health’s conduct of MBS reviews; or the Medical Services Advisory Committee (MSAC) application process for listing medical services on the MBS.
11. Relevant to this current audit, the ANAO undertook a performance audit of MRI policy development and implementation in 1999–2000.10 The audit examined the effectiveness and probity of the processes involved in the development and announcement of a 1998 Budget measure regarding MRI expansion, including negotiations with the diagnostic imaging profession, and the registration of eligible providers and equipment for receipt of Medicare payments. That audit found that the anticipated level of control over growth in diagnostic imaging outlays had not been achieved and that the then Department of Health and Aged Care’s management of the probity arrangements surrounding negotiations for the MRI measure was not adequate for the circumstances.11 The current audit has examined whether Health had regard to the lessons learned from the previous MRI expansion process and the ANAO audit report thereon, in the course of further extending access to Medicare for MRI services as part of the current reform package.
Overall conclusion
12. Diagnostic imaging, which includes the use of ultrasound, X-rays, computed tomography (CT) and magnetic resonance imaging (MRI), is used to support the diagnosis of a wide range of medical conditions. The Diagnostic Imaging Review Reform Package (the reform package), a 2011–12 Budget measure to be implemented over five years from 1 July 2011 to 30 June 2016, was intended to achieve multiple objectives, relating to accessibility, quality and financial sustainability, through the implementation of a balanced set of initiatives. Key initiatives included: improved patient access by increasing the number of MBS-eligible MRI machines12; improved ‘appropriate requesting’ by health professionals, to promote the more efficient and effective use of diagnostic imaging services; and regulatory changes to enhance quality and safety for patients. The reform package was also intended to contribute to the financial sustainability of Medicare13, with initiatives such as ‘appropriate requesting’ expected to help offset the cost of MRI expansion.14 The Department of Health (Health) has primary responsibility within government for implementing the reform package, which also relies for its success on effective consultation with the diagnostic imaging service industry and medical profession, and their adoption of key measures.
13. Some three years after the Budget measure was announced, Health’s overall implementation of the diagnostic imaging reform package has had mixed results. There has been an overall improvement in patient access to MRI services, including in regional areas; however, almost three times more MRI machines have been granted MBS-eligibility than originally estimated15, and the cost to Medicare of expanded MRI access is likely to be more than double the original Budget estimate of $94.5 million.16 While the introduction of complementary initiatives forming part of the reform package, such as ‘appropriate requesting’, was intended to help offset the cost of MRI expansion, those initiatives have not been adequately planned17 or substantively implemented and the anticipated benefits have not been realised. As a consequence, the cost impact of MRI expansion has been significantly greater than advised to government, with long-term implications for the Commonwealth Budget.18 In this context, the development of cost estimates for the MRI expansion initiative and the department’s implementation planning for the reform package as a whole, have not been fully effective.
14. In implementing the diagnostic imaging reform package, the expansion of MRI services has been the department’s primary focus to date. Implementation dates were established for the expansion process, providing an impetus which was missing for other elements of the reform package. As discussed, Health has significantly expanded the number of MRI machines eligible to receive MBS payments, improving overall patient access to MRI services.19 While improved patient access is expected to improve health outcomes20, it has been at a substantial cost to the Australian Government, with MRI expenditure growing 30 per cent between 2012–13 and 2013–14. The decision to allow General Practitioners (GPs) to request MRI services as part of the reform package21 has been the biggest driver of growth in MRI expenditure, and increased costs have not been offset by efficiencies expected from the take-up of ‘appropriate requesting’ practices by GPs; a behavioural reform initiative which has proven more difficult to implement than originally expected. Further unanticipated expenditure resulted from almost three times more MRI machines receiving MBS-eligibility under the reform package than originally estimated22, with ongoing budget implications.23
15. Health advised the ANAO that its ability to consult with industry – to develop an accurate estimate of the number of MRI machines likely to achieve MBS-eligibility – had been constrained by the need to avoid disclosing government policy intentions before the 2011–12 Budget. Health relied instead on available information and DHS data on the number of registered MRI machines. While this approach reflected the experience of an earlier MRI expansion process24, the limitations of the estimate became evident soon after the Budget measure was announced. At that time Health received numerous requests from providers seeking information on the eligibility of machines not identified by the department. Health subsequently recommended that the then Minister for Health (the Minister) broaden the eligibility criteria for approving MBS-eligibility25, in the belief that overall demand for MRI services and MBS expenditure would not be affected by making additional machines MBS-eligible. The department’s November 2011 advice contrasted with advice provided to the Minister in February 2011, which had noted that limiting the number of MBS-eligible machines had been an approach adopted to contain growth in Medicare expenditure.
16. Health also undertook a competitive invitation-to-apply process to identify and provide MBS-eligibility to 12 MRI machines in defined areas of need.26 The primary assessment process was not efficient, as it was re-run using an alternative scoring system developed late in the process to replace the original scoring system which was found to have shortcomings. Further, limited information was provided to inform the Minister’s decision-making on short-listed applicants – in particular, which 12 of the 21 machines on the short-list represented best value for money – and the department did not advise the Minister of the legal requirements applying to her in approving the commitment of public money. The department’s experience in administering the earlier MRI expansion process, which had demonstrated shortcomings, suggests that greater care should have been taken in these key aspects of the recent MRI expansion process.
17. The reform package included a commitment to review MBS fees for diagnostic imaging to help direct patients to the most clinically appropriate imaging service and avoid unintended incentives.27 The department has not commenced the review and restructure of MBS diagnostic imaging fees28, and this element of the reform package would benefit from the preparation of a targeted plan of action. Similarly, the department should prepare a plan identifying its proposed strategies and actions to improve the take-up of ‘appropriate requesting’ by health professionals. While Health has facilitated some well-targeted projects to improve ‘appropriate requesting’29, to date no enduring initiatives have been introduced to change, in a lasting way, the current practice of requesting diagnostic imaging services in Australia.
18. The ANAO has made two recommendations aimed at improving the effectiveness of Health’s implementation of remaining initiatives by: assessing progress made to date; developing an overall implementation plan to provide strategic direction and a basis for assessing the realisation of anticipated outcomes and benefits; and preparing targeted plans which identify proposed actions to progress key initiatives not yet implemented, including the review of MBS fees for diagnostic imaging and ‘appropriate requesting’ of diagnostic imaging services. To achieve full implementation of the reform package by 30 June 2016, as announced in the 2011–12 Budget context, will be challenging and will require a strong departmental focus and effective engagement with key stakeholders.
Key findings by chapter
Implementation Planning and Consultation (Chapter 2)
19. In its planning and implementation of the diagnostic imaging reform package, Health consulted widely and generally effectively with relevant stakeholders through the Diagnostic Imaging Review Consultation Committee and the Diagnostic Imaging Advisory Committee. The development of the reform package was also informed by two detailed reviews.30
20. Effective implementation planning requires a structured approach to thinking and communicating in the key areas of: planning; governance; engaging stakeholders; managing risk; monitoring review and evaluation; resource management; and management strategy.31 For long-term projects, strategic implementation plans are commonly prepared to provide structure to the implementation effort, prioritise activity and help maintain momentum. Contrary to advice provided to the Minister, Health did not develop an overall implementation plan, and did not provide its senior executive or Minister with advice on the prioritisation of implementation activity. While milestones were established for the implementation of MRI access initiatives, the department did not identify timeframes or milestones for the implementation of other key initiatives.
21. Some three years into the reform program, some key initiatives, including all those related to expanding MRI access, have been implemented by their due dates. However, significant elements of the reform package remain to be fully implemented, most notably initiatives relating to improving Medicare sustainability, such as ‘appropriate requesting’, which were intended to offset the cost of expanded access to MRI services. Health advised that a key reason for the limited progress on this issue was its complexity and the number of different stakeholders involved; which had made it difficult to identify and implement a solution which is effective and acceptable to all stakeholders.32
22. Following the MRI expansion, there has been a loss of momentum and implementation of the remaining measures is flagging. In these circumstances, the department should assess progress made to date in its implementation of the reform package to inform the development of an overall implementation plan to provide strategic direction going forward.
Enhancing Medicare Sustainability through Diagnostic Imaging Reforms (Chapter 3)
23. Evidence-based initiatives – including the development of clinical guidelines and educational resources to improve ‘appropriate requesting’ of diagnostic imaging services by health professionals – were a key element of the reform package. In particular, revised clinical practices were expected to help reduce the number of unnecessary, harmful or wasteful diagnostic imaging services requested by health professionals, and help offset the cost of improved patient access to diagnostic imaging through expanded access to MRI services.
24. Health has funded and undertaken some well-targeted research projects and trials to improve ‘appropriate requesting’, including funding for the development of guidelines for GP-requesting of MRI services and decision support tools. To date, however, no enduring initiative or group of initiatives has been introduced to change, in a lasting way, the current practice of requesting diagnostic imaging services in Australia. Health advised the ANAO that ‘appropriate requesting’– an initiative requiring take-up and behavioural change by health professionals such as GPs – has proven more difficult to implement than originally expected and may take some years to realise, notwithstanding the adoption of a multi-pronged approach. Given the importance of achieving better results here, the department should prepare a targeted plan identifying its proposed strategies and actions to improve the take-up of ‘appropriate requesting’ by health professionals.
25. The reform package included a commitment to review MBS fees for diagnostic imaging; acknowledging that some fees may not accurately reflect the cost of delivering imaging services. The review was also intended to avoid unintended incentives arising and help ensure that patients are directed to the most clinically appropriate imaging service.33 However, the department has not yet commenced the review and restructure of MBS diagnostic imaging fees, and this element of the reform package would also benefit from the preparation of a targeted plan of action identifying proposed strategies and actions to be undertaken.
Enabling Access to Affordable and Convenient MRI Services (Chapter 4)
26. To date, the MRI components of the reform package have been the department’s primary focus. Key initiatives have included: expanding the number of MRI machines with full or partial MBS-eligibility; standardising the conditions of those MRI machines that were already eligible; allowing GPs to request particular MRI items on the MBS; and providing additional incentives for diagnostic imaging providers to bulk bill for MRI services.
27. Health has implemented each of the MRI expansion initiatives in line with set dates. The total number of MBS-eligible MRI services was 829 000 in 2013–14, an increase of approximately 238 000 services (or 40 per cent) since 2011–12. In addition, the number of MRI machines has grown from amongst the lowest per capita in the OECD to above the OECD average. There has also been significant growth in the provision of services outside capital cities, with the number of MRI machines per capita more than doubling over a three year period. These initiatives have improved overall patient access to convenient MRI services. Further, MRI was made more affordable for patients overall, with the number of bulk billed MRI services almost doubling since 2009–10.34
28. The improvement in access to affordable and convenient MRI services has been achieved at a substantial cost to the Commonwealth Budget, with expenditure between 2011–12 and 2014–15 on these initiatives around twice the amount provided for in Budget estimates. Allowing GPs to request particular MBS-funded MRI services has been the biggest driver of growth in MRI services and expenditure.35 There is little doubt that an additional factor, acknowledged by Health as contributing to the unforeseen increase in expenditure, has been the significant growth in the number of MRI machines; with almost three times more machines approved for MBS-eligibility than originally estimated.
29. Some 125 MRI machines were MBS-eligible before the 2011–12 reform package was announced. The then Government’s decisions on the package were based on a Health estimate that this number would increase by 83 machines, bringing the total to 208 machines by 2014–15. In the event, 349 MRI machines are expected to become fully or partially MBS-eligible by January 2015. The department advised that its ability to consult with industry and develop an accurate estimate had been constrained by the need to avoid disclosing government policy intentions before the 2011–12 Budget36, and it relied instead on available information and DHS data on the number of registered MRI machines.
30. The limitations of the estimate became evident following the Minister’s announcement in the 2011–12 Budget context that 65 MRI machines would gain MBS-eligibility. Health subsequently received numerous requests from providers seeking information on the eligibility of other machines, and advised the Minister that an extra 139 machines would potentially qualify for full or partial MBS-eligibility, in addition to the 65 already announced. In November 2011, Health also recommended that the Minister broaden the eligibility criteria for approving MBS-eligibility37, in the belief that overall demand for MRI services and MBS expenditure would not be affected by making additional machines MBS-eligible. On the basis of this advice, in November 2011 the Minister approved the inclusion of newly identified MRI machines, noting that:
I am very surprised that there can be little/no cost change for such a large extra number of machines. I have approved, based on the assurance that DOFD agree to this cost. I will not approve a future increase if these calculations are wrong.38
31. The department’s November 2011 advice to the Minister contrasted with advice provided to the Minister in February 2011, which had noted that limiting the number of MBS-eligible machines had been an approach adopted to contain growth in Medicare expenditure. Health advised the ANAO during the course of the audit that it considers GP-requestors39 are the key driver for MRI services, but also acknowledged that the availability of additional MRI machines is a factor.
32. As discussed, Health advised the ANAO of the need to avoid signalling government Budget intentions in its development of estimates of MRI machines likely to achieve MBS-eligibility. While this approach reflected the experience of an MRI expansion process conducted in 1998, other aspects of the most recent MRI expansion did not have full regard to an ANAO performance audit of the earlier process40, which highlighted the importance of effective risk management, review of documentation and cross-checking of applications.41 At the planning stage for the recent MRI expansion initiative, a key risk was the development of robust budget estimates; and at the implementation stage, Health’s assessment plan did not identify risks to be managed in the assessment of applications, provide detailed guidance to help assessors determine whether applicants met eligibility criteria, or require cross-checking with other stakeholders on the accuracy of information supplied by applicants.42 The ANAO’s review of a sample of applications indicated that half43 of the planned machines that were approved were not supported by documentation, other than a statutory declaration, demonstrating that applicants had made a financial investment in a planned MRI machine.44
33. In addition, Health undertook a competitive, invitation-to-apply (ITA) process for MBS-eligibility to identify 12 applicants seeking to operate MRI machines in defined areas of need.45 The primary assessment process was not efficient, as it was re-run using an alternative scoring system developed late in the process to replace the original scoring system, which was found to have shortcomings. The ANAO’s review indicated that there was likely to have been little material difference in outcomes from the ITA process arising from the re-run of the primary assessment phase. However, the ANAO’s review of the secondary assessment phase indicated that limited information was provided by Health to the Minister on the 21 short-listed applicants to assist the Minister in selecting 12 machines on value for money grounds. In addition, the basis for the Minister’s selection was not documented. These factors limited the transparency of the decision-making process. Further, the department did not advise the Minister of the legal requirements applying to her in approving commitments of public money. The department’s experience in administering the 1998 MRI expansion process, which had demonstrated shortcomings, suggests that greater care should have been taken in these key aspects of the recent MRI expansion process.
Reforms to Enhance Quality and Safety in Diagnostic Imaging (Chapter 5)
34. To date, parts of two reform initiatives relating to improving quality and safety in diagnostic imaging have been implemented. Amendments to the diagnostic imaging regulations have strengthened minimum qualification requirements for diagnostic radiology46 and guidance has been developed relating to safe dosage levels for CT scans, including for children. However, the department has not established ongoing monitoring and evaluation activities to enable it to demonstrate whether these initiatives have been effective in reducing radiation and other risks.
35. Health has also commenced work on a number of other initiatives, including a review of supervisory requirements for diagnostic imaging, whose successful implementation will depend on the outcomes of stakeholder consultation and Ministerial consideration.47 A key initiative in this category is the implementation of enhancements to the DIAS accreditation approach, which Health has acknowledged was ‘in its infancy and cannot provide robust quality assurance’. At present, the accreditation process does not include risk-based on-site inspections or targeted phone interviews by accreditors to verify whether documented policies and procedures are being implemented by diagnostic imaging practices; and in consequence the current implementation of the DIAS provides only marginally more assurance than a self-assessment process.48 The department could provide additional assurance – on the consistency of accreditation assessments by the three approved accreditors, and the quality of the accreditation process overall – by reviewing and testing a sample of assessments from each accreditor. There is also scope to introduce a quality assurance process focusing on whether accreditors’ decisions are adequately supported by sufficient and appropriate evidence and that evidence is being assessed in a consistent manner.
36. The appropriateness of the existing accreditation approach is currently being discussed by Health and its stakeholders through the DIAS Monitoring and Implementation Committee, and Health has advised the ANAO of its intention that the DIAS accreditation process be strengthened over time. In particular, Health is considering whether on-site visits and a shorter accreditation cycle or mid-term assessments against some standards, such as infection control, is appropriate for the DIAS.
Summary of entity response
37. The proposed audit report issued under section 19 of the Auditor-General Act 1997 was provided to Health. In addition, relevant extracts of the proposed report were provided to the Department of Finance and the former Minister for Health, the Hon Tanya Plibersek MP.
38. A summary of Health’s response to the proposed audit report is below, with the full responses provided by Health and the Department of Finance at Appendix 1.
The Department of Health accepts the two recommendations. As the timeframe for implementation of the full package was 2011–12 to 2015–16, the findings of the report will inform the implementation activities remaining under the package. It is acknowledged that, as recommended in the report, implementation plans for all individual elements of the package, as well as an overall implementation and evaluation plan, would be of value. Consideration of the current Government’s priorities will be part of this process.
Recommendations
|
Recommendation No. 1 Paragraph 2.30 |
The ANAO recommends that the Department of Health:
Department of Health’s response: Agreed. |
|
Recommendation No. 2 Paragraph 3.34 |
The ANAO recommends that the Department of Health develop, as part of its implementation planning, targeted plans which identify proposed strategies and actions to progress key initiatives not yet implemented, including ‘appropriate requesting’ of diagnostic imaging services and the review of MBS fees for diagnostic imaging. Department of Health’s response: Agreed. |
1. Introduction
This chapter provides an overview of the Diagnostic Imaging Review Reform Package and the role of the Department of Health in its development and implementation. It also provides an outline of the audit objective, criteria and approach.
Background
1.1 Diagnostic imaging supports the diagnosis of a wide range of medical conditions, from musculoskeletal injuries to cancer. It requires specialised equipment, such as ultrasound, X-ray, computed tomography (CT), magnetic resonance imaging (MRI) and gamma cameras, and the professional input of a radiologist or other medical specialist to interpret the generated images. Some 15 per cent of all Medicare49 outlays in 2014–15, approximately $3.1 billion, are related to diagnostic imaging services.
1.2 Expenses for Medicare benefits, including diagnostic imaging services, continue to increase in real terms driven by higher demand for increasingly expensive health services and a growing and ageing population. Medicare outlays on diagnostic imaging services have grown at an annual average rate of nine per cent between 1984–85 and 2013–14, increasing from $225 million to $2.9 billion during this time.50 Against this background, successive governments have sought to maintain the sustainability of Medicare in the face of rising costs and demand for medical services.51,52 Medicare Benefits Schedule (MBS)53 service volumes, expenditure and growth for diagnostic imaging from 2005–06 to 2013–14 are outlined in Appendix 2 (Table A.1).
1.3 The reform package, a 2011–12 Budget measure, was introduced to help improve the overall sustainability of Medicare by reforming key aspects of diagnostic imaging following two detailed reviews, discussed in the following paragraphs.
Review of funding for diagnostic imaging services
1.4 In 2008, a taskforce54 was established to manage expenditure growth and to respond to concerns about the diagnostic imaging industry’s sustainability and the expiry of a previous memorandum of understanding (MoU) between Health and the radiology sector.55 As part of the taskforce’s advice to Government, it indicated that another MoU would not achieve the structural reform in diagnostic imaging considered necessary, and on that basis the then Government commissioned a detailed funding review as part of the 2009–10 Budget.56
1.5 The review of government funding for diagnostic imaging services focused on whether the Government was paying the right amount of support for patients to access quality diagnostic imaging services, and considered whether there was a need for structural changes to the way these services were provided through Medicare.57
1.6 The terms of reference for the funding review also indicated that it would ‘establish fee relativities for the MBS items across and within different diagnostic imaging modalities’.58 The ANAO has not examined the work of the funding review.
1.7 The funding review recognised that there were complex, interrelated issues beyond the funding arrangements that needed to be addressed in order to maintain ongoing access to appropriate, quality services for patients and to support the long term sustainability of the diagnostic imaging sector.59
The Diagnostic Imaging Review Reform Package
1.8 In response to the funding review findings, the then Government announced a five year Diagnostic Imaging Review Reform Package (the reform package) in the 2011–12 Budget, to be implemented between 1 July 2011 to 30 June 2016. The reforms are focussed on achieving six objectives for diagnostic imaging services funded through the MBS:
- patients’ access to affordable and convenient diagnostic imaging services be maintained;
- patients in rural and remote areas have continued access to quality diagnostic imaging services;
- requesting practitioners and imaging services communicate effectively to ensure that patients receive appropriate imaging;
- each diagnostic imaging service reflects best clinical practice, is performed by an appropriately qualified practitioner and is provided within a facility which meets all necessary accreditation standards, minimising exposure to unnecessary radiation;
- ongoing Government expenditure for diagnostic imaging services is sustainable; and
- the diagnostic imaging sector is sustainable.60
Reform initiatives
1.9 The key elements of the reform package61 are to:
- expand patient access to MRI through a number of measures, including an increase in the number of MBS-eligible MRI machines;
- improve the requesting practices of medical practitioners of diagnostic imaging services – known as ‘appropriate requesting’;
- address fee relativities and incentives by restructuring the Diagnostic Imaging Services Table (DIST)62 to align with current clinical practice and review MBS fees for diagnostic imaging services so that they are appropriate and do not provide unintended incentives; and
- enhance the Diagnostic Imaging Accreditation Scheme (DIAS)63 by introducing more stringent quality and safety standards.
1.10 Key reform implementation dates are outlined at Figure 1.1.
Figure 1.1: Diagnostic imaging reform timeline
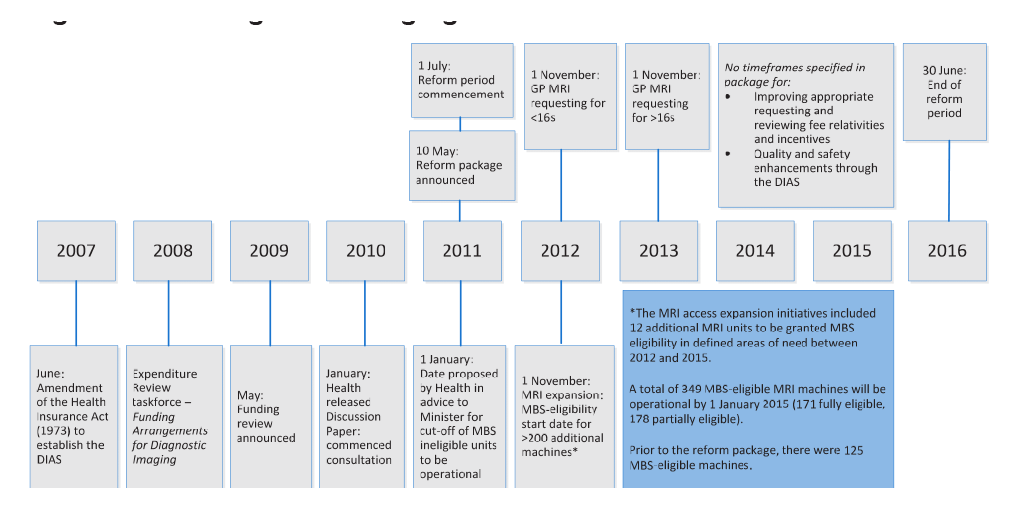
Source: ANAO analysis
Funding of diagnostic imaging reforms
1.11 The reform package included additional funding of $104.4 million over four years from 2011–1264 to improve and expand the provision of diagnostic imaging services, including $94.5 million in Medicare funding for MRI expansion initiatives. Initiatives such as ‘appropriate requesting’ were intended to help offset the cost to Medicare of expanding MRI services, following studies showing that some 20 to 50 per cent of services are potentially redundant or unnecessary.65 In addition, Health is funded several million dollars per annum to undertake diagnostic imaging-related policy and program implementation activities, including those related to the reform package as outlined at paragraph 1.14, to consult with industry and provide related policy and regulatory advice to Government.
1.12 Funding from two grant programs has also been used by Health to progress initiatives related to diagnostic imaging reform. The Diagnostic Imaging Quality Program (DIQP) commenced in 2012–1366 and funded more than $3 million worth of projects that have been identified by Health as directly relevant to implementation of reform initiatives.67 Prior to the announcement of the reform package, the National Prescribing Service (NPS) received $9.4 million over four years from 2009 to promote ‘appropriate requesting’ by medical practitioners of diagnostic imaging and pathology services.68
The Department of Health’s role
1.13 Health is responsible for health policy and program implementation, including Medicare–eligible services such as diagnostic imaging. The Department of Human Services (DHS) is responsible for administering Medicare payments on behalf of Health.69
1.14 In relation to the reform package, Health is responsible for ensuring the reforms are implemented, including working directly with the diagnostic imaging service industry and medical profession to:
- encourage more effective use of diagnostic imaging;
- amend regulations and guidelines for the standardisation of operating arrangements for MRI units;
- increase the bulk billing incentives for MRI;
- review the DIST with respect to fee relativities and incentives;
- assess MBS-eligibility for MRI units; and
- facilitate the implementation of the quality and safety enhancements through the DIAS.
ANAO audit coverage
1.15 In 1999–2000, the ANAO undertook an audit of MRI policy development and implementation.70 The audit examined the effectiveness and probity of the processes involved in the development and announcement of the 1998 Budget measure regarding MRI expansion, including negotiation with the diagnostic imaging profession, and the registration of eligible providers and equipment for receipt of Medicare payments. That audit found that the anticipated level of control over growth in diagnostic imaging outlays had not been achieved and that the then Department of Health and Aged Care’s management of the probity arrangements surrounding negotiations for the MRI measure was not adequate for the circumstances.71
1.16 As part of the ANAO’s wider coverage of Human Services’ management of risks to Medicare, in 2013–14 the ANAO undertook a performance audit of Medicare compliance audits, which examined risks related to health professionals’ MBS claiming at the post-payment stage.72
Audit objective, criteria and scope
1.17 The audit objective was to assess the effectiveness of Health’s implementation of the Diagnostic Imaging Review Reform Package, some three years into the five year reform period.
1.18 To conclude on this objective, the ANAO examined the progress and impact of the reform initiatives to date and whether Health has:
- worked effectively with stakeholders, planned for implementation of the reform measures, and monitors and reports on the progress and achievement of the objectives of the reform package;
- effectively progressed initiatives intended to improve the financial sustainability of Medicare, related to diagnostic imaging, in particular improving ‘appropriate requesting’ of imaging services and addressing fee relativities and incentives;
- effectively implemented reforms to enable patient access to affordable and convenient MRI services; and
- enabled diagnostic imaging service quality and safety enhancements by introducing relevant regulations and guidance in a timely manner.
1.19 The ANAO interviewed Health staff involved in the implementation of the reform package and key stakeholders, considered submissions from stakeholders received via the ANAO’s citizen input portal, and reviewed key reform implementation materials and documents. The ANAO also examined a sample of approved applications for MBS-eligibility of MRI machines and analysed key MBS service and expenditure data related to the diagnostic imaging reform initiatives.
1.20 The audit scope did not include an examination of: the administration of related grant programs (including DIQP); DHS’ roles and responsibilities in relation to Medicare payment processing and compliance, Health’s conduct of MBS reviews; or the Medical Services Advisory Committee (MSAC)73 application process for listing of medical services on the MBS.
1.21 The audit was conducted74 in accordance with ANAO audit standards at an approximate cost to the ANAO of $503 338.
Figure 1.2: ANAO visit to a diagnostic imaging facility

Source: ANAO fieldwork – audit team’s visit to the Canberra Imaging Group’s Deakin site, with CT machine shown, August 2014.
Report structure
1.22 The report structure is set out in Table 1.1.
Table 1.1: Report structure
|
Chapter |
Chapter Overview |
|
Chapter 2–Implementation Planning and Consultation |
This chapter examines Health’s implementation planning, and the effectiveness and probity of the department’s consultation with stakeholders during the reform review and subsequent implementation. |
|
Chapter 3–Enhancing Medicare Sustainability through Diagnostic Imaging Reforms |
This chapter examines the status and progress of Health’s implementation of the reform initiatives that aim to improve the sustainability of Australian Government expenditure for diagnostic imaging services. |
|
Chapter 4–Enabling Access to Affordable and Convenient MRI Services |
This chapter examines the extent to which Health has effectively implemented reforms to enable access to affordable and convenient MRI services. |
|
Chapter 5–Reforms to Enhance Quality and Safety in Diagnostic Imaging |
This chapter examines the status and progress of Health’s implementation of the reform initiatives that aim to enhance quality and safety in diagnostic imaging, in particular through the Diagnostic Imaging Accreditation Scheme (DIAS). |
2. Implementation Planning and Consultation
This chapter examines Health’s implementation planning and the effectiveness and probity of the department’s consultation with stakeholders during the reform review and subsequent implementation.
2.1 Effective stakeholder consultation and implementation planning are key enablers for achieving the diagnostic imaging reform objectives. Health advised the then Minister for Health (the Minister) that the reform package would be implemented in close consultation with the diagnostic imaging sector.
2.2 The ANAO examined the effectiveness of Health’s:
- stakeholder consultation process, including probity arrangements adopted as part of that process; and
- implementation planning process, including monitoring, evaluation and reporting, and advice to Government.
Stakeholder consultation
2.3 Appropriate levels of openness, transparency and integrity are required to provide stakeholders with confidence in public sector decision-making processes and actions. Openness and transparency involve meaningful consultation with stakeholders and the consistent communication of reliable information, having regard to charter responsibilities, privacy obligations and other legal and policy requirements.75
2.4 In determining the extent to which stakeholders were informed of the reform measures and their advice considered in implementation decisions, the audit examined Health’s arrangements and processes for consulting with stakeholders. The audit also considered probity arrangements adopted as part of the consultation process.76 This was important given the issues identified in relation to consultation with stakeholders over previous diagnostic imaging Budget measures, where probity was found not to be adequate in the circumstances.77
Consultation during the funding review
2.5 The reform package was developed in consultation with industry, the medical profession and consumer stakeholders, through five meetings of the Diagnostic Imaging Review Consultation Committee (DIRCC).78 The DIRCC was convened by Health in December 2009 following the 2009–10 Budget announcement of a detailed funding review of diagnostic imaging. Health considered that input from diagnostic imaging stakeholders was necessary to fully inform the review process of relevant issues for providers, requesters and consumers of diagnostic imaging.79 Following the release of a public discussion paper in January 2010, DIRCC meetings were held between March 2010 and May 2011. The last meeting was held on 13 May 2011 to inform stakeholders of the reform package announced in the 2011–12 Budget.80
2.6 To provide an evidence base for the diagnostic imaging funding review, Health drew on analysis of Medicare data, peer-reviewed scientific research and industry input and analysis. The funding review identified, with the support of stakeholders, that there were complex, interrelated issues beyond funding arrangements that needed to be addressed in order to maintain ongoing access to appropriate, quality services for patients and to support the long-term sustainability of the sector.
Consultation during implementation of the reform package
2.7 Following the finalisation of the funding review and announcement of the reform package, Health convened the Diagnostic Imaging Advisory Committee (DIAC)81 to ‘provide assistance to the Government on the implementation of the Diagnostic Imaging Reform Package and issues relating to diagnostic imaging and the diagnostic imaging sector’.82 The first DIAC meeting was held in December 2012 and a total of five meetings have been held to September 2014.
2.8 Matters that have been considered by DIAC include: examining radiation exposure to children from CT, ‘appropriate requesting’ and decision support tools, quality in diagnostic imaging, supervision of services, and capital sensitivity.83 Health has used the DIAC to inform its development of diagnostic imaging policy and as a forum for committee members to share perspectives and information on complex subject matter. Health also engages regularly with relevant stakeholders, through other forums84, on implementation of key elements of the package. While Health has established a range of consultative mechanisms, it advised the ANAO that progressing issues relating to diagnostic imaging has often been challenging and time consuming due to their complexity.
2.9 Stakeholders consulted during the course of the audit acknowledged Health’s efforts to effectively consult and engage on issues related to diagnostic imaging in both the funding review and reform implementation phases. However, a common concern expressed by several stakeholders involved in the reforms was that Health’s focus has been predominantly on the MRI expansion. Stakeholders indicated that, while the increased access to MRI services is an important improvement in access for patients, other equally important reform initiatives have not received appropriate attention. Health has acknowledged the substantial resources devoted to the MRI expansion, and noted that the process was more time consuming and resource intensive than anticipated. Health has further acknowledged that having specific timeframes for MRI-related implementation helped focus departmental attention on those reform elements.85
Probity arrangements
2.10 When consulting and negotiating with industry participants who may gain knowledge or insights that could benefit them materially, it is important that adequate probity arrangements are implemented. The audit considered whether arrangements to adequately preserve confidentiality and manage potential conflicts of interest were implemented in relation to the reforms.
2.11 The ANAO found that in establishing the DIRCC and DIAC, Health used departmental templates to obtain deeds of undertaking in relation to confidentiality of information and conflicts of interest from all committee members, and declarations of conflicts of interest were submitted by all members when appointed. Committee meeting minutes show that declarations of conflicts of interest were also sought verbally before the commencement of each committee meeting.86
2.12 The management of specific probity issues relating to the expansion of MRI services is discussed in Chapter 4 of this report.
Implementation planning, monitoring and reporting
Implementation planning
2.13 Effective implementation planning is a critical factor contributing to an entity’s ability to successfully prepare for the delivery of intended policy outcomes. Planning for successful implementation involves getting the implementation strategy, plan and design right before beginning time-critical and expensive implementation activities. Planning provides a ‘map’ of how an initiative will be implemented, addressing matters such as timeframe, dependencies with other policies or activities, phases of implementation, roles and responsibilities and resourcing.87 It is desirable that risk identification and treatment as well as arrangements for regular monitoring and review of key implementation deliverables be established as early as possible, preferably during the policy design and implementation planning phase.
Progress with implementation
2.14 Some three years into the reform period, Health has implemented a number of reform initiatives in line with the publicly announced timeframes, most notably those associated with improving MRI access. Other reform initiatives did not have publicly announced timeframes for implementation. To date, the department has:
- progressed some of the quality and safety initiatives outlined in the funding review, including strengthening qualification requirements for providers of some diagnostic imaging types;
- reviewed diagnostic imaging regulations and identified potential changes that are currently being assessed; and
- funded projects and trials that will inform the department’s approach to improving ‘appropriate requesting’ of diagnostic imaging services.
2.15 However, a number of important reform initiatives have not yet been implemented. These include: addressing fee relativities and incentives, and implementing effective arrangements to improve ‘appropriate requesting’ of diagnostic imaging services. Efforts to improve ‘appropriate requesting’ were intended to contribute to the financial sustainability of MBS expenditure and help offset the cost of the MRI expansion measures. Establishing appropriate fee relativities and incentives across different types of diagnostic imaging services was a task that the funding review was originally intended to achieve, however this was deferred to be implemented as part of the reform package. While this task was considered a priority by the then Government, no meaningful progress has been achieved some five years later.88 During this audit, Health advised the ANAO that the current government is also seeking to address the overall financial sustainability of Medicare through its 2014–15 Budget measure relating to patient co-payments, and that the delay to date in implementing that measure has impacted on the department’s ability to resolve issues in the diagnostic imaging sector in a way that the sector considers satisfactory.
Implementation planning
2.16 For each initiative that has been implemented to date, Health has developed and been guided by various annual team work plans, assessment plans and consultation with stakeholders. In some cases, risks and mitigation strategies have been identified. In addition, an initial implementation approach was provided to the Minister in December 2011. This identified that in addition to the MRI reforms, the remaining reform elements were to be delivered in a phased manner in close cooperation with the diagnostic imaging sector over the following five years. Health also advised the Minister that a more detailed implementation approach would be developed and that the Minister would be kept informed of its development.
2.17 To date, however, Health has not developed an overall implementation plan for the reform package as a whole that assesses risks, specifies timeframes, resources, proposed approaches and performance indicators for all reform initiatives. Further, in the absence of an overall implementation plan, the department has not provided advice to its executive or the Minister on the prioritisation of implementation activities over the five year reform period.
2.18 Health has not yet identified timeframes or milestones that will be achieved, either within the five year reform period or beyond, for implementation of the remaining key reform initiatives. Following the implementation of initiatives to expand access to MRI services, the ANAO has observed that there has been a loss of momentum for the reform process, and implementation of the remaining measures is flagging. The development of a five-year implementation plan covering each aspect of the reform package would have provided structure to the implementation effort, prioritised activity and helped maintain momentum for the package as a whole. Effective departmental planning would have been of particular benefit for reform initiatives that did not have publicly specified timeframes.
Monitoring, evaluation and reporting
2.19 Implementation, to be successful, requires ongoing and active management. Active management – informed by well-designed monitoring, review and evaluation activity – enables entities to ensure that adequate resources continue to be available and commensurate with the scope, risk and sensitivity of the implementation. It also enables entity leadership and ministers to assess implementation progress, identify and address problems and review the ongoing relevance and priority of the initiative.89
Monitoring and evaluation
2.20 In its 2011 advice to the Minister that led to the 2011–12 Budget decisions on the reform package, Health outlined the desired outcomes to be achieved within five years and the proposed monitoring and performance review arrangements for several proposed reform elements. In particular, although specific targets and performance indicators were not publicly identified for the reform package, Health advised the Minister that it would monitor the initiatives for improved patient access to MRI services and the development of clinical guidelines for general practitioners (GPs), on an ongoing basis through in-depth analysis of Medicare data and by working closely with stakeholders. In addition, Health advised the Minister that it would review arrangements for MRI access after three years from implementation to determine whether patient access and affordability problems had been addressed. More generally, in its 2013 Portfolio Budget Statement, Health indicated that it would:
Continue to monitor and evaluate the impact of the changes introduced on 1 November 2012, which require those performing the actual diagnostic imaging procedure to hold minimum qualifications for all MBS-funded X-ray, angiography and fluoroscopy (diagnostic radiology) services, addressing quality and safety concerns that arose from the funding review.90
2.21 Health has scheduled a review of the impact of MRI access reforms to be undertaken in 2014–15. However, this review has not yet commenced and ongoing monitoring of reform initiatives, as advised to the Minister, has not occurred. The intended monitoring and evaluation of the impact of the changes introduced on 1 November 2012, in relation to the qualifications of practitioners providing diagnostic radiology services, is in its early stages.91
2.22 In common with the department’s approach to overall implementation planning, there has not to date been a structured approach to monitoring and evaluating progress against intended objectives for the reform package as a whole.
2.23 The diagnostic imaging reform package was intended to achieve multiple objectives, relating to accessibility, quality and financial sustainability, through the implementation of a balanced set of initiatives. Given these objectives for the reform package, Health should assess progress made to date in its implementation of the reform package, to inform the preparation of an overall implementation plan providing strategic future direction.
Reporting
2.24 Maintaining accountability through clear reporting on performance and operations is a critical factor in building and retaining the trust of the government and community. Timely and transparent public reporting on expenditure, activities and outcomes allows parliamentarians and members of the public to scrutinise and to make informed judgements about the performance and contributions of public sector entities.92
2.25 As discussed, Health did not develop an implementation plan for the reform package as a whole. Similarly, the department did not implement a structured approach to reporting on progress and impact over the life of the initiative. Health advised the ANAO that the department does not undertake regular, structured risk or performance reporting to the department’s senior executive, including in relation to the diagnostic imaging reform package. Rather, reporting occurs as considered appropriate and relevant at a point in time on a particular issue.
2.26 Reporting to Health’s senior executive and successive Ministers has occurred when Health has required a decision to be made, in preparation for briefings with stakeholders, or in response to a direct ministerial request for information. To date, advice to the senior executive and Ministers has predominantly focussed on the activities associated with the initiatives to improve MRI access.
2.27 Public reporting in relation to some of the reform initiatives has occurred in Health’s annual report against KPIs in portfolio budget statements. Information has been provided on activities undertaken in relation to granting MBS-eligibility for MRI machines, the number of sets of symptoms for which GPs have MRI requesting rights, and strengthening minimum requirements for practitioners delivering diagnostic radiology services (discussed above). While these are key elements of the reform package, Health has not yet reported on the outcomes of the reform package as a whole. Further, there is a lack of specific targets and performance indicators for the various initiatives, to assess outcomes and benefits achieved by the package.
2.28 In its public annual reporting, Health has not provided quantitative data and in some cases there have been shortcomings in reporting in relation to individual reform initiatives, such as the impact of introducing GP-requested MRI items. Health reported in its 2013–14 annual report that since increasing access to MRI services for primary care patients, MBS data has shown ‘a steady rise in services’.93 The ANAO found, however, that following the introduction of GP-requesting rights for MRI scans for patients aged 16 years and over, the number of MRI services increased by 30 per cent; a sharp increase rather than the steady rise reported by the department. The graph at Figure 2.1 shows the impact of the MRI access initiatives, noting in particular the steep rise in total MRI services since GP-requesting rights for MRI services were introduced in November 2013 for patients over 16 years.94
Figure 2.1: MRI services and expenditure requested by specialists/ consultant physicians and total, 2009–10 to 2013–14

Source: ANAO analysis from Health and DHS data.
Note: Services and expenditure ‘less GP-requested’ refer to those requests by specialists and consultant physicians.
2.29 With the change of government in September 2013, and its policy focus on the sustainability of Medicare, the audit examined whether the new Government had been advised on the reform measures. Other than a minute to the Minister in November 2013, which briefly listed the elements of the reform package in the context of seeking a decision on the third round of MRI expansion applications, there has been no specific advice provided on the overall progress and impact of the reform package. The preparation of an overall implementation plan would provide a basis for assessing and reporting on the realisation of outcomes and benefits of the reform package.
Recommendation No.1
2.30 The ANAO recommends that the Department of Health:
- assess progress made to date in its implementation of the diagnostic imaging reform package; and
- develop an overall implementation plan to provide strategic direction and a basis for assessing the realisation of anticipated outcomes and benefits.
Department of Health’s response: Agreed.
Conclusion
2.31 In its planning and implementation of the diagnostic imaging reform package, Health consulted widely and generally effectively with relevant stakeholders through the DIRCC and the DIAC. The development of the reform package was also informed by two detailed reviews.95
2.32 Effective implementation planning requires a structured approach to thinking and communicating in the key areas of: planning; governance; engaging stakeholders; managing risk; monitoring review and evaluation; resource management; and management strategy.96 For long-term projects, strategic implementation plans are commonly prepared to provide structure to the implementation effort, prioritise activity and help maintain momentum. Contrary to advice provided to the Minister, Health did not develop an overall implementation plan, and did not provide97 its senior executive or Minister with advice on the prioritisation of implementation activity. While milestones were established for the implementation of MRI access initiatives, the department did not identify timeframes or milestones for the implementation of other key initiatives.
2.33 Some three years into the reform program, some key initiatives, including all those related to expanding MRI access, have been implemented by their due dates. However, significant elements of the reform package remain to be fully implemented, most notably initiatives relating to improving Medicare sustainability, such as ‘appropriate requesting, which were intended to offset the cost of expanded access to MRI services. Health advised that a key reason for the limited progress on this issue was its complexity and the number of different stakeholders involved; which had made it difficult to identify and implement a solution which is effective and acceptable to all stakeholders.98
2.34 Following the MRI expansion, there has been a loss of momentum and implementation of the remaining measures is flagging. In these circumstances, the department should assess progress made to date in its implementation of the reform package to inform the development of an overall implementation plan to provide strategic direction going forward.
3. Enhancing Medicare Sustainability through Diagnostic Imaging Reforms
This chapter examines the status and progress of Health’s implementation of the reform initiatives that aim to improve the sustainability of Australian Government expenditure for diagnostic imaging services.
3.1 Medicare expenditure on diagnostic imaging continues to grow at 9.3 per cent per annum.99 The reform package was designed to address the high rate of growth in diagnostic imaging services and the resultant impact on the Commonwealth Budget and the sustainability of Medicare.
3.2 The reform package included two key initiatives that were intended to directly address the rate of volume growth of diagnostic imaging services. They were:
- improving the requesting practices of medical practitioners of diagnostic imaging services – known as ‘appropriate requesting’; and
- reviewing the fees paid by the Government for diagnostic imaging services, including relativities and incentives and restructuring the DIST to align with current clinical practice.100
3.3 The ANAO examined implementation of the two initiatives, and their effect to date on the rate of growth of diagnostic imaging.
Improving ‘appropriate requesting’
3.4 Requesting appropriate imaging services means that patients are directed to the most clinically appropriate service and that images are of value in diagnosing patient conditions. In addition, it relates to there not being unnecessary, harmful or wasteful services undertaken during the course of caring for a patient. The reform package sought to address these elements, in particular as requesting relates to growth in diagnostic imaging expenditure and patient safety risks, particularly computed tomography (CT) scans for children.
The importance of ‘appropriate requesting’
3.5 International studies have shown that 20 per cent to 50 per cent of diagnostic imaging for a variety of conditions fails to provide information that improves patient diagnosis and treatment and may therefore be considered redundant or unnecessary.101
3.6 In Australia, radiologists deliver over 80 per cent of diagnostic imaging services at the request of other medical practitioners, such as general practitioners (GPs) or specialists.102 GPs request over 65 per cent of diagnostic imaging services, with the rate of imaging tests ordered having increased by 45 per cent over the decade to 2012.103, 104 Nuclear medicine specialists also provide diagnostic imaging services at the request of other medical practitioners.105 In addition, diagnostic imaging is undertaken by medical practitioners at the point-of-care106, without a request from another doctor.
3.7 As part of its reform package, the then Government recognised that there are some areas where diagnostic pathways could be better defined and that there are interacting factors that affect requesting behaviours. These factors include the level of understanding and awareness by medical practitioners of the risks, benefits and suitability of different diagnostic tools in different circumstances. In addition, patient expectations and their understanding of risks, benefits and costs107, as well as access to services and perceived medico-legal risk are relevant. Furthermore, Health and the sector have recognised that financial or other incentives for providers of diagnostic imaging services may lead to preferential requesting or provision of certain types of services over others.108
3.8 In advice to government in the lead-up to the 2009–10 Budget, the interdepartmental expenditure review taskforce109 included an expert consultant’s report that examined funding issues and concluded that any strategy to address long-term expenditure growth on diagnostic services would be incomplete if it did not include measures to address growth in demand driven by requesting practitioners. The report also concluded that there is considerable scope to make better use of electronic decision support tools.
Reform initiatives to improve ‘appropriate requesting’
3.9 The reform package contained the following initiatives to improve ‘appropriate requesting’ of diagnostic imaging:
- [Health] will work with the Royal Australian and New Zealand College of Radiologists, the Royal Australian College of General Practitioners and other stakeholder bodies to develop clinical guidelines and educational resources, such as professional development modules to increase GPs’ knowledge of diagnostic imaging, including the risks associated with radiation;
- the development of guidelines and educational resources will support the extension of requesting rights to GPs for a limited range of clinically appropriate magnetic resonance imaging (MRI) uses. These guidelines will also assist GPs when determining the most appropriate imaging modality across CT and MRI; and
- where interventions are effective in reducing the rate of volume growth for imaging, funding may be redirected to allow schedule fee increases for appropriate imaging items.110
3.10 It was recognised at the time that the success of initiatives to improve ‘appropriate requesting’ would rely on the involvement of the diagnostic imaging service industry and medical profession.
Implementation to date
3.11 The reform package did not provide specific timeframes for completion of ‘appropriate requesting’. To date, Health has undertaken a number of activities and funded a number of research projects to support ‘appropriate requesting’ of diagnostic imaging services and to inform future policy. However, Health advised that there has been limited progress in implementing the initiative because of its complexity and the number of different stakeholders, noting that:
Although the stakeholders agreed in principle that there could be more ‘appropriate requesting’, they have diverging perspectives and proposals. This has made it difficult for the department to identify and implement a solution which is effective and acceptable to all stakeholders.111
3.12 Activities undertaken include funding, through the Diagnostic Imaging Quality Programme (DIQP), seven projects related to improved requesting.112 These projects, outlined in Table 3.1, address matters raised in the funding review including educational modules, electronic decision support113, informed consumer consent and examining image ordering by GPs.
Table 3.1: Projects funded under the Diagnostic Imaging Quality Program (DIQP)
|
Organisation |
Project Title |
Funding Provided |
Project Outputs or completion date |
|
RadLogix |
Electronic Decision Support in a GP Practice |
$329 384 |
Completed July 2013 (no web link available) |
|
Australian Society for Ultrasound in Medicine (ASUM) |
Point of Care Ultrasound Qualifications |
$166 969 |
|
|
Royal Australian and New Zealand College of Radiologists (RANZCR) |
Inside Radiology – Update 2013 |
$294 589 |
|
|
RANZCR |
CT Dose Optimisation |
$196 255 |
http://www.ranzcr.edu.au/quality-a-safety/program/insideradiology |
|
RANZCR |
Educational Modules |
$1 023 587 |
Completion due May 2015 |
|
Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) |
Nuchal Translucency Reflections on Effectiveness |
$223 300 |
Completion due late 2014 |
|
QLD Health |
CT Dose optimisation |
$165 000 |
http://www.health.qld.gov.au/hsq/biomedical-tech/ct-optimisation.asp |
|
University of Sydney |
Evaluation of the linear feature extraction algorithm |
$297 410 |
Completion due mid-2016 |
|
University of Sydney |
BEACH – Image Ordering by Australian GPs |
$156 417 |
http://ses.library.usyd.edu.au/BItstream/2123/10610/3/9781743324141_ONLINE.pdf |
|
Consumer Health Forum (CHF) |
Diagnostic Imaging and Informed Consumer Consent |
$303 407 |
|
|
WA Health |
Diagnostic Imaging Pathways Consent |
$248 848 |
Completion due late 2014 |
|
Australasian Association of Nuclear Medicine Specialists (AANMS) |
Provision of enhanced information to referrers and patients |
$48 400 |
http://www.anzapnm.org.au/index.php?option=com_content&view=article&id=40&Itemid=49 |
|
Total |
|
$3 453 566 |
|
Source: ANAO analysis of information provided by Health
3.13 In addition, guidelines developed by the Royal Australian College of General Practitioners to support GP-requesting rights for MRI have been funded by the department in the amount of $1.2 million. These guidelines were made available to GPs to support the introduction of the new MBS-supported referral items in November 2013.
3.14 As a result of the taskforce’s advice to government leading up to the 2009–10 Budget114, the National Prescribing Service (NPS)115 was funded $9.4 million over four years (2009–2013) to promote high quality and appropriate requests for diagnostic imaging services and pathology tests.116 The outcomes of this initiative have provided Health with information on the impact of interventions to improve ‘appropriate requesting’ for diagnostic services. The NPS project is estimated to have saved $23 million from initiatives undertaken targeting one diagnostic imaging and one pathology MBS item.117 The NPS advised the ANAO that based on its experience, education and awareness-raising campaigns directed at health professionals need to be repeated periodically, approximately every 18 months to two years, otherwise requesting habits may revert to previous levels. There would be benefit in Health considering past experience, in the context of designing and delivering decision support tools, professional educational modules and related actions to improve ‘appropriate requesting’ as part of the reform package.
3.15 In addition to the DIQP-funded projects and other initiatives, Health has commenced planning a review of lower-back imaging. This is in response to growth in services and research that early imaging in the absence of certain risk factors and clinical indications is generally not the most appropriate diagnostic and treatment pathway. Findings from this departmental MBS review will be referred to the Medical Services Advisory Committee (MSAC) for independent expert advice and a recommendation to the Minister.118 This review may result in changes in MBS item descriptors, changes to MBS fees, new MBS items, and education programs for medical and allied health professionals.
3.16 A related measure identified in the reform package was the review of restrictions limiting imaging substitution and the role of radiologists in appropriate imaging. The current regulations broadly permit a provider of diagnostic imaging services (usually a radiologist) to substitute a service for the one originally requested when it is considered more appropriate for the diagnosis of the patient’s condition and when the provider has consulted with the requesting practitioner, or taken steps to do so.119 The ANAO found that restrictions limiting imaging substitution have not yet been changed, notwithstanding the inclusion of this initiative in the reform package. Health and key stakeholders have advised the ANAO that the current situation works reasonably well, as there is scope for the provider to communicate suggested changes to the requester, and consequently this reform element is considered a lower priority than others not yet implemented.
3.17 Health has advised the ANAO that the MRI guidelines developed for GPs and initiatives undertaken to date are considered a first step and that:
Before making a decision on the best way to improve ‘appropriate requesting’, Health will consider the outcomes of all DIQP projects and related initiatives when remaining projects are completed in 2015. Health has no current intent to mandate the use of any tools and agreement with the diagnostic imaging sector will be sought, through the DIAC120 and possibly broader consultation, on the way forward. As an initial task, the Diagnostic Imaging section has included on its 2014–15 annual team workplan the preparation of an information paper to inform itself of how it will proceed with evaluating DIQP project outcomes.
3.18 Some three years into the reform process however, no enduring initiative or group of initiatives has been introduced to change, in a lasting way, the current practice of requesting diagnostic imaging services in Australia. The continued high growth rate in diagnostic imaging service expenditure over the last three years (discussed in paragraph 3.1) confirms this. The growth in MRI expenditure of 30 per cent between 2012–13 and 2013–14121, which arose particularly as a result of changes to GP-requesting rights, reinforces the benefits, identified in the context of developing the reform package, of improving ‘appropriate requesting’ of higher-value services as a means of managing the rate of expenditure growth.
3.19 Given the importance of achieving better results here, the department should develop, as part of its implementation planning, a targeted plan which identifies proposed strategies and actions to progress ‘appropriate requesting’ of diagnostic imaging services.
Addressing fee relativities and incentives
3.20 The second main reform element aimed at improving the sustainability of Medicare was a review of Medicare fees for diagnostic imaging services and a restructure of the regulations that support those payments.
3.21 The Diagnostic Imaging Services Table (DIST)122 specifies the services that are eligible for MBS rebates, the fees applicable to each item, and rules for interpretation of the table. There are approximately 870 service items currently on the DIST categorised by imaging modality.
The need to address fee relativities and incentives
3.22 Health and stakeholders advised the ANAO that for a number of reasons the MBS fees payable for many items on the DIST do not reflect the current cost of delivering the service. These reasons include: annual indexation of imaging schedule fees not being applied since November 1998123; adjustments made to fees under the capped-volume agreements with the radiology sector prior to 2008; and changes in technology and clinical practice.
3.23 While some services are known by Health and stakeholders to be under-funded in the MBS, there is also recognition that other types of imaging services are over-remunerated and therefore more profitable to imaging providers.124 The risk that remuneration arrangements may provide unintended incentives to invest in higher end technologies was identified in the funding review. An interdepartmental taskforce125 provided advice to the Government on this matter in the lead-up to the 2009–10 Budget, highlighting the potential for providers to invest in more profitable services (such as basic X–ray, CT and some types of ultrasound services with high revenue potential) rather than comprehensive diagnostic imaging practices, which may then result in the contraction of imaging services available to patients.
3.24 Government interest in reviewing MBS fees for diagnostic imaging preceded the reform package. The 2009 terms of reference for the review that led to the diagnostic imaging reform package focused primarily on the funding of diagnostic imaging, with the first task of the review being ‘to establish appropriate fee relativities for MBS items across and within diagnostic imaging modalities’.126 However, MBS fees were not reviewed or adjusted during that review and the task was instead deferred as part of the five-year reform package.
Reform initiatives to address fee relativities and incentives
3.25 The reform package contained the following initiatives to address fee relativities and incentives:
- The DIST would be restructured to align with current clinical practice. This initiative was intended to provide a more robust platform from which to better structure quality initiatives127 and incentives128 as well as to address fee relativities. Implementation would involve working closely with the sector to assess each item for its clinical appropriateness; and
- The MBS fees paid by the Government for diagnostic imaging services would be reviewed to examine the appropriateness of fee relativities across and within modalities. The review would be undertaken within the current funding envelope for imaging.
3.26 The reform package did not provide specific timeframes within the reform period for completion of the initiatives related to a review of fees and the DIST restructure. However, Health’s 2011–12 Portfolio Budget Statements (PBS) indicated that the structure of diagnostic imaging MBS items would be reviewed in 2011–12 and, when completed, fee relativities would be assessed to examine their appropriateness. The PBS also indicated that any changes to the structure of MBS items would be completed in a cost-neutral manner and outcomes from the MBS review phased in gradually from 1 November 2013, and completed by 1 November 2015.129
Implementation to date
3.27 A small number of changes have been made to the DIST over the first three years of the reform period, including adding new items for GP-requested MRI services and a small number of Budget initiatives.130 A number of related initiatives, discussed below, have also been commenced by Health. However, the comprehensive review and restructure envisaged under the reform package, and expressed in the earlier government announcement of the funding review made in the context of the 2009–10 Budget, has not yet occurred.
3.28 The funding review identified particular concerns about fee relativities for ultrasound, and as a consequence some preparatory work has commenced that will assist Health in reviewing this area of the DIST. The funding review identified that fees for different ultrasound items are inconsistent and have different patterns of bulk-billing depending on the specialty group involved. Fees for some ultrasound items (in particular, obstetric, gynaecological and urological) are recognised as having some of the lowest correlation with the cost of delivery for any imaging modality. Other ultrasound items are recognised as being relatively well-funded (in particular, cardiac). Health has investigated131 the extent of non-radiologist ultrasound work and commenced related activities including in relation to credentialing of ultrasound providers to assist in ensuring that the quality of service delivery is maintained (discussed further in Chapter 5).
3.29 Health has also communicated to stakeholders that under the reform package, there must be a reduction in the rate of volume growth for diagnostic imaging before MBS fees will be increased and that any fee adjustments must be cost-neutral and within ‘the existing funding envelope’. Health advised the ANAO that as a reduction in the rate of volume growth has not yet occurred, MBS fees for diagnostic imaging have not yet been reviewed. In effect, Health considers that this element of the reform package cannot be advanced unless progress is made in respect to other elements of the package. However, as noted in Chapter 2, the department has not developed an overall implementation plan for the reform package as a whole, and there was no stated threshold for the reduction in volume growth that was to be achieved for a review and restructure of the DIST to be undertaken.
3.30 Further, Health’s decision to not review fees on the basis that there have not yet been savings in the diagnostic imaging budget reflects a narrow interpretation of the reform measure, which was not limited to reviewing MBS fees for upward movement. The review of relativities and incentives is also a vehicle for addressing anomalies relating to the DIST, for example, different fees for ultrasound services involving similar time and complexity.132
3.31 There are imaging types that are recognised as being relatively well-funded under the MBS and would benefit from review. There are also item numbers that were advised to the ANAO by senior medical practitioners as no longer clinically relevant but under which claims are still made, and items where clinicians are aware that there are cheaper, safer and more clinically-effective options available than those currently listed on the DIST.133 The average MBS benefit paid for diagnostic imaging services was 20 per cent higher in 2013–14 than in 2005–06.134 MBS fees have not been indexed during this time, indicating that the increase has resulted from growth in the use of higher-end technologies and/or changes in clinical practice. Understanding such changes and their impact on government outlays is important for managing the DIST, as highlighted in 2009 by the Expenditure Review Taskforce which examined diagnostic imaging:
The operation of the MBS does, however, distort the market for health services. Under the MBS, the Commonwealth is a monopsony purchaser, fixing a floor price for services and assisting some patients to pay prices they might otherwise not be able to afford. However, the Commonwealth as purchaser lacks significant information on both the supply and demand sides. The MBS pays fixed rebates to all providers who can meet minimum requirements and there is little competition on price. There is therefore no effective market mechanism to feeding back to the purchaser any information about the costs of providing services. While the bulk billing rates and levels of patient copayments could provide some indication of the relationship between rebates and actual costs, they can be distorted by cross-subsidisation between patients, between items, and between services rebated through the MBS and services funded by other sources. Providers will selectively provide information to support claims for increases to rebates where they consider their costs are not covered, but have a strong incentive not to share any information that indicates rebates are too high… Better policy in this area will therefore require the gathering of information about what diagnostic services are needed for different patients and the true costs involved. It is unlikely that a market mechanism for this information could be introduced without sacrificing key elements of equity, so regulatory powers may need to be used particularly for gaining more information about costs.135
3.32 Apart from commencing planning for an MBS review on lower back imaging and facilitating the MSAC process136, Health has not been pro-actively monitoring and managing the DIST to identify areas where reductions in MBS fees may be possible or where more clinically appropriate and/or cost-effective treatments may be available. Health and key stakeholders have advised the ANAO that to undertake a complete review of all diagnostic imaging MBS items would be a resource-intensive, multi-year task and that, in the early years of the reform process to December 2013, resources have been focussed predominantly on the MRI expansion. Health further advised the ANAO that since the change of government in September 2013, preparation for the 2014–15 Budget and working through its implications, most notably the proposed $7 medical services co-payment that will also apply to diagnostic imaging (if passed), has been a priority for the department in relation to implementation of MBS-related initiatives.
3.33 While recognising the need to prioritise effort, particularly in a resource constrained environment, review of the DIST and fees payable under the MBS has been identified as a necessary reform since at least 2009, and was a key component of the 2011–12 Budget measure. The review was intended to avoid unintended incentives arising and, along with initiatives to improve ‘appropriate requesting’, help ensure that patients are directed to the most clinically appropriate imaging service. These initiatives were designed to help offset the costs of MRI expansion initiatives and contribute to overall Medicare sustainability. However, the department has not yet commenced the review and restructure of MBS diagnostic imaging fees, and this element of the reform package would also benefit from the preparation of a targeted plan of action identifying proposed strategies and actions to be undertaken.
Recommendation No.2
3.34 The ANAO recommends that the Department of Health develop, as part of its implementation planning, targeted plans which identify proposed strategies and actions to progress key initiatives not yet implemented, including ‘appropriate requesting’ of diagnostic imaging services and the review of MBS fees for diagnostic imaging.
Department of Health’s response: Agreed.
Conclusion
3.35 Evidence-based initiatives – including the development of clinical guidelines and educational resources to improve ‘appropriate requesting’ of diagnostic imaging services by health professionals – were a key element of the reform package. In particular, revised clinical practices were expected to help reduce the number of unnecessary, harmful or wasteful diagnostic imaging services requested by health professionals, and help offset the cost of improved patient access to diagnostic imaging through expanded access to MRI services.
3.36 Health has funded and undertaken some well-targeted research projects and trials to improve ‘appropriate requesting’, including funding for the development of guidelines for GP-requesting of MRI services and decision support tools. To date, however, no enduring initiative or group of initiatives has been introduced to change, in a lasting way, the current practice of requesting diagnostic imaging services in Australia. Health advised the ANAO that ‘appropriate requesting’ – an initiative requiring take-up and behavioural change by health professionals such as GPs – has proven more difficult to implement than originally expected and may take some years to realise, notwithstanding the adoption of a multi-pronged approach. Given the importance of achieving better results here, the department should prepare a targeted plan identifying its proposed strategies and actions to improve the take-up of ‘appropriate requesting’ by health professionals.
3.37 The reform package included a commitment to review MBS fees for diagnostic imaging; acknowledging that some fees may not accurately reflect the cost of delivering imaging services. The review was also intended to avoid unintended incentives arising and help ensure that patients are directed to the most clinically appropriate imaging service.137 However, the department has not yet commenced the review and restructure of MBS diagnostic imaging fees, and this element of the reform package would also benefit from the preparation of a targeted plan of action identifying proposed strategies and actions to be undertaken.
4. Enabling Access to Affordable and Convenient MRI Services
This chapter examines the extent to which Health has effectively implemented reforms to enable access to affordable and convenient MRI services.
4.1 An MRI scan is often favoured over other types of diagnostic imaging options due to the quality of the information it provides, and for its reduced safety risk as it does not expose the patient to ionising radiation. Unlike other forms of diagnostic imaging, for an MRI scan to be eligible for an MBS payment, it must be performed on an MBS-eligible machine and, until recently, could only be requested by a specialist or a consultant physician. In the lead up to the 2011–12 Budget, the then Government was advised by Health that Australia had significantly fewer MRI machines per million population (at 5.9 per million) compared with the OECD average (at 12.1 per million). The diagnostic imaging reform package announced as part of the 2011–12 Budget included a number of measures to improve patient access to MRI services.
Development of MRI access reforms
4.2 In January 2010, Health’s publicly released discussion paper138, prepared as part of the funding review, identified that one of the four key tasks of the funding review was to ‘review current arrangements for MRI, particularly restrictions around MBS-eligible/ineligible units’.139 Included in this discussion paper were options related to:
- opening access to the MBS for all MRI machines; and
- standardising conditions on existing MBS-eligible MRI machines. At the time, particular conditions such as hours of operation and billing practices were in place – these related to the year in which the particular machine was granted eligibility.140
4.3 Subsequently, the MRI aspect of the funding review was discussed by the DIRCC141 at their June 2010 meeting. The following key issues were raised:
- the inconsistency and complexity of MRI MBS funding arrangements;
- general support by stakeholders for MRI requests by general practitioners (GPs), to improve access more generally as well as to minimise children’s exposure to radiation by substituting MRI scans for other scans such as CT scans; and
- the need for GP referrals to be in parallel with improved access to services.
4.4 The funding review identified that by extending MRI requesting rights to GPs, additional demand for MRI services would be created. The existing 125 MBS-eligible MRI machines were not expected to have sufficient capacity to meet that demand.142 Health’s February 2011 advice to the then Minister outlined that new capacity would be required and there would be a budgetary impact. Health’s advice noted that:
The existing approach of containing growth in expenditure on MRI services by limiting the number of Medicare-eligible machines will not provide sufficient patient access to this important imaging modality in the longer term. In addition, the extension of requesting rights to GPs for some MRI services will result in a shortfall in the capacity of current Medicare eligible MRI units to meet increased patient demand. It is therefore necessary to consider ways in which eligibility might be extended to meet this demand.
… [the reform package’s MRI expansion initiatives would, in a staged approach, extend] Medicare eligibility in a gradual, fiscally responsible manner.
4.5 The reform package announced on 10 May 2011 included the following elements related to patient access to MRI services:
- allowing GP-requesting of MBS-eligible MRI services for clinically appropriate symptoms;
- standardising operating arrangements for existing MBS-eligible MRI machines;
- extending access to MBS-eligible MRI services for MBS-ineligible machines; and
- further expanding the bulk billing incentive for diagnostic imaging which was introduced in November 2009 to provide an additional five per cent for MRI services, bringing the payments that radiologists received to 100 per cent of the schedule fee.143 This element was to off-set an expected decrease in bulk billing from standardising operating arrangements for existing MBS-eligible MRI machines. Under arrangements existing at the time, some providers had bulk billing rates that they were required to meet.
4.6 To assess the extent to which Health has effectively implemented the above reforms to enhance the affordability and convenience of access to MRI services, the ANAO examined each of the elements of the reforms144, as well as monitoring and reporting on the impact of these reforms.
Extending Medicare MRI requesting rights to GPs
4.7 The reform package included two elements that extended MBS requesting rights for MRI scans to GPs for:
- patients under 16 years for a small set of clinically appropriate symptoms from 1 November 2012; and
- patients 16 years and over for a small set of clinically appropriate symptoms from 1 November 2013.
4.8 To examine Health’s effectiveness in implementing these elements of the reform package, the ANAO assessed the extent to which the department drew on expert opinion in determining which symptoms would be most clinically appropriate for MBS-eligible MRI scans requested by GPs and whether the measures took effect from 1 November 2012 and 2013 respectively.
Process to determine appropriate symptoms
4.9 Allowing GPs to request MRI scans with unlimited restrictions, particularly given the slow progress in implementing clinical guidelines and decision-support tools for GPs, increases the risk of inappropriate requesting of scans145, and the risk that the costs of MRI services would increase significantly given the number of potential GP-requestors. In order to assess and treat these risks, Health sought expert advice on the potential management of requests by GPs.
4.10 For both GP-requesting elements, expert working groups were established. These comprised representatives from general practice, as well as the diagnostic imaging profession and industry. These groups examined specific symptoms, focussing particularly, but not exclusively, on those sets of symptoms raised in stakeholder consultations conducted as part of the funding review. In considering which symptoms should be included, the groups determined whether MRI scans would assist in diagnosis, the most appropriate diagnostic image type, and whether the symptoms were better managed by a specialist. The groups also advised on the MBS item description for each set of symptoms.
4.11 The funding review had highlighted the health risks from the exposure of children to ionising radiation from CT scans. Given this, a particular focus of the expert working group considering MBS items for those under 16 years, were the symptoms on which GPs were basing requests for CT scans, and the extent to which MRI scans could be substituted.
4.12 The MBS items for those 16 years and over were also separately considered by a Royal Australian College of General Practitioners (RACGP) advisory group, who recommended that items relating to two sets of symptoms be excluded.146 Health accepted the recommendations of the expert working group excluding the two MBS items as recommended by the RACGP advisory group. Prior to making legislative changes for MBS items for those 16 years and over, the Medical Services Advisory Committee (MSAC)147 Executive Committee considered the clinical effectiveness and cost-effectiveness, and delayed the inclusion of one item until a full review could be conducted.148 This input, together with that received from the engagement with professional and industry groups, indicates that there was relevant consultation to ensure that only those symptoms where a GP’s management of a patient’s condition would benefit from an MRI scan were included in GP-requested MBS items.
Implementation of the measures against timelines announced in the 2011–12 Budget
4.13 In both cases the final list of MBS items was approved by the Minister and regulatory changes were made in sufficient time to allow GPs to request MBS-eligible services in line with the timeframe set in the reform package.149 As a result, GPs have been able to request MBS-eligible MRI scans for six sets of symptoms in patients under 16 years from 1 November 2012 and four sets of symptoms in patients 16 years and over from 1 November 2013, as anticipated under the reform package (see paragraph 4.7).
4.14 In summary, the ANAO found that Health acted on expert advice in developing MBS items that allowed GPs to request MBS-eligible MRI scans for clinically appropriate symptoms. In addition, Health progressed the necessary changes to regulation to allow MBS payments for these items to be made by the timeframes set by Government.
Standardising and extending MBS-eligibility for MRI machines
4.15 In order to improve access to MBS-eligible services from MRI machines, the Government announced a number of measures as part of the reform package, namely:
- standardising MBS-eligible operating arrangements for all MBS-eligible machines;
- extending access to the MBS for MRI machines that were MBS-ineligible. This was to allow: machines operating in regional and remote areas to become eligible for all MBS MRI services (full eligibility); and machines operating in major cities to become MBS-eligible for the new GP-requested MRI services (partial eligibility)150; and
- extending full MBS-eligibility to 12 additional machines, based on defined areas of need.
4.16 The ANAO examined Health’s implementation of each of the above measures, including the department’s approach to planning, assessing, and monitoring improvements in patient access to MRI services resulting from the increase in MRI machines that are MBS-eligible.
Standardising operating arrangements for existing MBS-eligible machines
4.17 The implementation of standardised operating arrangements required a number of legal steps. Health facilitated changes to the Health Insurance (Diagnostic Imaging Services Table) Regulations 2011 to deregulate bulk billing and hours of operation requirements relating to specific MRI machines. The amendment also required owners/lessees of such equipment to provide to the Commonwealth a record of the location and specifications of the equipment, and for this information to be included in a deed between the Commonwealth and service provider. The deed was required to facilitate and standardise the transferability of MBS-eligibility arrangements. The regulations came into effect as planned on 1 May 2012, and the department entered into the relevant deeds with service providers.
Expanding access to MBS for planned or existing MBS-ineligible MRI machines
4.18 On Budget night (10 May) 2011, the Government announced an expansion of access to the MBS for MRI machines. Eligibility was to be expanded from 125 machines to an estimated 208 machines by 2014–15 – an increase of up to 83 machines. The 83 affected machines comprised:
- 71 current MBS-ineligible151 MRI machines152, with those operating153 outside major cities becoming fully MBS-eligible and those operating in major cities becoming partially eligible (that is, MBS-eligible for some services) from 1 November 2012; and
- an additional 12 MRI machines becoming MBS-eligible between 2012 and 2015 (two per annum in the first two years then an additional four per annum for two years), extended to providers who applied to provide services in defined areas of need.
4.19 The Budget announcement specified the date on which the ineligible machines were able to start providing MBS-eligible services (1 November 2012) and the years in which the 12 additional MRI machines in defined areas of need would become MBS-eligible. However, the Budget announcement, and the Government decision supporting it, did not specify:
- the number of MBS-ineligible machines that would become eligible. This was relevant for the department’s planning for the assessment process and to respond to stakeholder questions following the announcement;
- the cut-off date for assessing the entitlement of MRI machines to MBS-eligibility. The date by which MRI machines were required to be operational in order to be covered by the Budget measure would be important in determining eligibility;
- the matter of whether ‘planned’ MRI machines would be included in the reform package MRI expansion; or
- the process and basis on which eligibility would be assessed, including the criteria by which ‘areas of need’ would be assessed.
4.20 Health provided advice to the Minister to clarify these matters as part of its planning for implementation of the MRI reform initiatives.
4.21 While 71 machines were advised to the Minister154 as being reasonably expected to be granted MBS-eligibility as part of the reform package, a total of 216155 machines were eventually granted MBS-eligibility as part of the expansion process, at significant additional cost to the MBS.156 Given this significant difference in the number of machines granted MBS-eligibility, the ANAO examined:
- the department’s advice and planning for the expansion, particularly:
- the basis on which Health had determined the number of MRI machines, to inform Government decision-making in the lead up to the 2011–12 Budget;
- Health’s subsequent advice to Government on the eligibility criteria for expansion, against the Government’s Budget decision; and
- Health’s planning for the conduct of assessments against the eligibility criteria to ensure a structured implementation approach and to manage key risks; and
- the assessment of applications157 against the eligibility criteria and management of the assessment risks.
Advice and planning on the expansion
Determining the number of MBS-ineligible MRI machines
4.22 Determining the number of machines that would become MBS-eligible as a result of the 2011–12 Budget initiative was undertaken by Health in a number of steps, and initially without industry consultation due to the need to avoid disclosing government policy intentions.
4.23 The estimate of 71 MBS-ineligible MRI machines was based on the number of MRI machines that had been registered with Medicare Australia as at 10 December 2010. Diagnostic imaging service providers with MBS-ineligible machines were (and still are) able to register their machines with the Department of Human Services (DHS), but are not obliged to do so.
4.24 In April 2011, following the Government decision to expand MBS-eligibility as part of the Budget but before the Budget announcement, Health prepared advice for the Minister’s office indicating that – based on machines registered with DHS, correspondence with providers and internet research – there were 83 MBS-ineligible machines in operation and a further nine machines with secure funding and for which building works had commenced. In this advice, Health informed the Minister that it had not gained information directly from industry or the profession on the number of MBS-ineligible units in operation at the time, given the need to preserve the confidentiality of matters under consideration in the 2011–12 Budget context, and acknowledged that it may not be aware of all MBS-ineligible machines. Health advised the Minister’s office that it planned a communication exercise after Budget night to ensure all operational machines were captured.
4.25 Health advised the ANAO that:
In compiling the initial estimate of the number of MRI units which would become Medicare-eligible under the proposed expansion, the department was acutely aware of gaming of particular parts of the sector in previous expansions and previous ANAO reports on the matter. Information gathering from the sector, particularly in the initial stages, was necessarily circumspect and informal to avoid providing confirmation the Government was actively considering an expansion process. As the [ANAO notes], there was already speculation within the sector that another expansion process could occur. The department sought to balance the competing objectives of providing suitable advice to the Minister about the number of MRI units against managing the risk of gaming by the sector if more transparent communication occurred.158
4.26 While acknowledging the need to avoid signalling policy intentions and the potential risks of providing industry stakeholders with privileged information that may provide a material benefit, the ANAO notes that the reform package was the product of a consultative review process in 2010, which had provided opportunities to collect appropriate relevant information on the state of the MRI sector.
4.27 Following the Budget announcement, Health checked the status of the 71 machines registered with DHS; 65 of these registered machines were found to be in operation and part of a comprehensive159 diagnostic imaging service. On 16 May 2011, the Minister announced that these 65 machines would have access to MBS-eligibility from 1 November 2012.
4.28 In the event, Health’s advice to the Minister before and after the Budget announcement significantly understated the likely number of MBS-eligible machines. Almost three times more MRI machines were eventually approved than originally estimated.160
Advice to Government on the criteria for expanding MBS-eligibility, including cut-off
4.29 As discussed at paragraph 4.19, the criteria for assessing eligibility, including the cut-off date and the matter of whether ‘planned’ MRI machines would be included in the reform package MRI expansion, were not specified in the Government decision.
4.30 In its advice to the Minister, in the context of the 2011 Budget and leading to the announcement of the reform package, Health proposed that access be extended to MBS-eligible MRI services for ‘current MBS-ineligible MRI units operating’ in non-major cities (full eligibility) and major cities (partial eligibility) from 1 November 2012. In addition, Health proposed a cut-off date that would precede the Budget night announcement:
There will be a requirement that all ineligible MRI units must have been operational (i.e. provided MRI services to patients) prior to 1 January 2011.
4.31 The Government’s decision to extend MBS-eligibility to more MRI machines specified that eligibility would be extended ‘to current MBS-ineligible MRI units’. However, the reform package publicly announced indicated that MBS-eligibility would be extended to ‘operating’ MRI units.161
4.32 Following the Minister’s announcement of the initial 65 MRI machines gaining MBS-eligibility162, Health received numerous requests from providers seeking clarity of the MBS-eligibility of other machines. In particular, providers sought information on the treatment of machines which were not operational on the date of the government announcement, 10 May 2011. Health undertook discussions with the Minister’s office in June 2011, and in September 2011 advised the Minister that there were approximately a further 139 machines that potentially would qualify for full or partial MBS-eligibility in addition to those already announced. Health advised the ANAO that its estimate of the number of extra machines was based on feedback received from a request to diagnostic imaging providers via the Royal Australian and New Zealand College of Radiologists (RANZCR) and the Australian Diagnostic Imaging Association (ADIA).
4.33 In September 2011, Health sought approval from the Minister on the following criteria for assessing and approving MBS-eligibility. These criteria proposed two categories, ‘in operation’ and ‘planned’, with a cut-off date later than the date of 1 January 2011 proposed by Health in its early advice to the Minister. The proposed criteria for MBS-eligibility were that MRI machines had to:
- be operational or ‘planned’ as at 10 May 2011 (Budget night), as follows:
- operational MRI machines must have been providing MRI services to patients; and
- diagnostic imaging providers of ‘planned’ machines must have evidence of an arrangement or contractual obligation which demonstrates a financial investment163 in the provision of an MRI service. Evidence would include:
- the purchase of an MRI or associated equipment for a new machine,
- evidence of capital works to house a new machine,
- evidence of an agreement between a hospital and a private provider to install an MRI machine at the hospital, or
- evidence of a committed planning process, e.g. a development application to local council;
- be part of a comprehensive diagnostic imaging practice or radiology department164;
- have a strength of a minimum of 1.5 tesla165;
- for regional machines located in ASGC-RA2 to RA5 and for metropolitan machines had to be in ASGC-RA1166; and
- be part of a practice that had diagnostic imaging accreditation.
4.34 By including planned as well as operational MRI machines167, and specifying 10 May 2011, rather than 1 January 2011 as the cut-off date, Health broadened the eligibility criteria on which the Government’s decision had been based. In advising the Minister on different cut-off dates, Health provided a number of options, but recommended 10 May 2011 as the cut-off date on the following basis:
Industry expects a majority of pre-Budget existing and planned MRI units will be granted Medicare eligibility from 10 May 2011. The department recommends 10 May 2011 as it has a minor impact on the overall number of MRI units (i.e. an additional four units from 1 January 2011); it grants access to all current MRI practices that meet the criteria; and it best aligns with the objective of the DI reforms package to expand patient access to MRI.
4.35 The Minister requested further information on the financial implications. Health advised the Minister in November 2011 that the extra machines would have no Budget implications, as the Budget costings had been based on the expansion of MBS-eligible MRI services rather than additional MRI machines. In its assumptions for the Budget costings, Health based its estimates only on the increase in the number of requestors, that is, from allowing GPs to request MBS-eligible MRI services. Once the demand for services from these requestors had been met by a threshold number of MRI machines, Health assumed that a further increase in the number of MBS-eligible MRI machines over that threshold would have little impact on the request for services. In essence, Health considered that demand would be satisfied across a larger number of machines instead of concentrated across a smaller number of machines and that beyond a certain threshold, particularly in metropolitan areas, demand for MRI services was not affected by additional machines being made available. On the basis of this advice, in November 2011 the Minister approved the inclusion of newly identified MRI machines, noting that:
I am very surprised that there can be little/no cost change for such a large extra number of machines. I have approved, based on the assurance that DOFD agree to this cost. I will not approve a future increase if these calculations are wrong.168
4.36 Health’s November 2011 advice to the Minister contrasted with advice earlier that year, which argued against providing MRI machines with open access to MBS-eligibility as such an approach ‘involves risks of over-investment by the sector, with impacts to MBS expenditure’.169 Health advised the ANAO during the course of the current audit that it now considers the key driver for MRI services to be the availability of requestors170, but also recognises that increased supply of MRI machines is a factor. Therefore, Health’s advice to the Minister (November 2011) that there would be no budgetary effects from an increased number of MRI machines over that which the Government made its decision did not take into account supplier-induced demand by diagnostic imaging providers, a factor recognised in its earlier (February 2011) advice and its current view. There is little doubt that the significant expansion of MRI machines has contributed to the significant growth in MBS expenditure over Budget forecasts for GP-requested MRI services, which is shown in Table 4.3. Overall, it indicates that, at the time, Health did not take account of the risks to expenditure from its expanded eligibility criteria in its advice to the Minister in November 2011.
4.37 During the course of the audit, Health commented to the ANAO that:
With the benefit of hindsight, the anticipated level of correlation between demand and supply was not anticipated and, together with the apparent supplier induced demand by diagnostic imaging providers, appears to have resulted in higher utilisation and expenditure than estimated.171
Planning for the conduct of assessments against the eligibility criteria
4.38 Planning for an assessment process allows timelines, resources, and risks to be identified and addressed in an orderly way.
4.39 Following the Minister’s agreement on the eligibility criteria, Health disseminated information on the MRI expansion process to potential MRI service providers through RANZCR and ADIA, and through direct communication with state and territory health departments as well as a large number of providers known to the department. No closing date for applications was included in the information.
4.40 In its 1999–2000 performance audit report on the expansion of MBS-eligible MRI machines, the ANAO also identified no closing date in that earlier process, which resulted in a continued trickle of applications and machines being registered in even greater numbers than were predicted.172 Similarly, applications for the MRI expansion announced as part of the 2011–12 Budget were not processed in one round prior to the implementation date of 1 November 2012. Rather, applications for eligibility trickled into Health from December 2011 to December 2013 and were assessed in four rounds.173 Multi-round application processes can affect the efficiency with which an entity is able to undertake assessments, as it needs to undertake separate assessments and briefing processes for each round. Further, multiple rounds can result in inconsistent outcomes unless care is taken to align assessment processes.
4.41 On 30 March 2012, the departmental delegate approved an assessment plan that reflected the approved criteria.174 This assessment plan did not include:
- any identification and analysis of risks that may need to be managed when undertaking assessments – such as the risk of inconsistent assessments across rounds, and a number of other risks which had arisen in past MRI assessment processes, discussed below; and
- detailed guidance to assist assessors to determine whether applicants met the eligibility criteria. The lack of such guidance has resulted in applications being approved based on incomplete information.175
4.42 The importance of risk management was highlighted in the ANAO’s 1999–2000 report on MRI services. In that report, assessments for planned machines to be MRI-eligible were based on signed statutory declarations and signed contracts with machine suppliers. The audit found that ‘some of the contracts for these ordered machines were apparently backdated’ to meet the eligibility criteria.176 The lessons of that audit relating to risk assessment were not fully considered by the department in its planning for the 2012 MRI expansion assessment process.
Assessing applications for MBS-eligibility
4.43 The MRI expansion assessment process was a gateway to the receipt of Commonwealth payments, in the form of MBS rebates. In circumstances where entity processes are likely to result in long-term financial commitments for the Commonwealth, it is important that entities undertake a robust assessment to ensure that applications clearly meet the eligibility criteria and take account of the risks of inaccurate or fraudulent claims. Furthermore, all potential applicants should have equitable access to the assessment process.
4.44 Health assessed the applications for eligibility in four rounds:
- an original assessment of applications undertaken in April to May 2012, based on applications received at that time;
- a second round of applications received from May to December 2012. Following the completion of the assessment of applications, Health sought Ministerial approval to close-off applications, but this was not agreed;
- a third round of applications received from December 2012 to November 2013. At the time of approving these applications, the Minister also approved closing-off applications on 31 December 2013; and
- a fourth round of applications received from November 2013 to 31 December 2013, announced in June 2014. The cut-off date was advertised on Health’s web-site.
4.45 One hundred and twenty-six177 machines were approved through the original assessment round and 25 further machines were approved in the subsequent rounds. In each of the rounds, applications were rejected (for example, 13 applications were identified as non-compliant in the original assessment round); although some of these were subsequently approved following the submission of further information in later rounds. Health sought Ministerial approval for each of the rounds subsequent to the original round in mid-2012. However, unlike the original round, Health did not widely disseminate information178 that applications would continue to be assessed following the original round and, if they met the eligibility criteria, Ministerial approval would be sought. Inconsistency in the approach adopted to disseminate information, and unnecessarily limiting the information available to potential applicants, can raise questions about the transparency and fairness of a process. Such a view was expressed to the ANAO by a key industry stakeholder who advised that it had not been made aware that the application process remained opened, and therefore questioned the transparency of the process.
4.46 To undertake the assessments, Health required providers to submit the following information:
- for MRI machines in operation as at 10 May 2011, a statutory declaration to include:
- details of the machine’s location to enable Health to determine in which ASGC-RA the machine was based179,
- the practice’s DIAS accreditation status,
- whether the diagnostic imaging practice was comprehensive, and
- details of the machine, including tesla strength, make, model, date of manufacture and date of operation;
- for MRI machines not operational as at 10 May 2011, a statutory declaration as above was required, along with evidence of financial investment into the provision of an MRI service on or before 10 May 2011. The evidence required was that outlined for a ‘planned’ machine approved by the Minister.180
4.47 While the proper assessment of applications against guidelines required judgment by assessors, the guidelines included examples of acceptable information to demonstrate financial investment.
4.48 To determine the extent to which Health had assessed applications in a manner consistent with the eligibility criteria, the ANAO examined all available documentation for a sample of 73 approved applications from the original assessment round, comprising:
- nineteen applications randomly selected from the 66 operational machines; and
- all 54 ‘planned’ machines, which had been approved based on submitted evidence of financial investment.181
4.49 The documentation provided to Health by applicants for determining decisions on eligibility for the 73 applications sampled is outlined in Table 4.1.
Table 4.1: Evidence for decisions of MBS-eligibility for sampled applications of approved machines from original round
|
Basis of assessment |
Number of machines |
Evidence provided |
ANAO finding – completeness of evidence |
|
Operational |
19 |
Statutory declarations only |
Complete – evidence provided in line with eligibility criteria. |
|
Planned |
25 |
Evidence of financial investment, including a binding contract with MRI suppliers or with state government, or building plans and permits that include the provision of an MRI machine, prior to 10 May 2011. Statutory declaration. |
Complete – evidence provided in line with eligibility criteria. |
|
29 |
Evidence provided included:
Statutory declaration |
Incomplete – evidence provided by diagnostic imaging practices to Health was not sufficient to demonstrate that the eligibility criteria were met. In these cases, there was no documentation to substantiate a clear and unqualified financial commitment on or prior to 10 May 2011. |
|
|
Total |
73 |
|
|
Source: ANAO analysis of Health documentation.
4.50 ANAO sampling indicated that, while all applications had each been assessed against consistent selection criteria, 54 per cent of applications for planned machines (29 applications) had been approved using evidence that did not sufficiently demonstrate a clear and unqualified financial commitment182 on or prior to 10 May 2011. The assessment for 14 of these 29 applications was primarily based on a quote from an MRI machine supplier that had been accepted by the diagnostic imaging provider prior to 10 May 2011; however, the terms and conditions of accepting the quote were not included as evidence. In its assessment, the ANAO found four such quotes with terms and conditions attached:
- two quotes included cancellation clauses that imposed no financial penalty for the provider and therefore do not include any contractual obligation to purchase a machine; and
- two quotes imposed a financial penalty and therefore provide evidence of a financial investment.
4.51 The ANAO’s analysis indicates that, as there was a risk that the terms and conditions of the quotes supporting 14 applications did not impose financial penalties for cancellation, Health did not obtain sufficient evidence of a clear and unqualified financial commitment for these approved applications.
4.52 As part of its assessment process, Health was able to follow up with applicants to gain clarification and further evidence. While this was done on a regular basis, Health did not address all the limitations in documentation found by the ANAO in its review. The ANAO’s findings in relation to the approval of 29 ‘planned’ machines are consistent with the view of a key industry stakeholder who advised that, for the ‘planned’ machines, there seemed to be ‘a tenuous link in a lot of cases regarding the level of (financial) commitment’.
Cross-checking the accuracy of information with other stakeholders
4.53 The importance of cross-checking with other stakeholders the accuracy of information submitted by the applicant was highlighted in the ANAO’s previous MRI audit.183 In the current audit, Health advised that they did not follow up with suppliers, for example, to cross-check the information provided by applicants in copies of contracts or statutory declarations. Furthermore, in the original assessment round, Health did not confirm agreements between hospitals and private practice providers. Subsequently, the risk that an approved applicant did not meet the MBS-eligibility criteria materialised. In particular, one of the regional applications identified as eligible in the original expansion assessment and included in early advice to the Minister, was found to be non-compliant. This was only identified when the Minister’s office requested that an on-site announcement by the Minister be scheduled. A phone discussion with the hospital in which the provider was placing the machine raised doubts as to the eligibility of the machine. This machine was subsequently identified as ineligible.
4.54 The ANAO found that Health’s assessment process improved over subsequent rounds. In particular, the department recognised that a supplier’s quote is not in itself sufficient evidence on which to determine compliance with the eligibility criteria. In the last two assessment rounds, Health documented and separately assessed each piece of evidence against the eligibility criteria, outlining the reasons why it might meet/or not meet the criteria. This more structured process provided greater assurance that the application was accurately assessed against the eligibility criteria, and that the evidence base was adequate and documented. Furthermore, Health advised the ANAO that the department confirmed that agreements between hospitals and private MRI providers were in place through phone calls in assessment rounds subsequent to the original round.
Assessing the risk of backdating orders for MRI machines
4.55 In the 1998 expansion of MBS-eligibility to planned or existing MRI machines, some 33 machines were ordered in the four working days between 7 and 12 May (Budget night), according to providers’ statutory declarations. This was in the context of some 60 machines in operation at the time. As a result, there were significant risks that contracts to order the machines were backdated and that there was some prior knowledge of the inclusion of machines on order as part of the Budget measure.184 To determine whether there was a parallel increase in ordering prior to the 2011–12 Budget announcement of the reform package, the ANAO examined when MRI machines in use became operational. The results are shown in Figure 4.1.
Figure 4.1: MRI machines in use at 30 June 2014 by period in which they became operational

Source: ANAO analysis of Health records.
4.56 Figure 4.1 shows that there was significant percentage growth in MBS-eligible MRI machines in operation in the two years following the 1998–99 Budget night announcement of an MRI expansion, a process that was characterised by a significant risk of backdated orders. In contrast, in the recent expansion, the most significant growth in MRI machines in operation occurred in the two years following the 2009–10 Budget when the funding review (not the reform package containing the MRI expansion measures) was announced, with a decrease in growth in the three years following the announcement of the reform package. The growth of MRI machines in the two years following the funding review announcement is likely to have arisen from an anticipated opening up of MBS-eligibility of MRI machines following the announcement of the funding review, and particularly from Health making publicly available a discussion paper which raised this as an option.185 A further increase in growth subsequent to the 2011–12 Budget would have indicated a significant increased risk of backdated orders following Budget night 2011. This did not occur.
4.57 In summary, the ANAO’s assessment of the expansion identified a number of areas in Health’s administration that could have been improved had the risks highlighted in the ANAO’s earlier report on MRI expansion (that resulted from the 1998–99 Budget) been addressed. These risks related to: the impact on MBS expenditure from increased supply of MBS-eligible MRI machines; the importance of a close-off date for the application process to manage the number of applications; and the need to cross-check the accuracy of information provided by diagnostic imaging providers. That said, a key lesson from the earlier MRI expansion process, relating to the importance of maintaining confidentiality of Budget measures under consideration by the Government, does appear to have been addressed.
4.58 A number of shortcomings not apparent in the earlier expansion process were identified by the ANAO during this audit. Health recommended to the Minister broadening the eligibility criteria without fully identifying the risks to MBS expenditure of doing so. Furthermore, based on its sampling, the ANAO’s analysis indicates that Health’s assessment of over half the applications for MBS-eligibility of ‘planned’ machines from the original expansion round was not based on complete information, providing the potential for machines to be approved that did not meet the eligibility criteria.
Extending full MBS-eligibility to 12 additional machines in areas of need
4.59 To give effect to the Budget decision to extend full MBS–eligibility to 12 additional MRI machines in areas of need over the period 2012 to 2015, the Minister agreed the following implementation matters based on departmental advice provided in December 2011:
- criteria for determining areas of need based on geographic distribution of MBS-eligible MRI machines, patient needs and health service integration;
- a single expression of interest to be conducted in early 2012 to identify machines to becomes eligible in line with the Government decision to allow two machines to become eligible in both 2012 and 2013, and four machines in both 2014 and 2015; and
- organisations to identify areas of need in their applications rather than Health determining the areas of need and calling for an expression of interest in these specific areas.
4.60 Health undertook an invitation to apply (ITA) process to identify the MRI machines which would be granted full MBS-eligibility – these could include machines in metropolitan areas that were otherwise partially MBS-eligible, and planned machines which did not meet the requirements under the expansion process. The ITA process was not required to be subject to the Commonwealth Grant Guidelines (CGGs) applicable at the time186 as MBS-eligibility does not have the character of a discretionary grant, but establishes ongoing access to Commonwealth funding based on the provision of defined services to patients. Nevertheless, the CGGs represent good practice for the conduct of competitive assessment processes. Their adoption promotes transparency and can assist an entity in demonstrating and satisfying value for money requirements.187 Health advised the financial delegate that the assessment process had been conducted in accordance with the CGGs. The ANAO assessed the design and planning processes and the selection and decision-making processes adopted for the ITA against the relevant CGG principles.
Planning and design for the ITA
4.61 The CGGs identify the importance of robust planning and design of the granting activity. These include having proper regard for:
- establishing performance and evaluation measures;
- communicating effectively with potential recipients;
- developing appropriate documentation, such as selection and assessment guidelines; and
- undertaking risk management.
4.62 In April 2012 the delegate approved an ITA process to identify MRI locations. Information on the ITA was disseminated via a widely based advertisement, and followed up by an evaluation, involving compliance primary and secondary assessments. A timeframe based on an estimated 80 applications to complete the process by the end of July 2012 was included in the briefing to the delegate.
4.63 An assessment plan identified the evaluation process, which was communicated to potential applicants in the ITA documents. Key elements were:
- compliance assessment which assessed the completeness of applications against all requirements;
- primary assessment based on: a weighted assessment of the two nominated criteria from the three bases on which areas of need would be determined – ‘geographic distribution’, ‘health service integration’, and ‘population group’ – and on improvement to patient access and infrastructure to provide the full range of MRI services on the MBS; and non-weighted assessment of sub-criteria related to diagnostic imaging practice business structure and operations in which the unit would be placed; and
- secondary assessment of applications shortlisted from the primary assessment, based on a 100 per cent weighting against the criteria, ‘Government priorities for health’. Against this criterion, applicants needed to demonstrate knowledge, understanding and awareness of the Australian Government’s broader health reforms and how access to Medicare-eligible MRI services in the applicant’s identified area would contribute to these reforms.
4.64 The assessment plan identified that the departmental delegate could accept or reject (with reasons) the assessment committee’s recommendation on applicants to receive full MBS-eligibility188, seeking endorsement of the decision from the Minister.
4.65 The assessment plan also addressed risks. These included the means of managing applications in excess of the initial estimate of 80 applications; and the means of managing actual or perceived discrepancies in the ITA assessment process. To assist in establishing a consistent and transparent assessment process, a scoring system was provided for assigning scores to the weighted criteria for the primary assessment, to which the committee members were required to have regard. This scoring system provided scores of 0 to 10 based on the number of ‘factors’ the application addressed. However, an explanation of what ‘factors’ were relevant was not identified.
4.66 In line with the timeline in the assessment plan, advertisements were placed nationally on 26 May 2012, with the ITA process closing on 22 June, providing 27 days in which applicants could respond.
4.67 The design and planning for the ITA relating to the 12 additional MRI machines in areas of need was largely in line with the better practice discussed in the CGGs. However, key aspects of the assessment process were not well developed and subsequently affected the efficiency and transparency of the assessment process. These related to:
- the scoring system for assessing weighted criteria. An explanation of what ‘factors’ were relevant was not identified;
- the specific criteria for determining which applications would be subject to secondary assessments. The assessment plan did not specify the short-listing basis, but implied that it should be on the top-scoring applications from the weighted criteria and meeting the unweighted criteria; and
- the specific requirements that applicants were expected to address for the ‘Government priorities’ criterion which were to form the basis of the secondary assessment.
Assessment and decision-making
4.68 Health received 185 applications, indicating significant interest from the sector. The department’s initial assessment of compliance identified duplicate and late applicants, resulting in 181 applications that were subject to formal primary assessment.
4.69 The primary assessment was undertaken by a three person committee on all the compliant applications. The initial assessment of applications was found to be inconsistent across committee members as the scoring system was not sufficiently detailed. As a result, the committee developed a more detailed scoring system, and undertook a reassessment of all applications using this new scoring system.
4.70 The more detailed scoring system provided significantly more guidance to the primary assessment committee, resulting in more consistent scoring across applications. The scoring system for one of the sub-criteria under the ‘geographic distribution’ criterion, provided greater weight to applications that included a map as well as a description of the catchment area, a factor not required for applications. A reassessment undertaken by the ANAO eliminating the requirement for a map, found that it is unlikely that this issue impacted materially on the assessment outcome.
4.71 To assess whether Health’s primary assessment was consistent with the assessment plan and revised scoring system, the ANAO examined a random sample of 19 applications to compare the assessment scores against the applications. Two applications from the sample were not on Health’s files. Of the remaining 17 applications, 15 were found to be consistent with the scoring system. Of the two remaining applications, in one case there had been a very narrow interpretation of a selection criterion, and in the other case there was an error in transposing the scores from hard to electronic formats. In both cases, the ANAO found that it is unlikely that the inconsistencies would have impacted materially on the assessment outcome.
4.72 In late August 2012, Health sought and gained agreement and clarity on the secondary assessment from the Minister’s office prior to its commencement. Particular issues related to:
- which applications should be subject to secondary assessment. It was recognised that there had been insufficient guidance to applicants for some criteria and had resulted in applications receiving poor overall scores which would otherwise have strong cases based on geographic location. Health proposed that the following be included in the secondary assessment:
- all applications that had a 50 per cent or higher weighted score, and passed the non-weighted criteria; and
- for those that did not have a 50 per cent or higher weighted score, but had a geographic distribution criteria amended at 50 per cent or higher; and
- the basis for evaluating the ‘Government priorities for health’ criterion for the secondary assessment process. These were:
- populations with low socio-economic status, for which Health used the socio-economic indices for areas (SEIFA) from the ABS189;
- locations that still did not have adequate access to MRI services, for which Health used the direct distance to the nearest fully MBS-eligible MRI unit; and
- co-location with a public or private hospital service or in a private practice.
4.73 This late clarification resulted in no assessment of the responses that applicants provided to the ‘Government priorities for health’ criterion. Within the sample selected (see paragraph 4.71), the ANAO found that applicants had written between 1 and 22 pages to address this criterion, indicating that some applicants had made considerable effort to address this matter in their application. In addition, the basis for applications being subject to secondary assessment effectively provided greater weight to the geographic distribution criterion than was specified in the documentation provided to applicants.
4.74 On the basis agreed with the Minister’s office, Health identified 82 applications for secondary assessment. The ANAO found that one application that met the criteria for secondary assessment had been excluded, with no documented explanation. Nonetheless, the ANAO found that it is unlikely that its inclusion would have impacted on the final assessment outcome.
4.75 Health advised the ANAO that in order to short-list applications for approval, the secondary assessment committee drew on spreadsheets that ranked the 82 applications by SEIFA and by distance to the nearest fully MBS-eligible MRI unit. However, Health was unable to provide documentation to support this approach. Health further advised the ANAO that the Minister requested that a short-list of 21 applications be provided to allow the Minister choice in selecting the successful applicants and, consequently, a short-list of 21 applications was developed. This approval process was different to that outlined in the assessment plan.190
4.76 Of the 21 short-listed applications provided to the Minister, one secondary assessment could not be located by Health to provide to the ANAO, either in hard copy or electronically. Of the remaining 20 applications, only ten included justification for inclusion on the list – these justifications were in line with the criteria used for assessment.
4.77 In respect to the list of 21 short-listed applications that went to the Minister, the ANAO noted that:
- All the applicants in the secondary assessment whose location was at least 50 km from a fully MBS-eligible MRI machine, were included on the short-list. This approach reflected the Minister’s preference for addressing geographic need. Most alternates from the same location were largely excluded, with justification. One exception to this general approach was the exclusion from the short-list of an application for an MRI machine located 53km from an MBS-eligible MRI machine, but this area had a relatively high socio-economic score, a SEIFA of 6.
- Three applications related to MRI machines up to 23 km from a fully MBS-eligible MRI machine (the machines were 23km, 11km and 0km away respectively). There was a justification to include those at 23 km and 11 km in line with the criteria, but no justification to include the one at 0 km. The ANAO notes that the one at 0 km had a SEIFA of 4, and was co-located at a public hospital. However, two applications with SEIFA of 3 or less and greater distances from an existing fully MBS-eligible MRI machine (one part of a private practice and the other a public hospital) were not included in the shortlist.
4.78 While it is possible to identify a rationale for elements of the short-list, the basis of the advice offered to the Minister was not fully documented.
4.79 In September 2012, an assessment report was prepared by Health. Advice to the Minister was prepared that included the list of 181 applications, the secondary assessment list of 82 including SIEFA, practice location, and distance, and the short-list of 21 applications. The short-list was ranked by SEIFA within each state/territory, and included information on the distance to alternative fully MBS-eligible services, and whether the machine was located at a public or private hospital, or in a private practice. In recommending that the Minister approve 12 machines from the short-list of 21 applications, Health advised that the short-listed applications ‘most strongly demonstrated unmet need against one or more of the three priorities’. While Health provided some information to the Minister to assist in decision-making, the department did not advise that in approving the 12 machines, the Minister would be exercising the role of approver for the purposes of Regulation 9 of the FMA Act.191 The relevant Department of Finance guidance on the interpretation and operation of Regulation 9 stated that in such circumstances ‘the relevant agency should take appropriate steps to advise their Minister of the legal requirements established by the FMA regulation’.192
4.80 The Minister approved 12 machines on 30 October 2012 with no documented record for choosing these machines over the remaining machines on the shortlist.193 Health then ranked these applications in order of their MRI machine’s actual or expected operating date to determine the timing at which they would become full MBS-eligible. The process was completed in time for two machines to be granted full MBS-eligibility in 2012, in line with the Budget announcement.
4.81 As there was no documented basis provided by the Minister in approving eligibility for the 12 machines from the list of 21, the ANAO assessed the extent to which the distribution of machines favoured locations in particular Federal electorate types194 over others. The results are shown in Table 4.2.
Table 4.2: Distribution of recommended and approved applications by 2010 electorate type
|
Electorate type |
Shortlist of 21 applications |
Applications approved (12) |
||
|
Number |
Percentage |
Number |
Percentage |
|
|
ALP – marginal |
3 |
14 |
3 |
25 |
|
Coalition – marginal |
4 |
19 |
2 |
17 |
|
Coalition – safe/fairly safe |
11 |
52 |
5 |
42 |
|
Independent1 |
3 |
14 |
2 |
17 |
|
Total |
21 |
100 |
12 |
100 |
Source: ANAO analysis of information from Health and the Australian Electoral Commission
Note 1: The electorates in which the applicants were located were New England and Lyne, held at the time by independent members who made agreements with the Australian Labor Party to support the minority Government in confidence motions and supply bills.
4.82 The final distribution shows a reduction in the machines approved for the Coalition/Independent groupings but in the absence of documentation the basis for this reduction is not clear.
4.83 In summary, in documentation provided to applicants to frame their responses, applicants were provided with an expectation that their applications would be assessed on a particular basis. Better advice to applicants, consistent with the assessment process undertaken, would have assisted applicants by removing the need for responses to criteria that were not assessed. It would have also encouraged applicants to place more emphasis on responding to the ‘geographic distribution’ criteria and thus improving the opportunity for a secondary assessment. Furthermore, knowing the criteria for the secondary assessment, would have better informed applicants of the likelihood of their success, and may have resulted in fewer applications.
4.84 The primary assessment of applications for MRI machines in areas of need was not an efficient process, as it needed to be repeated using an alternative scoring system that was developed late in the process to replace the original scoring system. There were a number of shortcomings in the assessment and the selection process. These related to a narrow view in scoring the criteria that would not have been apparent to an applicant, and to poor recording of the scoring results. Nevertheless, the ANAO found that there is likely to have been little material difference in outcomes from the ITA process from these factors.
4.85 In the secondary assessment, the ANAO found that poor record-keeping limited the transparency of short-listing applications for Ministerial decision. Nevertheless, for all but one of the short-listed applications, there was either explicit or implied justification of their inclusion. In providing the short-list to the Minister, Health did not advise the Minister of the legal requirements relating to exercising the role of a financial approver, as provided for in Department of Finance guidance. Furthermore, advice from Health as to which 12 of the 21 machines in the short-listing represented best value for money would have assisted the Minister in undertaking the role of approver. Moreover, the basis for the selection approved by the Minister was not documented. While not mandated by the financial management framework, recording the basis for the approval would have improved the transparency of the decision-making process.
Expanding bulk billing incentives
4.86 From November 2009, the percentage of the schedule fee that providers were paid for bulk billing MRI services went from 85 per cent to 95 per cent. As part of the reform package, the bulk billing rate for MRI services went up a further 5 per cent to 100 per cent of the scheduled fee from 1 May 2012. This change was introduced by amending the Health Insurance (Diagnostic Imaging Services Table) Regulations 2011. The initiative was implemented within the timeframes of the Government’s Budget announcement.
4.87 The financial impact and effectiveness of this initiative and all other MRI access initiatives announced as part of the reform package are discussed in the following section.
Monitoring and reporting on the impact of MRI reform initiatives
4.88 The importance of monitoring the impact of the initiatives to improve MRI access was recognised in Health’s February 2011 advice to the Minister, on which the Government’s decision on the reform package was based. Health advised that it would monitor the proposals on an ongoing basis, through an in-depth analysis of Medicare data and extensive consultation with expert stakeholders and patient representatives. In addition, Health advised that it proposed to review these arrangements three years after the 2012 implementation to ensure patient access and affordability problems had been addressed.
4.89 As discussed in Chapter 2, Health did not implement on-going monitoring of the MRI initiatives and introduced only limited reporting. In the absence of this monitoring, the ANAO undertook some analysis to determine the effectiveness of the initiatives in enabling access to affordable and convenient services. In particular, the ANAO assessed:
- the financial impact of the MRI initiatives;
- the take-up of MRI requesting by GPs;
- the access to convenient MRI services through expansion of MBS-eligible MRI machines in regional and metropolitan areas; and
- the increase in affordability of MRI services from the expansion of the bulk billing incentive.
Financial impact of the MRI initiatives
4.90 Overall expenditure relating to the MRI initiatives, taking into account all elements, against forecast reform package Budget costings is outlined in Table 4.3.
Table 4.3: Reform package Budget costings versus expenditure associated with the initiatives to expand access to MBS-eligible MRI scans ($m)
|
|
Item |
2011–12 |
2012–13 |
2013–14 |
2014–15 |
Total |
|
Budget Costings |
Expansion of MBS-eligibility to MRI units incl. Bulk Billing extra incentive and GP-requested items for under 16 years1 |
0.6 |
5.0 |
6.6 |
6.8 |
19.0 |
|
GP-requested MBS-eligible MRI items 16+ years |
|
|
29.5 |
46.1 |
75.5 |
|
|
Total ($m) |
0.6 |
5.0 |
36.1 |
52.9 |
94.5 |
|
|
Expenditure |
GP-requested MBS-eligible MRI items under 16 years |
|
4.2 |
8.9 |
8.92 |
22.02 |
|
GP-requested MBS-eligible MRI items 16+ years |
|
|
56.4 |
84.62 |
141.02 |
|
|
Bulk-billing extra incentive3 |
1.3 |
9.0 |
10.1 |
10.12 |
30.52 |
|
|
Total ($m) |
1.3 |
13.2 |
75.4 |
103.62 |
193.52 |
|
|
Additional expenditure over Budget costings (per cent) |
117% |
164% |
109% |
96% |
105% |
|
Source: ANAO analysis of information from Health
Note 1: This costing was for the whole item, ‘expansion of MBS-eligibility to MRI units’; this item included:
GP-requesting of MRI scans for under 16 year olds; the expansion of MBS-eligible MRI machines; and the bulk billing incentive. Early departmental costings of GP-requested MRI scans for under 16 year olds was estimated at approximately $4.0 million for a full year in 2012–13.
Note 2: The 2014–15 expenditure figure is a conservative ANAO estimate, based on the (estimated) full year expenditure for 2013–14, with no growth included, and no change in bulk billing rates.
Note 3: The bulk-billing extra incentive excludes the contribution to expenditure from GP-requested items to prevent double counting.
4.91 Table 4.3 shows that expenditure related to expanding MRI access has significantly exceeded the costings prepared for the 2011–12 Budget in every year since the reform package was introduced, by between 96 and 164 per cent. That is, over the four years since 2011–12, expenditure has been around twice the amount provided for in Budget estimates. The ANAO has estimated that the MRI expansion initiatives will add more than $50 million per annum to government expenditure over the original Budget forecasts, on an ongoing basis, unless offsetting measures are implemented.195
4.92 In particular, by the end of 2013–14, the expenditure on GP-requested MRI items for under 16 year olds had exceeded Budget cost estimates for the whole of the Budget item ‘expansion of MBS-eligibility’ by approximately $1 million since implementation on 1 November 2012, and for those 16 years and over by $26.9 million (or 91 per cent) since the implementation on 1 November 2013. In addition, the bulk billing extra incentive had exceeded the whole of the Budget item ‘expansion of MBS-eligibility’ by $8.2 million (or 67 per cent) to the end of 2013–14. The figures suggest that each element of the MRI reforms has contributed to the growth in expenditure over Budget forecasts. In particular, there has been a stronger than expected take-up of requesting by GPs, especially for those 16 years and over, and of bulk billing with the extra incentive, as well as a fiscal impact from expanding the number of MBS-eligible MRI machines significantly over the number on which the Budget decision was based.
4.93 In approving the broader criteria for MBS-eligibility of MRI machines, the Minister did so on the basis that there would be no increase in expenditure over and above the Budget costings.196 Had Health monitored the expenditure early and on an on-going basis, it would have been in a position to advise successive Ministers of the impact to date against Budget costings of the initiatives. Such information is critical for decision-makers and should have been included by Health in seeking approval for applications for MRI-eligibility assessed in subsequent rounds.197
Take-up of MRI requesting by GPs
4.94 To assess the effect of MRI requesting by GPs, the ANAO examined the growth in MRI services and expenditure in the five years to the end of 2013–14, with and without the contribution of GP-requested services.
Figure 4.2: MRI services and expenditure requested by specialists/ consultant physicians and total, 2009–10 to 2013–14

Source: ANAO analysis from Health and DHS data.
Note: Services and expenditure ‘less GP-requested’ refer to those requests by specialists and consultant physicians.
4.95 Figure 4.2 shows that there has been a substantial increase in both MRI services and expenditure between 2012–13 and 2013–14 of 30 per cent. Most of this growth is attributable to the GP-requested services, particularly related to those aged 16 years and older, introduced in November 2013, and so has occurred over an eight month period rather than a full year. Once MRI services and expenditure are adjusted, to remove the effects of GP-requesting, there is a marginal fall in the rate of growth from specialist and consultant physician requesting over the period. Such a fall contributes only marginally to offset the significant growth in the number of requests and the cost overruns against Budget forecasts from the increase in GP-requesting of MRI scans.
4.96 During the audit, a specialist clinician, whose work relies on ‘appropriate requesting’ of diagnostic imaging, advised the ANAO that he was increasingly seeing patients who had MRI scans requested by their GP, when plain X-rays, available at a substantially lower cost, could have resulted in a similar or better diagnosis. The emergence of revised GP practices can result in significant growth in expenditure from GP-requested MRI scans; an outcome which the department’s planning and consultative arrangements, discussed in paragraphs 4.9 to 4.14, had sought to avoid.
Changes in requesting by GPs of CT scans for children
4.97 As the measure to allow GPs to request MRIs for patients under 16 years was partially aimed at minimising children’s exposure to ionising radiation when subject to a CT scan, the rate of growth in MRI services was expected to be offset by a decrease in the rate of growth of CT requesting. The ANAO therefore examined GP-requested CT and MRI services for children, to consider whether MRI requests were offset by a reduction in CT service requests, shown in Figure 4.3.
Figure 4.3: GP-requested diagnostic imaging services (CT and MRI) for children

Source: ANAO analysis of Department of Human Services (Medicare Australia) and Health data and an assessment (unpublished) undertaken by the Australian Commission on Safety and Quality in Health Care.
Note: While MRI services are for patients under 16 years, CT scan services are for patients under 15 years.
4.98 Figure 4.3 shows that estimated GP-requesting of CT scans for those under 15 years198 fell by 1050 services in 2012–13 over the previous year, and by 1627 services in 2013–14. In contrast, the number of GP-requested MRI services for those under 16 years was 10 354 in its first part year (2012–13), increasing to 21 842 in its first full year (2013–14). The ANAO’s analysis indicates that this measure contributed to relieving the risk of children’s radiation exposure through CT scans by improving access to MRI services and reducing the use of CT scans for this cohort. Further, the decrease in the number of CT scans partially off-set the $1 million overspend against the Budget estimate for the MRI item by approximately $600 000 for the period 2012–13 to 2013–14.
4.99 To date, Health has gathered data but not yet monitored and reported upon the take-up of GP-requesting of MRI scans, as they consider it premature given the length of time (less than 12 months) since some items were introduced.199 However, reviewing the impact of GP-requesting of MRI services for both patients under 16 years, and those aged 16 years and over is a key activity for Health planned in 2014–15. In summary, ANAO analysis indicates that the take-up of GP-requesting has significantly exceeded Budget forecasts, and has not been offset by the efficiencies expected from the take-up of ‘appropriate requesting’ (discussed in Chapter 3), but has succeeded to some extent in lessening children’s exposure to ionising radiation from CT scans. Furthermore, there are some concerns arising in the medical profession that MRI scans have replaced less expensive diagnostic imaging services, notwithstanding the cost-benefit and effectiveness of these scans.
Accessing convenient MRI services through expansion of MBS-eligible MRI machines
4.100 Extending MBS-eligibility to additional MRI machines has almost tripled the number of machines that are at least partially MBS-eligible, as shown in Table 4.4.
Table 4.4: MBS-eligibility of MRI machines: May 2011 to January 2015
|
Process |
Metro: full eligibility |
Metro: partial eligibility |
Regional: full eligibility |
Total |
|
Eligible prior to 10 May 2011 |
93 |
|
32 |
125 |
|
Registered with Medicare Australia prior to 10 May 2011 (i.e. known to be ‘operational’) |
|
57 |
8 |
65 |
|
Original expansion (applicable from 1/11/12) |
|
104 (-11) |
22 |
125 |
|
2nd round regional and metro expansion (December 2012) |
|
11 |
2 |
13 |
|
3rd round regional and metro expansion (November 2013) |
|
02 (+13) |
2 (-13) |
2 |
|
4th round regional and metro expansion (June 2014) |
|
9 |
1 |
10 |
|
ITA (applicable from 1/11/12) |
2 |
-24 |
|
0 |
|
ITA (applicable from 1/1/13) |
1 |
-14 |
1 |
1 |
|
ITA (applicable from 1/1/14) |
|
|
4 |
4 |
|
ITA (applicable from 1/1/15) |
|
|
4 |
4 |
|
Total |
96 |
178 |
75 |
349 |
|
Percentage increase to 1 January 2015 |
195% |
|
134% |
180% |
Source: ANAO analysis of Health data.
Note 1: One provider subsequently relinquished their partially eligible machine as it was decommissioned.
Note 2: One hospital provider in an outer metropolitan area relinquished their partial eligibility as they had assumed that they would be granted full eligibility.
Note 3: A previously approved fully eligible MRI machine was found subsequently to be in a metropolitan location and therefore only partially eligible.
Note 4: The three metropolitan machines that gained full eligibility had previously been granted partial eligibility.
4.101 As shown in Table 4.4, the reform package measure to extend MBS-eligibility for MRI has resulted in significant growth in the number of MBS-eligible MRI machines, from 125 fully eligible machines to 171 fully eligible. A further 178 partially eligible machines will be in operation by 1 January 2015, a total of 349 MBS-eligible MRI machines. By the beginning of 2015, there will be approximately 15.0 MRI machines per million people200, exceeding the most recent OECD average of 13.3 machines per million.201
Regional access to MRI services
4.102 The ANAO found that the MRI expansion initiative has increased the number of MRI machines per capita from 5.9 per million to 16.8 per million in metropolitan areas and from 4.8 per million to 11.0 per million in regional areas.
4.103 Since 2011, MBS-eligible MRI machines have increased by 181 machines (or 195 per cent) in metropolitan areas including those with partial eligibility, and by 43 machines (or 134 per cent) in regional areas. To assess the effect on regional access to services, the ANAO compared the number of MBS-eligible MRI services by geographic location of service provider, in the three months leading up to 1 November 2012, the time when MRI machines operating as at 10 May 2011 became MBS-eligible; as compared with the three months to 30 June 2014, once increased eligibility was established. The results are shown in Table 4.5.
Table 4.5: Metropolitan and regional MBS-eligible MRI services: August-October 2012 and April-June 2014 by region
|
Period |
Metro (ASGC-RA1) |
Regional (ASGC-RA2 to RA5) |
Total |
|
August to October 2012 |
134 628 |
25 551 |
160 179 |
|
April to June 2014 |
188 868 |
43 469 |
232 337 |
|
Percentage increase |
40% |
70% |
45% |
Source: ANAO analysis of Health data.
4.104 Table 4.5 shows that the number of MRI services increased 45 per cent overall between the period immediately prior to implementing the expansion of MRI services to the final quarter of 2013–14, with a 70 per cent increase in services delivered in regional areas. The initiative to expand MRI services has had an impact in making the accessibility of MRI services more convenient for patients in regional areas by improving the geographic distribution of MRI machines.
Expanding the bulk-billing incentive
4.105 To assess the effectiveness of this initiative in increasing the affordability of MRI services, the ANAO examined the percentage of MRI services receiving an MBS payment that had been bulk billed (that is, the MRI bulk billing rate) for the period 2009–10 to 2013–14 (see Figure 4.4).
Figure 4.4: Bulk billing rates for MRI services for the period 2009–2010 to 2013–14

Source: ANAO analysis of Health data.
4.106 Figure 4.4 shows that in the two years following the introduction of the 95 per cent bulk billing rebate, the bulk billing rate for MRI services rose an average of four per cent per annum. In the two full years since the introduction of the 100 per cent bulk billing rebate, the bulk billing rate for MRI services increased by an average of 3.5 per cent per annum. Overall, the bulk billing rate increased from 62 per cent of MRI services to 75 per cent over five years.202 In 2009–10 the number of bulk-billed MRI services was 317 000, rising to 622 000 by 2013–14, an increase of 96 per cent over the period. The substantial increase in bulk billing rates indicates that the 95 per cent and 100 per cent rebates made MRI services more affordable for patients, a key objective of this initiative.
4.107 In summary, the bulk billing extra incentive of five per cent improved take-up rates indicating that requesters and providers were aware of and took advantage of the incentive. The bulk billing incentive (rising from 85 to 100 percent between November 2009 and May 2012) has resulted in a significant increase in the number of services bulk billed, reflecting the improved affordability of MRI services. However, as noted earlier203, the cost of the bulk billing extra incentive has well exceeded Budget forecasts.
4.108 The ANAO notes that in the 2014–15 Budget, the new Government announced that the bulk billing incentive for diagnostic imaging services will cease for general and concessional patients and that a ‘Low Gap Incentive’ for concessional patients will be introduced instead.204
Conclusion
4.109 To date, the MRI components of the reform package have been the department’s primary focus. Key initiatives have included: expanding the number of MRI machines with full or partial MBS-eligibility; standardising the conditions of those MRI machines that were already eligible; allowing GPs to request particular MRI items on the MBS; and providing additional incentives for diagnostic imaging providers to bulk bill for MRI services.
4.110 Health has implemented each of the MRI expansion initiatives in line with set dates. The total number of MBS-eligible MRI services was 829 000 in 2013–14, an increase of approximately 238 000 services (or 40 per cent) since 2011–12. In addition, the number of MRI machines has grown from amongst the lowest per capita in the OECD to above the OECD average. There has also been significant growth in the provision of services outside capital cities, with the number of MRI machines per capita more than doubling over a three year period. These initiatives have improved overall patient access to convenient MRI services. Further, MRI was made more affordable for patients overall, with the number of bulk billed MRI services almost doubling since 2009–10.205
4.111 The improvement in access to affordable and convenient MRI services has been achieved at a substantial cost to the Commonwealth Budget, with expenditure between 2011–12 and 2014–15 on these initiatives around twice the amount provided for in Budget estimates. Allowing GPs to request particular MBS-funded MRI services has been the biggest driver of growth in MRI services and expenditure.206 There is little doubt that an additional factor, acknowledged by Health as contributing to the unforeseen increase in expenditure, has been the significant growth in the number of MRI machines; with almost three times more machines approved for MBS-eligibility than originally estimated.
4.112 Some 125 MRI machines were MBS-eligible before the 2011–12 reform package was announced. The then Government’s decisions on the package were based on a Health estimate that this number would increase by 83 machines, bringing the total to 208 machines by 2014–15. In the event, 349 MRI machines are expected to become fully or partially MBS-eligible by January 2015. The department advised that its ability to consult with industry and develop an accurate estimate had been constrained by the need to avoid disclosing government policy intentions before the 2011–12 Budget207, and it relied instead on available information and DHS data on the number of registered MRI machines.
4.113 The limitations of the estimate became evident following the Minister’s announcement in the 2011–12 Budget context that 65 MRI machines would gain MBS-eligibility. Health subsequently received numerous requests from providers seeking information on the eligibility of other machines, and advised the Minister that an extra 139 machines would potentially qualify for full or partial MBS-eligibility, in addition to the 65 already announced. In November 2011, Health also recommended that the Minister broaden the eligibility criteria for approving MBS-eligibility208, in the belief that overall demand for MRI services and MBS expenditure would not be affected by making additional machines MBS-eligible. On the basis of this advice in November 2011, the Minister approved the inclusion of newly identified MRI machines. The department’s November 2011 advice to the Minister contrasted with advice provided to the Minister in February 2011, which had noted that limiting the number of MBS-eligible machines had been an approach adopted to contain growth in Medicare expenditure. Health advised the ANAO during the course of the audit that it considers GP-requestors209 are the key driver for MRI services, but also acknowledged that the availability of additional MRI machines is a factor.
4.114 As discussed, Health advised the ANAO of the need to avoid signalling government Budget intentions in its development of estimates of MRI machines likely to achieve MBS-eligibility. While this approach reflected the experience of an MRI expansion process conducted in 1998, other aspects of the most recent MRI expansion did not have full regard to an ANAO performance audit of the earlier process210, which highlighted the importance of effective risk management, review of documentation and cross-checking of applications.211 At the planning stage for the recent MRI expansion initiative, a key risk was the development of robust budget estimates; and at the implementation stage, Health’s assessment plan did not identify risks to be managed in the assessment of applications, provide detailed guidance to help assessors determine whether applicants met eligibility criteria, or require cross-checking with other stakeholders on the accuracy of information supplied by applicants.212 The ANAO’s review of a sample of applications indicated that half213 of the planned machines that were approved were not supported by documentation, other than a statutory declaration, demonstrating that applicants had made a financial investment in a planned MRI machine.214
4.115 In addition, Health undertook a competitive, invitation-to-apply (ITA) process for MBS-eligibility to identify 12 applicants seeking to operate MRI machines in areas of need.215 The primary assessment process was not efficient, as it was re-run using an alternative scoring system developed late in the process to replace the original scoring system, which was found to have shortcomings. The ANAO’s review indicated that there was likely to have been little material difference in outcomes from the ITA process arising from the re-run of the primary assessment phase. However, the ANAO’s review of the secondary assessment phase indicated that limited information was provided by Health to the Minister on the 21 short-listed applicants to assist the Minister in selecting 12 machines on value for money grounds. In addition, the basis for the Minister’s selection was not documented. These factors limited the transparency of the decision-making process. Further, the department did not advise the Minister of the legal requirements applying to her in approving commitments of public money. The department’s experience in administering the 1998 MRI expansion process, which had demonstrated shortcomings, suggests that greater care should have been taken in these key aspects of the recent MRI expansion process.
5. Reforms to Enhance Quality and Safety in Diagnostic Imaging
This chapter examines the status and progress of Health’s implementation of the reform initiatives that aim to enhance quality and safety in diagnostic imaging, in particular through the Diagnostic Imaging Accreditation Scheme (DIAS).
5.1 The reform package contained a number of initiatives that were intended to improve the quality and safety of diagnostic imaging and stated that these would be progressed through enhancing the Diagnostic Imaging Accreditation Scheme (DIAS). This chapter examines the extent to which Health has implemented the quality and safety-related initiatives of the reform package.
The Diagnostic Imaging Accreditation Scheme
5.2 The DIAS has been recognised by successive governments as an important means of ‘ensuring that Medicare funding is directed to diagnostic imaging services that are safe, effective and responsive to the needs of healthcare consumers’.216 The main quality and safety risks relevant to diagnostic imaging are summarised in Table 5.1.
Table 5.1: Diagnostic imaging safety and quality risks
|
Safety risks in diagnostic imaging relate primarily to radiation and infection:
Quality risks in diagnostic imaging relate primarily to poor image quality, reporting accuracy, and patient information:
|
Source: ANAO analysis of Health documents, reform initiatives and DIAS standards.
Note 1: Ionising radiation can damage living tissue. Radioisotopes – radioactive variants of an element – are used in the medical sector for diagnostic and therapeutic purposes. Radiopharmaceuticals and X-ray are used to diagnose and treat diseases such as cancer. Exposure to radiation during medical procedures represents the largest non-natural radiation exposure to the Australian population. Further explanation of the benefits and risks associated with the use of radiation was outlined in ANAO Audit Report No. 29, 2013–14, Regulation of Commonwealth Radiation and Nuclear Activities. In particular see paragraphs 1.5 to 1.9.
5.3 It is a requirement for medical practices to be accredited under the DIAS in order to provide MBS-eligible diagnostic imaging services. Health advised the ANAO that the DIAS was designed to leverage off existing regulatory regimes by allowing licenses issued under those regimes to be used as evidence for accreditation in a consolidated and streamlined way.217 If effectively implemented, such an approach is consistent with better regulatory practice.
5.4 The DIAS was established by the Australian Government in 2007218 and introduced progressively in two stages219, to ensure that diagnostic imaging providers ‘had ample time to prepare for accreditation and to enable the continuation of Medicare benefits during the transition’ to the DIAS.220 Practices that are not accredited under the DIAS must inform patients prior to performing a procedure that the procedure is not MBS-eligible.
5.5 Health administers the DIAS and works in close cooperation with stakeholders including medical colleges and industry through the DIAS Monitoring and Implementation Committee (DIAS-MIC). The DIAS-MIC meets approximately two to three times per year to examine the effectiveness and progress of the DIAS and to provide advice and feedback to Health on implementation matters and review of existing and future standards.
5.6 During the staged introduction of the DIAS there were different timeframes for providers, to receive either ‘entry level accreditation’ against three standards or accreditation against the full suite of 15 standards. Table 5.2 shows the full suite of DIAS standards.
Table 5.2: The Diagnostic Imaging Accreditation Scheme Standards
|
Accreditation standard coverage |
|
Part 1: Organisational Standards
|
|
Part 2: Pre-procedure Standards
|
|
Part 3: Procedure Standards
|
|
Part 4: Procedure Standards
|
Source: Health Insurance (Diagnostic Imaging Accreditation) Instrument Schedule 2010; and Department of Health and Ageing, Diagnostic Imaging Accreditation Scheme: User Guide for Practices Applying for Accreditation, February 2012, p. 1.
5.7 For a diagnostic imaging practice to receive full accreditation under the DIAS, a practice must undergo an assessment by one of three approved accreditors221 against 15 accreditation practice standards every four years.
5.8 In addition, a voluntary accreditation program established by the Royal Australian and New Zealand College of Radiologists (RANZCR), known as the Medical Imaging Accreditation Program (MIAP), is recognised under the DIAS.222 The recognition provides MIAP-registered practices full DIAS accreditation.
5.9 As at 30 June 2014, 4008 diagnostic imaging practices were accredited under the DIAS223, with 3873 accredited against the full suite of standards, 59 against entry level standards, and 76 accredited under MIAP.
Quality and safety concerns in Australian diagnostic imaging identified in the funding review
5.10 The funding review identified several areas where quality and safety in diagnostic imaging could be improved. In particular, the funding review identified that there are: no minimum training requirements for non-radiologist specialists performing Medicare-eligible ultrasound services; no rules or standards around radiation dose; no minimum requirements for digital imaging and information sharing; and differences in interpretation of requirements for professional supervision, such as instances of CT being provided where there is no radiologist on site.224 As discussed in Chapter 4, concerns over radiation exposure, particularly in children, was a substantial driver in the reform package for improving access to MRI services.225
5.11 To address these concerns, the then Government agreed that as part of the reform package the DIAS would be strengthened by introducing more stringent quality and safety standards. The key focus areas, as identified by the funding review, related to:
- practitioner credentialing;
- practice and patient safety;
- professional supervision requirements;
- management of patient radiation dose; and
- other areas that impact on the delivery of a safe and quality service.226
5.12 Consistent with the approach proposed by the funding review, the introduction of more stringent quality standards through the DIAS was intended to:
Ensure that each diagnostic imaging service reflects good clinical practice, is performed by an appropriately qualified practitioner and is provided within a facility which meets all necessary accreditation standards.227
5.13 In addition, the reform package stated that Health would review current diagnostic imaging legislation and the merits of moving quality and safety requirements into the DIAS to ensure consistency across different types of diagnostic imaging services and providers.228
5.14 The reform package did not provide specific timeframes within the reform period (2011–2016) for completion of the initiatives related to quality and safety enhancements.
5.15 To examine Health’s effectiveness in administering those parts of the reform package related to quality and safety, the ANAO examined the extent to which implementation of initiatives outlined above has occurred229 as well as the level of assurance that Health obtains from the DIAS accreditation processes.
Strengthening the DIAS
Implementation of reform initiatives to enhance the DIAS
5.16 As discussed in Chapter 2, Health has not developed an overall implementation plan for the reform package. While a small number of activities to strengthen the DIAS have been outlined and allocated to staff in annual team work plans, Health has not prepared a project-level operational plan to inform implementation. Operational plans can usefully outline key activities and priorities, timelines for delivery, and project-level monitoring and reporting arrangements.
5.17 In the absence of specific arrangements for monitoring progress with implementation, the ANAO has examined the status and progress of the reform initiatives relating to enhancing the DIAS. Specific areas that have been progressed relate to practitioner credentialing, reviewing requirements for supervision and management of patient radiation dose. Tangible improvements to practice and patient safety in other areas are yet to be observed.
Practitioner credentialing
5.18 Practitioner credentialing relates to the technical and educational qualifications and skills that are held by professionals performing diagnostic imaging services. Appropriate credentialing is important because it impacts on the quality of the images obtained, and therefore the diagnostic potential of the images.
5.19 The funding review identified that there are no minimum training requirements for non-radiologist specialists providing Medicare-eligible ultrasound services. The reform package stated that Health would work with professional bodies to ensure that appropriate credentialing schemes or essential educational requirements for each diagnostic imaging modality were implemented and that ultrasound would be the first modality reviewed.
5.20 To date, Health has not yet introduced more stringent credentialing requirements for providers of ultrasound services, although work has commenced to progress this initiative. Heath funded, through the Diagnostic Imaging Quality Program (DIQP), the Australasian Society for Ultrasound in Medicine (ASUM) to develop minimum qualification requirements for the practice of point-of-care ultrasound by practitioners who are not imaging specialists, such as cardiologists, obstetricians and gynaecologists, vascular surgeons and urologists. The ASUM identified suitable qualification requirements and a curriculum as well as an e-learning platform for online distance delivery. The project has been completed and recommendations were provided to Health in January 2014.
5.21 In addition, Health introduced the Strengthening the Provision of Quality Diagnostic Radiology Services initiative on 1 November 2012. This was implemented through a change in the regulations230 and requires those performing diagnostic imaging procedures to hold minimum qualifications for all MBS-funded X-ray, angiography and fluoroscopy services. Those minimum qualifications are determined by the registration requirements of the relevant medical board of the Australian Health Practitioner Regulation Agency (AHPRA).231
5.22 In summary, there has been some progress to date to improve the qualifications and training requirements of providers of some types of diagnostic imaging services. However, in the absence of an implementation plan and stated timeframes for review of all other modalities, it is not clear whether Health intends to undertake any further activities related to this initiative, or the priority of this work relative to other reform initiatives.
Professional supervision requirements
5.23 An appropriate level of supervision is important to ensure that diagnostic imaging services are of high quality and are safe and appropriate for the patient. The funding review identified that while the diagnostic imaging regulations232 define requirements around professional supervision for each type of diagnostic imaging service, concern was expressed by a key stakeholder as to differences in their interpretation. One such example related to comprehensive diagnostic imaging practices providing CT services in metropolitan areas without a radiologist on site.233
5.24 Health has reviewed, with stakeholder input through the DIAC and directly with the radiology sector, the diagnostic imaging regulations. Health has identified that the regulations do not clearly state the level of supervision that is required for diagnostic imaging services including CT, ultrasound, mammography and MRI services. Stakeholders have estimated that around 80 diagnostic imaging providers in metropolitan areas do not have a radiologist on site when performing CT services, arguing that some providers are putting patient safety at risk by exploiting a loophole in the current legislation to gain a competitive advantage by not employing an on-site radiologist and continuing to provide CT and ultrasound services.
5.25 Health is preparing a draft ‘options-stage’ regulation impact statement (RIS) which addresses these matters. The RIS identifies a number of options to improve supervision, including applying practice-based supervision requirements rather than defining supervision for individual services in the regulations. Depending on the outcome of consultation and Government decisions on the proposals in the RIS, there may be amendments to the relevant regulations, to remove the current ambiguity in relation to supervision.234
Management of patient radiation dose
5.26 Based on work by the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA), the funding review identified that there were no rules or standards around radiation dose.235
5.27 ARPANSA subsequently developed in 2012–13 national radiation diagnostic reference dose levels (reference levels) for CT scans and guidance about cancer risks for children from CT scans.236 The reference levels must be adopted by various standards and accreditation programs, including the DIAS and MIAP. The incorporation of the reference levels into the DIAS and MIAP was achieved indirectly and without change to those programs through pre-existing requirements for compliance with state and territory radiation regulation, which in turn requires compliance with the ARPANSA Code of Practice.237
5.28 Health relies on the DIAS accreditation process to ensure that MBS funding is directed to diagnostic imaging services that are being delivered in accordance with the national radiation dose reference levels. However, the level of assurance provided to Health through the DIAS is not strong, as discussed later in this chapter. Furthermore, the better practice identified by ARPANSA in relation to monitoring compliance of a sample of patient radiation doses against reference levels238 has not been included as part of the DIAS accreditation process and there are no stated plans by Health to do so.
5.29 The development of reference levels and guidance for referrers and parents on risks from CT scans for children was an important achievement to address long-identified concerns239 about cancer risks from exposure to ionising radiation in medical procedures. A further and related initiative being undertaken by Health, on the advice of the Chief Medical Officer240, was approved in the 2013–14 Budget and is currently in the scoping stage.241 The project has the potential to provide Health with valuable baseline data and identify monitoring indicators. However it is unlikely, on its own and without an ongoing structured approach to monitoring and reporting, to provide Health with information as to whether the implementation of reform and other initiatives to reduce patient radiation exposure from diagnostic imaging services has been effective at the practice level and whether any further ongoing actions are required, for example through education and awareness raising.
Practice and patient safety
5.30 A range of other initiatives were undertaken by Health during the reform period that relate to enhancing the DIAS. The ANAO identified that progress has been made, although changes have not yet been implemented, in relation to reviewing ultrasound accreditation arrangements and equipment standards, reviewing DIAS standards, including for alignment with the National Safety and Quality Health Service (NSQHS) Standards242 and progressing digital imaging standards.
5.31 Health proposed in 2014 (in consultation with the DIAC) the introduction of an ultrasound-only accreditation scheme. This was in response to some ultrasound-only providers finding the current DIAS accreditation standards somewhat ‘irrelevant’ and ‘burdensome’. In addition, Health has proposed the introduction of an ultrasound equipment DIAS standard. This is to address a concern that the increasing availability of cheap, portable ultrasound machines has resulted in the production of images not of a suitable diagnostic quality. Consultation on these initiatives is in progress at the date of this audit through the RIS process, discussed at paragraph 5.25.
5.32 Health has undertaken a review of the wording and coverage of current DIAS standards in close consultation with key professional, industry and consumer stakeholders through the DIAS-MIC. The views of DIAS accreditors have also been taken into account. In addition, Health has commenced reviewing DIAS alignment with the NSQHS Standards and is engaging in discussions with the Australian Commission on Safety and Quality in Healthcare, which has responsibility for the NSQHS Standards. Health has identified that minor changes to the DIAS standards are likely to be implemented in the first half of 2015, with major changes such as the NSQHS Standards alignment and implementation of ultrasound-only accreditation standards to be implemented in the second half of 2015.243
5.33 The funding review also identified that the diagnostic imaging regulations and DIAS do not prescribe any minimum requirements around digital imaging and information sharing. The funding review considered that the continued development and implementation of standards244 has the potential to further improve the quality of diagnostic imaging, noting that the take-up and evolution of e-health would need to be underpinned by appropriate standards to ensure that quality is maintained.245 During the reform period, Health has reviewed and considered digital imaging standards through the DIAC in consultation with the DIAS-MIC.246 At the time of this audit, Health was considering, in consultation with stakeholders, whether a new technical imaging standard247 will be adopted into the DIAS standards.
Reviewing legislation – regulations versus standards
Review of diagnostic imaging legislation
5.34 The reform package specified that Health would review the diagnostic imaging legislation and consider the merits of moving quality and safety requirements into the DIAS; intended to address complexity and ambiguity in the regulations that was identified during the review. In the funding review, Health noted that most of the standards and rules relating to quality and safety in the provision of MBS-eligible diagnostic imaging services were outlined in regulation through the Diagnostic Imaging Services Table (DIST), rather than in the DIAS. Health noted that, as a result, enforcement and compliance monitoring of quality and safety aspects of diagnostic imaging services has proven difficult because of the complexity and in some instances the ambiguity of the regulations.248
5.35 Health has reviewed, with stakeholder input through the DIAC and directly with the radiology sector, the diagnostic imaging regulations. This review identified in particular that the existing regulations do not consistently specify who can assist in the performance of an MBS-funded diagnostic imaging procedure or the qualification requirements for ultrasound, and that the regulations do not clearly state the level of supervision that is required. As outlined at paragraph 5.25, Health is preparing a draft ‘options-stage’ RIS which addresses these matters. Depending on the outcome of consultation and Government decisions on the proposals in the RIS, there may be amendments to the relevant regulations to move aspects of quality that are currently specified in the regulations into the DIAS. Should this occur, the process for assuring compliance with the DIAS standards would become of increased importance.
Assurance on DIAS compliance and the quality of accreditation processes
5.36 The type of accreditation process adopted by a regulatory scheme determines the nature and quality of evidence that is gathered to support the assessment result. This in turn determines the level of assurance that can be obtained from the regulatory scheme regarding the extent to which standards have been met. The level of assurance is also influenced by the consistency of assessments undertaken as part of the accreditation process.
5.37 In the funding review, Health acknowledged that the DIAS was ‘in an embryonic form’ and ‘cannot provide robust quality assurance’.249 However, the DIAS was considered an appropriate means to further stipulate quality and safety standards as it allowed involvement from the diagnostic imaging sector and could accommodate changes in clinical practice.250
5.38 The type of accreditation process that Health has chosen for the DIAS is a desktop assessment251 (also known as a desk-based review) every four years. Diagnostic imaging practices select an approved accreditor who undertakes a desktop assessment of documentation provided by the practice. Documentary evidence requirements are specified in the standards252 and include policies and procedures, copies of practitioner registration documentation or registration numbers that are verifiable to a publicly available register, equipment licences and service records, radiation safety plans and samples of requests documenting clinical need for an imaging procedure and samples of consumer information available at the practice.253
5.39 Health and accreditors interviewed during the audit advised the ANAO that, to their recollection, all diagnostic imaging practices that have sought accreditation have passed. However, the process will often involve further requests for information.254 Approximately 1000 practices, including many obstetric and gynaecology ultrasound-only practices have opted-out of the DIAS because they consider it too burdensome for the level of diagnostic imaging services they provide as part of their practice. The number of practices opting-out from the scheme was not foreseen by Health during the DIAS’ establishment, and the ultrasound-only accreditation standards discussed in paragraph 5.31 are aimed at bringing such practices into the scheme.
5.40 Health and stakeholders, including accreditors, have highlighted to the ANAO the benefits arising since 2008 from implementing DIAS accreditation. The main benefits are considered to be raised awareness among diagnostic imaging practices of quality and safety issues. Accreditors have also noted an improvement in the ability of practices to provide compliant documentation since the scheme’s introduction.
5.41 The accreditors approved under the DIAS also undertake accreditation assessments for other programs in the health care sector, including for the NSQHS Standards, RANZCR’s MIAP and Health’s more established pathology accreditation program.255 On the basis of their experience with other accreditation schemes, DIAS accreditors advised the ANAO that there are inherent limitations in a process based on desktop assessment. In particular, without onsite inspections or the ability to undertake targeted phone calls with a range of practice staff, it is not possible to determine whether the policy and procedure documents provided for assessment, such as those related to infection control, are actually being implemented in the practice. One accreditor advised that:
Without an on-site visit to determine whether a practice is implementing the procedures as advised to the accreditor, the positive impacts of the Scheme on safety and quality cannot be determined.
5.42 Health and stakeholders have stated that the key reason for not conducting on-site inspections is the additional cost for practices, particularly those in rural and regional areas. Health advised the ANAO that in order to encourage practices to initially join the scheme, it designed the DIAS to have a phased introduction and an accreditation process that involved less cost and regulatory burden for diagnostic imaging practices. Health further advised the ANAO that the department is only one of the heath care participants concerned with quality and safety in diagnostic imaging and that others, including the professional colleges and other regulatory agencies, have an important role.
5.43 Nevertheless, the appropriateness of the existing accreditation approach is currently being discussed by Health and its stakeholders through the DIAS-MIC. In particular, Health is considering whether on-site visits and a shorter accreditation cycle or mid-term assessments against some standards, such as infection control, is appropriate for the DIAS.256 Health has advised the ANAO of its intention that the DIAS accreditation process be strengthened over time, and that:
While the pathology accreditation scheme is viewed as a key benchmark for the healthcare sector, and it can be used for guidance, the form and timing of changes to DIAS will depend upon balancing the costs and benefits. The changes will be tailored to suit diagnostic imaging, in consultation with stakeholders and will not necessarily follow pathology in terms of application and timeframe.
5.44 In the absence of a program of risk-based on-site inspections or targeted phone interviews by accreditors – to verify whether documented policies and procedures are being implemented by diagnostic imaging practices – the current implementation of the DIAS provides only marginally more assurance than a self-assessment process. Self-assessment processes have inherent limitations with respect to the level of assurance they can provide on adherence to a regulatory framework.
5.45 To monitor the quality and consistency of assessments by accreditors, Health receives annual reports from the three approved accreditors, outlining whether they have complied with their contractual requirements and notifying Health of issues found in relation to systemic non-compliance by practices or challenges in the interpretation of standards by practices or assessors during the previous year. These reports are of varying detail and quality. Accreditors attend the DIAS-MIC and answer questions of Health and other stakeholders when their annual reports are tabled in that forum. In addition, Health has commissioned three research reports to assess the impacts and perceptions of the DIAS on accredited practices.
5.46 Achieving consistency between accreditation providers was identified as a continuing risk by one accreditor, who commented to the ANAO that:
There is no mechanism in place to ensure that all three accreditors are assessing material to the same standard. The introduction of the accreditation scheme was to ensure that Medicare funds were being used appropriately. How can this be assured when the standard for achieving accreditation varies between the accreditors?
5.47 Health could provide additional assurance on the consistency of accreditation assessments across the three approved accreditors, and of the quality of the accreditation process overall, by reviewing and testing a sample of assessments from each accreditor. A quality assurance process could focus on whether accreditors’ decisions are adequately supported by sufficient and appropriate evidence and that evidence is being assessed in a consistent manner.
Conclusion
5.48 To date, parts of two reform initiatives relating to improving quality and safety in diagnostic imaging have been implemented. Amendments to the diagnostic imaging regulations have strengthened minimum qualification requirements for diagnostic radiology257 and guidance has been developed relating to safe dosage levels for CT scans, including for children. However, the department has not established ongoing monitoring and evaluation activities to enable it to demonstrate whether these initiatives have been effective in reducing radiation and other risks.
5.49 Health has also commenced work on a number of other initiatives, including a review of supervisory requirements for diagnostic imaging, whose successful implementation will depend on the outcomes of stakeholder consultation and Ministerial consideration.258 A key initiative in this category is the implementation of enhancements to the DIAS accreditation approach, which Health has acknowledged was ‘in its infancy and cannot provide robust quality assurance’. At present, the accreditation process does not include risk-based on-site inspections or targeted phone interviews by accreditors to verify whether documented policies and procedures are being implemented by diagnostic imaging practices; and in consequence the current implementation of the DIAS provides only marginally more assurance than a self-assessment process.259 The department could provide additional assurance – on the consistency of accreditation assessments by the three approved accreditors, and the quality of the accreditation process overall – by reviewing and testing a sample of assessments from each accreditor. There is also scope to introduce a quality assurance process focusing on whether accreditors’ decisions are adequately supported by sufficient and appropriate evidence and that evidence is being assessed in a consistent manner.
5.50 The appropriateness of the existing accreditation approach is currently being discussed by Health and its stakeholders through the DIAS-MIC, and Health has advised the ANAO of its intention that the DIAS accreditation process be strengthened over time. In particular, Health is considering whether on-site visits and a shorter accreditation cycle or mid-term assessments against some standards, such as infection control, is appropriate for the DIAS.
Appendices
Appendices
Please refer to the attached PDF for the Appendices:
- Appendix 1: Entity Responses
- Appendix 2: MBS Diagnostic Imaging Service and Expenditure Growth by Financial Year
- Appendix 3: MBS Diagnostic Imaging Services and Value of Benefits by Provider Type 2013–14
- Appendix 4: Medicare Services and Expenditure by Diagnostic Imaging Modality by Financial Year
Abbreviations and Glossary
|
‘appropriate requesting’ |
Requests by medical practitioners for diagnostic imaging services that are clinically appropriate and cost-effective. |
|
ASGC–RA |
A geographic classification system that was developed in 2011 by the Australian Bureau of Statistics (ABS) as a statistical geography structure which allows quantitative comparisons between ‘city’ and ‘country’ Australia. |
|
Bulk billing |
When a medical services provider bills Medicare directly for any medical or allied health service that the patient receives. The provider accepts the relevant Medicare benefit as a full payment for the service and the patient assigns their right to a Medicare benefit to the service provider, allowing the benefit to be paid directly to the provider. |
|
CT |
Computed tomography |
|
DHS |
Department of Human Services |
|
DIAC |
Diagnostic Imaging Advisory Committee |
|
DIAS |
Diagnostic Imaging Accreditation Scheme |
|
DIQP |
Diagnostic Imaging Quality Program |
|
DIRCC |
Diagnostic Imaging Review Consultation Committee |
|
DIST |
Diagnostic Imaging Services Table |
|
Fully MBS-eligible MRI |
Fully MBS–eligible MRI machines are those that attract MBS funding for all MBS–eligible MRI services performed on the machine (full eligibility). |
|
GP |
General practitioner |
|
Health |
Australian Government Department of Health |
|
MBS |
The Medicare Benefits Schedule (published by Health) lists the services that are subsidised by the Australian Government under Medicare. |
|
Medicare |
Australia’s universal healthcare system which provides people with access to free or subsidised health and hospital care. |
|
MIC |
Monitoring and Implementation Committee for the DIAS |
|
MRI |
Magnetic resonance imaging |
|
MSAC |
Medical Services Advisory Committee |
|
Partially MBS-eligible MRI |
Partially MBS–eligible MRI machines are those that attract MBS funding for a limited number of services performed on the machine, including GP-requested MRI services and for MBS MRI items for the staging of rectal and cervical cancers and breast screening for women aged under 50 years (partial eligibility). |
|
PET |
Positron emission tomography |
|
Provider |
A medical practitioner who renders, or carries on a business that renders, diagnostic imaging services. |
|
RACGP |
Royal Australian College of General Practitioners |
|
RANZCR |
Royal Australian and New Zealand College of Radiologists |
|
Schedule fee |
A ‘schedule fee’ is the amount pertaining to a particular service on the MBS. |
|
The funding review |
Review of Funding for Diagnostic Imaging Services (undertaken in 2010–11, final report published November 2011). |
|
The reform package |
Diagnostic Imaging Review Reform Package (announced 10 May 2011, as outlined in pp. 23–26 of the funding review report). |
|
X-ray |
Diagnostic radiography |
Footnotes
1 Medicare is Australia’s universal healthcare system which provides people with access to free or subsidised health and hospital care.
2 Department of Health and Ageing, Medical Benefits Reviews Task Group, Review of Funding for Diagnostic Imaging Services, final report November 2011 (‘the funding review’) p. 4, available from <http://www.ranzcr.edu.au/> [accessed 6 June 2014] and ANAO analysis for data from 2010–11 to 2013–14 at Appendix 2, Table A.1.
3 Successive Budget Papers for the Health portfolio have discussed these matters, see for example, Australian Government, Portfolio Budget Statements 2009–10, Health and Ageing portfolio, Budget related paper 1.10, Commonwealth of Australia, Canberra, p. 119 and Australian Government, Portfolio Budget Statements 2014–15, Health and Ageing portfolio, Budget related paper 1.10, Commonwealth of Australia, Canberra, p. 79.
4 The MBS lists the services that are subsidised by the Australian Government under Medicare and the schedule fee.
5 The objectives and specific measures agreed by Ministers in the 2011–12 Budget context were reproduced in the publicly released funding review report, ‘Outcomes of the Review of Funding for Diagnostic Imaging Services’ (‘the reform package’), p. 23.
6 The DIST is the part of the MBS that specifies the diagnostic imaging services that will be subsidised by the Australian Government, the schedule fee and the requirements to be met in order for services to be MBS-eligible.
7 Since 2008, accreditation under the DIAS has been required for the payment of Medicare benefits for diagnostic imaging services.
8 The funding review, p. 21.
9 ANAO analysis of Health data at Appendix 4: ‘Medicare services and expenditure by diagnostic imaging modality by financial year’.
10 ANAO Audit Report No.42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation.
11 That audit examined accusations that some persons placing orders for machines in the period preceding the Budget had access to information to be announced in the Budget, thus providing those persons with a significant financial advantage (p. 11).
12 $94.5 million was provided under the reform package over four years from 2011–12 for MBS expenditure related to expanded patient access to Medicare–funded MRI services.
13 Some 15 per cent of all Medicare outlays in 2014–15, approximately $3.1 billion, are related to diagnostic imaging services. The average annual growth of diagnostic imaging expenditure over the three years to 2013–14 of 9.3 per cent exceeds the Australian Government’s general receipts growth of 6.3 per cent over the same period.
14 ‘Appropriate requesting’ for diagnostic imaging services has the potential to contribute to Medicare sustainability by reducing the number of unnecessary, harmful or wasteful diagnostic imaging services requested by health professionals.
15 While Health estimated that an additional 83 MRI machines would receive government funding, 224 additional machines were approved. See footnote 22.
16 The ANAO has estimated that actual expenditure between 2011–12 and 2014–15 associated with the introduction of initiatives to allow GP-requesting of MRI services and the bulk billing extra incentive may reach $193.5 million. See Table 4.3.
17 As at mid-October 2014, the department had not prepared an overall implementation plan for the reform package.
18 The ANAO has estimated that the MRI expansion initiatives will add more than $50 million per annum to government expenditure over the original Budget forecasts, on an ongoing basis, unless the reform measures expected to help offset the cost of MRI expansion are implemented. See Table 4.3.
19 Under the reform package, the number of MRI machines in Australia has grown from amongst the lowest in the Organisation for Economic Co-operation and Development (OECD) to above the OECD average, including a significant expansion in non-metropolitan areas. Between 2012–13 and 2013–14, the number of MBS-funded MRI scans increased by 190 655—from 638 064 to 828 719 scans
20 The MRI expansion initiative was intended to improve access to convenient MRI services and enhance patient health outcomes by enabling faster diagnosis and reducing exposure to unnecessary radiation, particularly for children, as MRI scans can be substituted in some circumstances for CT scans which expose patients to ionising radiation and have been linked to an increased cancer risk.
21 Previously, only medical specialists and consultant physicians could request MBS-funded MRI scans for patients.
22 There were 125 MBS-eligible MRI machines before the 2011–12 reform package was announced. Health estimated that the MRI access reforms would result in an increase of 83 MRI machines eligible for MBS payments (comprising 71 machines registered with DHS and 12 additional machines in areas of need), bringing the total to 208 machines. However, 224 additional MRI machines were provided with MBS-eligibility as part of the MRI access reforms, bringing the expected total to 349 machines by January 2015.
23 See footnote 18.
24 Health’s heightened awareness of the need for Budget confidentiality and the importance of not giving stakeholders access to financially advantageous information, through consultation processes, reflected the experience of the 1998 MRI expansion process examined in ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation. See footnote 11.
25 Ministers had originally agreed that ‘current’ (operational) MRI machines be granted MBS-eligibility under the reform package. Following the Budget announcement, the Minister agreed to include ‘planned’ as well as operational MRI machines and to extend the cut-off date for applications from 1 January 2011 to 10 May 2011.
26 Criteria for defining areas of need were not announced as part of the reform package, but were subsequently determined on the basis of geographic distribution of MBS-eligible MRI machines, patient needs and health service integration. See Chapter 4, from paragraph 4.59 onward.
27 One of the objectives of the reform package was to review MBS fees for diagnostic imaging so that the fees paid by the Australian Government do not result in unintended incentives, for example patients being directed to services with higher profitability for the service provider, rather than on the basis of the most clinically appropriate imaging service.
28 Health advised the ANAO that the current government is also seeking to address the overall financial sustainability of Medicare through its 2014–15 Budget measure relating to patient co-payments, and that the delay to date in implementing that measure has impacted on the department’s ability to resolve issues in the diagnostic imaging sector in a way that the sector considers satisfactory.
29 Projects have included funding for the development of guidelines for GP-requesting of MRI services and decision support tools.
30 In 2008, an interdepartmental Expenditure Review Taskforce for the Health and Ageing Portfolio was formed to examine Future Funding Arrangements for Diagnostic Imaging and Pathology. It considered options for addressing expenditure growth, access, affordability, sustainability and competition, and led to the 2009–10 Review of Funding for Diagnostic Imaging Services, which was undertaken in consultation with stakeholders.
31 Department of the Prime Minister and Cabinet, Guide to Implementation Planning, 2014, p. 1, available from <http://www.dpmc.gov.au/implementation/planning.cfm> [accessed 26 October 2014]. See also ANAO Better Practice Guide, Successful Implementation of Policy Initiatives, October 2014, Canberra.
32 Health advice to the ANAO, 16 October 2014.
33 The two detailed reviews that informed the reform package (discussed at footnote 30) recognised the risk of MBS fees providing unintended incentives, for example patients being directed to services with higher profitability for the service provider, rather than on the basis of the most clinically appropriate imaging service. The initiative outlined in the reform package to address MBS fee relativities and incentives was intended to address this.
34 In 2009–10, the number of bulk-billed MRI services was 317 000, rising to 622 000 by 2013–14 – an increase of 96 per cent over the period.
35 There was a sharp increase in MRI services after GP-requesting rights commenced for patients over 16 years on 1 November 2013. The number of services grew by 30 per cent in 2013–14 compared with the previous year.
36 Health’s heightened awareness of the need for Budget confidentiality, and the importance of not giving stakeholders access to financially advantageous information through the consultation process, reflected learnings from an earlier MRI expansion process examined in ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation. See footnote 11.
37 Ministers had originally agreed that ‘current’ (operational) MRI machines be granted MBS-eligibility under the reform package. Following the Budget announcement, the Minister agreed to include ‘planned’ as well as operational MRI machines and to extend the cut-off date for applications from 1 January 2011 to 10 May 2011, following the normal practice of seeking the agreement of senior ministers or the Prime Minister when varying significant budget or policy parameters.
38 ANAO comment: DoFD was the then Department of Finance and Deregulation (now Finance). In its advice, Health also advised the Minister that Finance had confirmed that there would be ‘no additional cost impact to the Budget bottom line’. During this audit, Finance advised the ANAO that the costings for the MRI expansion were agreed with Health on the basis of two key assumptions: that the key driver of demand for MRI services under the measure was not the number of MRI machines but the number of new requests due to the introduction of GP-requested items; and that, while there would be increased expenditure due to the additional MRI machines, this would be offset in part by a reduction in the use of other diagnostic modalities. In discussing changes to the measure, Finance was advised by Health that the assumptions from the Budget costing were still relevant and that there would be no net impact from the proposed changes. On the basis of Health’s advice, Finance was in ‘broad agreement with Health that it is unlikely that any material financial impact would result from the higher than anticipated number of MBS-eligible MRI units’.
39 Prior to introducing the GP-requested items, only medical specialists and consultant physicians could request MBS-funded MRIs. By allowing GPs to request MRI services, the potential requestor numbers more than doubled. In 2011, there were 25 056 general practitioners and 24 415 medical specialists working in Australia, noting that not all medical specialists would use MRI scans to assist their diagnosis. See Australian Institute of Health and Welfare (2013) Medical Workforce 2011, p 14.
40 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation. See paragraph 11.
41 In that audit report, assessments for planned machines to be MRI-eligible were based on signed statutory declarations and signed contracts with machine suppliers. The audit found that ‘some of the contracts for these ordered machines were apparently backdated’ to meet the eligibility criteria.
42 Health did not confirm agreements between hospitals and private practice providers in the first of its four assessment rounds, and subsequently, the risk that an approved applicant did not meet the MBS-eligibility criteria materialised. Health’s assessment process improved over subsequent rounds. In particular, the department recognised that a supplier’s quote is not in itself sufficient evidence on which to determine compliance with the eligibility criteria. In the last two assessment rounds, Health documented and separately assessed each piece of evidence against the eligibility criteria, outlining the reasons why it might meet or not meet the criteria. This more structured process provided greater assurance that the application was accurately assessed against the eligibility criteria, and that the evidence base was adequate and documented.
43 The ANAO examined all available departmental documentation for a sample of 73 approved applications from the original assessment round, comprising 19 applications randomly selected from the 66 operational machines and all 54 planned machines approved in that round.
44 Applicants were required to demonstrate a financial investment in the planned MRI machine prior to the Budget announcement (10 May 2011) by providing statutory declarations and other relevant documentation, such as receipts for purchase, evidence of capital works or a signed purchase order with a financial penalty for cancellation.
45 See footnote 26 and from paragraph 4.59 onward.
46 Diagnostic radiology includes X-ray, angiography and fluoroscopy.
47 These initiatives may result in amendments to the diagnostic imaging regulations to improve practitioner credentialing and supervision of diagnostic imaging services, as well as ultrasound equipment standards, amongst others. Health has also reviewed the wording of the existing DIAS standards and commenced work on aligning DIAS standards with the National Safety and Quality Health Service Standards.
48 Self-assessment processes have inherent limitations with respect to the level of assurance they can provide on adherence to a regulatory framework.
49 Medicare is Australia’s universal healthcare system which provides people with access to free or subsidised health and hospital care.
50 Department of Health and Ageing, Medical Benefits Reviews Task Group, Review of Funding for Diagnostic Imaging Services’, final report November 2011 (‘the funding review’) p. 4, available from <http://www.ranzcr.edu.au/> [accessed 6 June 2014] and ANAO analysis for data from 2005–06 to 2013–14 at Appendix 2, Table A.1.
51 The 2014 National Commission of Audit identified that ‘Health care spending represents the Commonwealth’s single largest long-term fiscal challenge, with expenditure on all major health programmes expected to grow strongly to 2023–24 and beyond’ (p. xxi). The Commission recommended that ‘the Commonwealth Government pursue reforms to improve the health system as soon as practicable’. In addition to recommending the introduction of co-payments for all Medicare-funded services, the Commission of Audit recommended ‘reviewing the Medicare Benefits Schedule to identify and remove ineffective items, replace expensive items with less expensive alternatives where available and investigate options for cost recovery for applications to list items on the Schedule’ (p. xl). Towards Responsible Government, the Report of the National Commission of Audit, Phase One, February 2014, Canberra, available from <http://www.ncoa.gov.au/report/docs/phase_one_report.pdf> [accessed 23 September 2014].
52 Successive Budget Papers for the Health portfolio have discussed these matters, see for example, Australian Government, Portfolio Budget Statements 2009–10, Health and Ageing portfolio, Budget related paper 1.10, Commonwealth of Australia, Canberra, p. 119 and Australian Government, Portfolio Budget Statements 2014–15, Health and Ageing portfolio, Budget related paper 1.10, Commonwealth of Australia, Canberra, p. 79.
53 The Medicare Benefits Schedule (MBS) lists the services that are subsidised by the Australian Government under Medicare and the schedule fee.
54 In 2008, an interdepartmental Expenditure Review Taskforce comprising the then Departments of Health and Ageing and Finance and Deregulation was established to consider options for addressing expenditure growth, access, affordability, sustainability and competition for pathology and diagnostic imaging. The taskforce consulted with and coordinated comments from the then Departments of Innovation, Industry, Science and Research, Prime Minister and Cabinet, Treasury, Veterans’ Affairs, Human Services, Attorney-General’s and the Office of Best Practice Regulation.
55 The MoU, and others before it, was a price-volume agreement that capped expenditure growth at an agreed rate of approximately five per cent per annum. The MoU provided a framework for the Commonwealth and the radiology sector to work cooperatively to help manage both parties’ financial risks. The MoU allowed for a reduction in MBS fees when service volumes exceeded the cap. It also guaranteed a minimum level of expenditure growth so that if volumes dropped below 4.5 per cent MBS fees could be increased.
56 A review of funding for pathology services was announced at the same time.
57 The funding review, Attachment A, Terms of Reference.
58 ibid. Reviewing MBS fees was the first of four key tasks outlined for the funding review.
59 ibid., Outcomes of the review, p. 23–26.
60 ibid.
61 ibid., pp. 23–25.
62 The DIST is the part of the MBS that specifies the diagnostic imaging services that will be subsidised by the Australian Government, the schedule fee and the requirements to be met in order for services to be MBS-eligible.
63 Since 2008, accreditation under the DIAS has been linked to the payment of Medicare benefits for diagnostic imaging services.
64 Funding was appropriated to Health’s Program 3.1 – Medicare benefits, for payment under the MBS.
65 The funding review, p. 21.
66 The DIQP was announced in the 2011–12 Budget, however the first projects did not commence until the 2012–13 financial year.
67 DIQP projects are listed in Table 3.1 in Chapter 3. Cessation of the DIQP was announced in the 2014–15 Budget and no new projects will be funded under the program.
68 The NPS was funded from the Quality Use of Diagnostics, Therapeutics and Pathology Fund, which is one of 16 funds (following consolidation in 2011–12) that is used by Health as a means of responding to priority and emerging health policy matters. The purpose of the NPS is to raise awareness and provide evidence–based information for health professionals and consumers on the quality use of medicines and medical services. The outcomes of this project, as they relate to improving ‘appropriate requesting’ by medical practitioners of diagnostic imaging services, are discussed in Chapter 3.
69 Department of Human Services, Medicare Services, available from <www.humanservices.gov.au/customer/subjects/medicare-services> [accessed 29 May 2014], in accordance with the Bilateral Head Agreement established between the Departments of Health and Human Services, 2012–2015.
70 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation.
71 That audit examined accusations that some persons placing orders for MRI machines in the period preceding the Budget had access to information to be announced in the Budget, thus providing those persons with a significant financial advantage (p. 11). Specifically, there were no agreed procedures for managing confidentiality and conflicts of interest. As a result, 33 MRI units were ordered in the four working days in the lead up to the Budget announcement and cut–off for Medicare eligibility. Until the 1998 Budget measure, Commonwealth funding for MRI was restricted to 18 publicly–owned MRI units.
72 ANAO Audit Report No.26 2013–14, Medicare Compliance Audits.
73 While not examined as part of this audit, the nature and purpose of the MSAC application process is outlined in Chapter 3 as the MSAC process can result in changes to diagnostic imaging items that are listed on the DIST and funded under the MBS.
74 The ANAO engaged Vista Advisory Pty Ltd to provide audit services for the conduct of this audit.
75 ANAO Better Practice Guide – Public Sector Governance, June 2014, Canberra, p. 17.
76 The matter of some parties having access to information to be announced on Budget night that may have provided a financial advantage arose in relation to a previous decision on MBS-eligible MRI units from the 1998–99 Budget. Referred to as the ‘scan scam’, the matters of concern and the findings of the ANAO’s audit, including lessons learned, are documented at ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation.
77 See paragraph 1.15 and footnote 71.
78 Organisations represented on DIRCC included the Australian Diagnostic Imaging Association (ADIA), the Royal Australian and New Zealand College of Radiologists (RANZCR), the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), the Cardiac Society of Australia and New Zealand (CSANZ), the Australasian Association of Nuclear Medicine Specialists (AANMS, formerly known as the Australian and New Zealand Association of Physicians in Nuclear Medicine, ANZAPNM), the Consumer Health Forum (CHF), the Australian Medical Association (AMA), the Australian Institute of Radiography (AIR), the Australasian Sonographers Association (ASA), Allied Health Professionals Australia (AHPA), the Australasian Society for Ultrasound in Medicine (ASUM), the Royal Australasian College of Surgeons (RACS) and the Royal Australian College of General Practitioners (RACGP).
79 The terms of reference were outlined in the review report, Attachment B, ‘Purpose and role of the Diagnostic Imaging Review Consultation Committee’.
80 The Budget was announced on the evening of 10 May 2011.
81 The DIAC is a subset of the DIRCC discussed in footnote 78. DIAC membership includes key stakeholders with the addition of ARPANSA.
82 DIAC terms of reference, November 2012.
83 Capital sensitivity was introduced prior to the reform package to promote patient access to new technologies by reducing by 50 per cent the level of Medicare rebate for certain diagnostic imaging services that were performed on machines that had reached a certain age; 10–15 years depending on the type of machine. Consultation with stakeholders through the DIAC on capital sensitivity is an example of consultation on broader diagnostic imaging-related matters beyond the reform package.
84 Health has consulted with stakeholders and drawn on their technical and clinical knowledge in relation to a range of specific diagnostic imaging matters. Working groups of clinicians were formed to provide advice on the symptoms for which GPs would be able to request MRI scans (discussed in Chapter 4), and for the Monitoring and Implementation of the Diagnostic Imaging Accreditation Scheme (DIAS-MIC, discussed further in Chapter 5). Furthermore, the Medical Services Advisory Committee (MSAC) provides advice to the Minister for Health on evidence relating to the safety, clinical effectiveness and cost-effectiveness of new medical technologies and procedures and informs Australian Government decisions about public funding for new, and in some cases existing, medical procedures, available from <http://www.msac.gov.au/internet/msac/publishing.nsf/Content/about-us-lp-1> [accessed 21 August 2014].
85 Health advice to ANAO, 15 and 26 August 2014. Matters related to the MRI expansion are discussed in Chapter 4.
86 Further information on the Department of Health’s overall approach to managing conflicts of interest relating to advisory committees is outlined in Chapter 3 of ANAO Report No.47 2013–14, Managing Conflicts of Interest in FMA Agencies.
87 ANAO Better Practice Guide – Successful Implementation of Policy Initiatives, October 2014, Canberra, p. 43.
88 The status and progress of implementation of each diagnostic imaging reform initiative is discussed in Chapters 3, 4 and 5.
89 ANAO Better Practice Guide – Successful Implementation of Policy Initiatives, October 2014, Canberra, p. 57.
90 Australian Government, Portfolio Budget Statements 2013–14, Budget related paper No. 1.9, Health and Ageing portfolio, Commonwealth of Australia, Canberra, 2013, p. 97.
91 Health advised the ANAO that initial acquisition of data occurred in March 2014 and that the department has liaised with the Office of Best Practice Regulation in relation to the process and timeframes for the post-implementation review.
92 ANAO Better Practice Guide – Public Sector Governance, June 2014, Canberra, p. 52.
93 Department of Health, Annual Report 2013–14, Volume 1, Canberra, 2014 p. 70, available from <http://www.health.gov.au/internet/main/publishing.nsf/Content/DC5839D1C54A92C3CA257D50001CB666/$File/2.1%20Outcome%203%20%20Access%20to%20Medical%20Services.pdf> [accessed 3 November 2014].
94 The analysis of the impact of the MRI access reform initiatives is discussed in paragraphs 4.88 to 4.108 in Chapter 4.
95 In 2008, an interdepartmental Expenditure Review Taskforce for the Health and Ageing Portfolio was formed to examine Future Funding Arrangements for Diagnostic Imaging and Pathology. It considered options for addressing expenditure growth, access, affordability, sustainability and competition, and led to the 2009–10 Review of Funding for Diagnostic Imaging Services, which was undertaken in consultation with stakeholders.
96 Department of the Prime Minister and Cabinet, Guide to Implementation Planning, 2014, p. 1, available from <http://www.dpmc.gov.au/implementation/planning.cfm> [accessed 26 October 2014].
97 As at mid-October 2014.
98 Health advice to the ANAO, 16 October 2014.
99 Average annual growth of diagnostic imaging expenditure over the three years to 2013–14, based on ANAO analysis of MBS data, shown at Appendix 2.
100 Health advised the Minister that the DIST would be restructured so that items are listed by body system (for example, the skeletal system) rather than the current modality structure (that is, listing items by ultrasound, MRI and others) to better reflect current clinical practice.
101 Health cited three studies in its funding review, noting that those studies were performed in the United States and may not be indicative of the extent to which inappropriate requesting is a problem in Australia (funding review report p. 21). A small Australian study of 50 consecutive CT requests received by two Australian private radiology practices in 2004–05 identified that 34 scans (68 per cent) were considered to be inappropriate, per G Simpson and G Hartrick, Use of Thoracic CT by General Practitioners, Medical Journal of Australia 2007; 187, pp. 43–46, available from <http://www.ncbi.nlm.nih.gov/pubmed/17605703> [accessed 19 August 2014].
102 See Appendix 3 for details of providers of MBS-funded diagnostic imaging services by specialisation in 2013–14.
103 H Britt, GC Miller, L Valenti, J Henderson, J Gordon, AG Pollack, C Bayram, C Wong, ‘Evaluation of imaging ordering by general practitioners in Australia, 2002–03 to 2011–12’, General practice series no.35, Sydney University Press, 2014, available from <http://hdl.handle.net/2123/10610> [accessed 19 August 2014].
104 This substantial increase in imaging ordering has attracted public interest, see for example: S Parnell, ‘Doctors blamed as public X-rays soar’, The Australian, 23 July 2014, p. 4.
105 Nuclear medicine specialists are generally physicians with sub-specialty training in nuclear medicine. Nuclear medicine involves injection of a radioactive isotope into a patient for diagnostic or treatment purposes, providing information about both the anatomy of the body and its function.
106 ‘Point-of-care’ conduct of diagnostic imaging services is where a medical practitioner provides an imaging service without an arms-length request for service from another medical practitioner. This includes cardiac, vascular and obstetric ultrasound performed by medical specialists as part of a consultation and may also include X-rays in a regional GP practice.
107 There is strong evidence that with proper information and shared decision-making, patients will choose a more conservative treatment option, including not having tests or procedures performed. The Australian Commission on Safety and Quality in Health Care (ACSQHC) has developed national safety and quality health service standards, which include standards to involve patients in treatment and care decisions. ACSQHC has identified the benefits of shared decision-making: see for example Patient-Centred Care: Improving Quality and Safety through Partnerships with Patients and Consumers, ACSQHC, Sydney, 2011, p. 15: “Patient-centred care has been associated with a reduction in the number of diagnostic orders and other referrals, better adherence to treatment regimens, greater patient satisfaction and greater patient enablement”.
108 This was recognised in the funding review, in relation to fee relativities being reviewed to encourage practices to direct patients to the most appropriate imaging modality (p. 24) and addressing unintended incentives to invest in higher end technologies (p. 25) or patients being directed to services with higher profitability for the service provider, rather than on the basis of the most clinically appropriate imaging service.
109 The taskforce that led to the funding review is discussed in Chapter 1.
110 The funding review, p. 24.
111 Health advice to the ANAO, 15 October 2014.
112 Five of the twelve DIQP projects funded from October 2012 related to improving quality and safety in diagnostic imaging. The ANAO notes that ‘appropriate requesting’ is related to improved patient safety, for example in relation to reduced radiation exposure. Following a decision taken in the 2014–15 Budget, the DIQP will end and no new grants will be offered.
113 The most developed decision-support tool in Australia is the Western Australian Government-sponsored ‘Diagnostic Imaging Pathways’ (DIP) tool, which the ANAO has been advised is used in public and many private hospitals in Western Australia, and has been adopted by several other public health services in other jurisdictions. DIP is aimed at GPs and junior doctors and contains flowcharts, information on the uses and risks of different types of imaging tests, and provides example images and further references. It has been developed over the last 12 years by a group of over 60 medical specialists and researchers. This tool was cited by the July 2014 Britt et al study (see reference at footnote 103 above) as the most appropriate and useful guidance for GPs in Australia. The Australian Government Department of Health funded a DIQP project to investigate its adoption into GP-practices, including embedding it in practice software. A report is due to the department in late 2014. The tool is available from <http://www.imagingpathways.health.wa.gov.au/> [accessed 20 August 2014].
114 The taskforce that led to the funding review is discussed in Chapter 1.
115 The National Prescribing Service is funded by the Australian Government Department of Health to provide practical tools (including evidence-based information educational activities) with the intention of improving the way health technologies, including how medicines and medical tests, are prescribed and used, available from <http://www.nps.org.au/about-us> [accessed 27 August 2014]. The NPS diagnostic imaging project was funded under the Quality Use of Diagnostics, Therapeutics and Pathology Fund, which is one of 16 flexible funds established within Health. Flexible funds are described as a means of responding to priority and emerging health policy matters.
116 The funding review, p. 22.
117 The NPS MedicineWise MBS project report estimated savings, based on MBS data and practitioner requesting patterns, of $5.4 million from reduced expenditure on diagnostic imaging (lumbosacral CT scans for the period December 2010 to December 2011) and $17.6 million from reduced expenditure on pathology (Vitamin D tests for the period April 2011 to June 2011).
118 The Medical Services (Benefits) Advisory Committee comprises independent experts, established under the Health Insurance Act 1973 to provide advice to the Minister for Health on evidence relating to the safety, clinical effectiveness and cost-effectiveness of new medical technologies and procedures. MSAC advice informs Australian Government decisions about public funding for new, and in some cases existing, medical procedures. Health provides secretariat support for MSAC, available from <http://www.msac.gov.au/internet/msac/publishing.nsf/Content/about-us-lp-1> [accessed 11 September 2014].
119 Australian Government Department of Health, Medical Benefits Schedule Book, Operating from 1 August 2014, p. 584, ‘Substituted Services’.
120 The role and composition of the Diagnostic Imaging Advisory Committee is discussed in Chapter 1.
121 See Appendix 4, Table A.6 and discussion in Chapter 4 (Figure 4.2 and paragraph 4.95).
122 The DIST is prescribed under regulations made under section 4AA of the Health Insurance Act 1973 (Cth). There are similar tables and provisions for pathology and general medical services.
123 The funding review, p. 8.
124 Advice to the then Government in the context of the 2009–10 Budget that led to the funding review identified that, over time, more and more items on the DIST do not reflect the cost of providing services and providers have needed to cross-subsidise between over-remunerated and under-remunerated services.
125 Paragraph 1.4 provides further information on the taskforce.
126 The funding review, Attachment A, Terms of Reference.
127 Chapter 5 discusses quality initiatives, including the Diagnostic Imaging Accreditation Scheme, and the extent to which quality initiatives are provided for in the DIST and proposed changes in this area.
128 Quality incentives refer to Government initiatives that are aimed to incentivise and reward good standards of clinical practice, such as the program that operates to provide incentive payments to accredited GP practices – the Practice Incentives Program, information available from <http://www.medicareaustralia.gov.au/provider/incentives/pip/> [accessed 27 November 2014]. While the reform package did not provide for implementation of such a measure for diagnostic imaging, the funding review discussed proposals by the sector for incentives aimed at supporting higher levels of clinical input from onsite radiologists. Medical practitioners during audit interviews further considered that, given DIST fees have not been indexed for many years, incentives are important for distinguishing between and rewarding higher-quality practices, including those that use lower-radiation imaging equipment and record patient radiation doses on each imaging report.
129 Australian Government, Portfolio Budget Statements 2011–12. Health and Ageing portfolio, Budget related paper 1.10, Commonwealth of Australia, Canberra, p. 152.
130 These included initiatives resulting from MSAC recommendations and not directly related to the reform package. Refer to footnote 118 of this chapter for discussion of the role of MSAC. Although section 4AA (2) of the Health Insurance Act 1973 requires the DIST to be remade every 12 months, and this is done in the form of a new regulation (legislative instrument) made in November each year, this has occurred without reviewing its structure or fees.
131 Health has funded a study of ultrasound use by (non-radiology) medical specialists (Aspex Consulting, October 2011) and has received advice from ADIA and RANZCR about the highest priorities for reviewing ultrasound items.
132 The funding review, p. 12.
133 The ANAO interviewed and received written submissions from senior representatives of medical colleges and specialist associations, including current and past presidents, chairs of diagnostic imaging and education committees of those organisations, and professors of medicine.
134 See Appendix 2, Table A.1, growth in diagnostic imaging services, expenditure and cost since 2005–06. The ANAO calculated that the average cost of service has grown from $108 in 2005–06 to $129 in 2013–14.
135 Advice to Ministers, Expenditure Review Taskforce, Health and Ageing Portfolio, Future Funding Arrangements for Diagnostic Imaging and Pathology, Attachment E.
136 The Government is currently considering the approach through which MSAC recommendations are progressed outside the annual Budget process.
137 The two detailed reviews that informed the reform package (outlined at paragraph 1.4) recognised the risk of MBS fees providing unintended incentives, for example patients being directed to services with higher profitability for the service provider, rather than on the basis of the most clinically appropriate imaging service. The initiative outlined in the reform package to address MBS fee relativities and incentives was intended to address this.
138 Department of Health and Ageing, Detailed Review of Funding for Diagnostic Imaging Services – Discussion Paper, January 2010, p 2.
139 ibid.
140 ibid., pp 13–15.
141 The Diagnostic Imaging Review Consultative Committee’s terms of reference and membership is outlined in Chapter 2.
142 The funding review, p. 17.
143 Prior to November 2009, radiologists received a payment of 85 per cent of the schedule fee. In November 2009, this increased to 95 per cent of the schedule fee. The 2011–12 Budget measure increased the amount that they received for MRI scans to 100 per cent of the schedule fee.
144 The ANAO notes that there are earlier related measures, including capital sensitivity, which had the potential to affect the affordability and convenience of services – these aspects were not included in the ANAO’s examination as they did not form part of the reform package.
145 These matters are discussed in Chapter 3.
146 These MBS items related to symptoms of hips and shoulders. The advisory group considered that these symptoms were either one of the following: from a rare condition not commonly diagnosed by a GP; usually referred to specialists; or having insufficient evidence to indicate that an MRI scan would be useful in diagnosis.
147 The role of MSAC is outlined in Chapter 3, footnote 118.
148 This MBS item related to lower back pain. The proposed lower back MRI item was delayed until a comprehensive review of the evidence on the efficacy of lower back pain diagnostic imaging could be undertaken as part of the suite of MBS reviews under the comprehensive management framework, a 2013–14 Budget measure. This review is outlined at paragraph 3.15.
149 These MBS items are part of the Diagnostic Imaging Services Table (DIST) of the Medicare Benefits Schedule. The DIST is enacted under regulation of the Health Insurance Act 1973 – this regulation is in force for a 12 month period, with new regulations approved on an annual basis, coming into effect on 1 November each year. The GP-requested MBS items for MRI scans for those under 16 years were included in Health Insurance (Diagnostic Imaging Services Table) Regulation 2012. With the settling in period for the newly elected government in September 2013, the equivalent legislative instrument for 2013 could not be considered by Executive Council in sufficient time. As a result, the Departmental delegate made a determination under subsection 3C(1) of the Health Insurance Act 1973 to include the GP-requested MBS items for MRI scans for those 16 years and older in the DIST through a legislative instrument, Health Insurance (MRI for Patients 16 Years and Over and Capital Sensitivity Consequential Amendments) Determination 2013.
150 For MRI machines that were to be given partial MBS-eligibility in metropolitan areas, the reform package also provided for several items, in addition to the GP-requested MRI services, that would be MBS-eligible on those machines. The items were for the staging of rectal and cervical cancers and breast screening for women aged under 50 years. Staging describes the extent or severity of a person’s cancer. Knowing the stage of disease helps the doctor plan treatment and estimate the patient’s prognosis.
151 For scans that are either not listed on the MBS or are undertaken on MBS-ineligible machines, patients are required to pay the full fee charged by the service provider and are unable to claim an MBS rebate.
152 Diagnostic service providers with MBS-ineligible machines used for MBS-ineligible services were (and still are) able to register their machines with the Department of Human Services (DHS), but are not obliged to do so. The 71 machines which could be made MBS-eligible were those that had been registered with Medicare Australia as at 10 December 2010.
153 Different words ‘current’ and ‘operating’ MBS-ineligible MRI units were used in the Government decision documentation and the reform package respectively.
154 The advice to the Minister in the context of the 2011 Budget formed the basis on which the Government made its decision.
155 This figure does not include those MRI machines granted MBS-eligibility as part of the Invitation to Apply (ITA) process for 12 additional machines in areas of need (discussed from paragraph 4.59 onward), noting that some of the machines granted partial MBS-eligibility as part of the expansion process subsequently gained full eligibility as part of the ITA process. See Table 4.4 and footnote 160.
156 Discussion of the impact of the MRI access reforms on MBS expenditure, and consideration of the extent to which the existence of additional MBS-eligible MRI machines may have impacted on service numbers and expenditure exceeding departmental estimates, is discussed at paragraphs 4.36 and paragraphs 4.90 to 4.99, and footnote 168.
157 Based on Health’s advice, the Minister agreed to undertake the expansion process through application.
158 Health advice to the ANAO, 15 October 2014.
159 A comprehensive diagnostic imaging practice or radiology department was defined as one that provided X-ray, CT and ultrasound services as well as planned or actual MRI services.
160 There were 125 MBS-eligible MRI machines before the 2011–12 reform package was announced. Health estimated that the MRI access reforms would result in an increase of 83 MRI machines eligible for MBS payments (comprising 71 machines registered with DHS and 12 additional machines in areas of need), bringing the total to 208 machines. However, 224 additional MRI machines were provided with MBS-eligibility as part of the MRI access reforms, bringing the expected total to 349 machines by January 2015. The break-down in numbers and process through which additional MRI machines were approved is shown in Table 4.4.
161 The funding review, Outcomes of the Review, p 25.
162 See paragraph 4.27.
163 Health in its advice to the Minister and its assessment process used a number of terms for ‘financial investment’. The terminology approved by the Minister was that planned units ‘must have evidence of clear and unqualified financial commitment by 10 May 2011’. The guidelines developed by Health, which required a financial investment to be demonstrated, were less rigorous than the requirement approved by the Minister and therefore required the exercise of judgment by assessors. This included the need for assessors to consider the relevance and quality of evidence provided by applicants.
164 A comprehensive diagnostic imaging practice or radiology department is defined at footnote 159.
165 A tesla is a unit measurement of the strength of the magnetic field produced by the MRI unit. The length of time a patient requires for an MRI scan of equivalent clarity is inversely related to the unit’s tesla strength.
166 ASGC-RA is a geographic classification system that was developed in 2001 by the Australian Bureau of Statistics (ABS), as a statistical geography structure which allows quantitative comparisons between ‘city’ and ‘country’ Australia. ASGC-RA1 is ‘major cities of Australia’ and ASGC-RA2 to RA5 are regional and remote locations, available from <http://www.doctorconnect.gov.au/internet/otd/publishing.nsf/Content/ra-intro> [accessed 18 August 2014].
167 Ministers had originally agreed that ‘current’ (operational) MRI machines be granted MBS-eligibility under the reform package. Following the Budget announcement, the Minister for Health agreed to include ‘planned’ as well as operational MRI machines and to extend the cut-off date for applications from 1 January 2011 to 10 May 2011, following the normal practice of seeking the agreement of senior ministers or the Prime Minister when varying significant budget or policy parameters.
168 ANAO comment: DoFD was the then Department of Finance and Deregulation (now Finance). In its advice, Health also advised the Minister that Finance had confirmed that there would be ‘no additional cost impact to the Budget bottom line’. During this audit, Finance advised the ANAO that the costings for the MRI expansion were agreed with Health on the basis of two key assumptions: that the key driver of demand for MRI services under the measure was not the number of MRI machines but the number of new requests due to the introduction of GP-requested items; and that, while there would be increased expenditure due to the additional MRI machines, this would be offset in part by a reduction in the use of other diagnostic modalities. In discussing changes to the measure, Finance was advised by Health that the assumptions from the Budget costing were still relevant and that there would be no net impact from the proposed changes. On the basis of Health’s advice, Finance was in ‘broad agreement with Health that it is unlikely that any material financial impact would result from the higher than anticipated number of MBS-eligible MRI units’.
169 The advice was part of the advice provided in February 2011, outlined at paragraph 4.4.
170 Prior to introducing the GP-requested items, only medical specialists and consultant physicians could request MBS-funded MRIs. By allowing GPs to request MRI services, the potential requestor numbers more than doubled. (In 2011, there were 25 056 general practitioners and 24 415 medical specialists working in Australia, noting that not all medical specialists would use MRI scans to assist their diagnosis. (Australian Institute of Health and Welfare (2013) Medical Workforce 2011, p 14)
171 Health advice to the ANAO, 15 October 2014.
172 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation, p 35.
173 Paragraph 4.44 outlines each of the rounds. 125 MRI machines were approved for MBS-eligibility in the first round, and 13, 2 and 10 machines in the second, third and fourth rounds respectively. The number of MBS-eligible machines approved as a result of the expansion is shown in Table 4.4.
174 The eligibility criteria are discussed at paragraph 4.33.
175 See paragraph 4.50.
176 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation, p 21.
177 One provider subsequently relinquished their partially-eligible machine as it was decommissioned.
178 Following the original assessment process, Health did not advertise or communicate publicly that further applications would be assessed. Applicants who had not been approved in the original round, were advised by letter from Health that they may wish to provide further information to support their claim. These letters and the fact that no close-off date was announced until after the approval of the third round of applications, resulted in an ongoing trickle of applications being received by Health.
179 Health sought this information from the departmental DoctorConnect web-site, available from http://www.doctorconnect.gov.au/internet/otd/publishing.nsf/Content/locator [accessed 19 August 2014]).
180 See paragraph 4.33.
181 A further six ‘planned’ machines were approved for public hospitals in the original assessment round. These had received funding through COAG agreement and therefore were not sampled by the ANAO.
182 The Minister specifically approved planned units on the basis of having ‘evidence of clear and unqualified financial commitment by 10 May 2011’. See footnote 163.
183 See paragraph 4.42.
184 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation, p. 21.
185 See paragraph 4.2. The probity arrangements put in place for consultations during the funding review are discussed in Chapter 2.
186 The CGGs do not apply to MBS benefits as these are an entitlement established by legislation. See sub-clause 2.8d. of the CGGs. On 1 July 2014, the CGGs were replaced with Department of Finance (2014), Commonwealth Grants Rules and Guidelines, available from http://www.finance.gov.au/sites/default/files/commonwealth-grants-rules-and-guidelines-July2014.pdf [accessed 22 August 2014].
187 Under the Financial Management and Accountability Act 1997 (FMA Act), which applied at the time, FMA Act agencies were required to satisfy the approver of a proposal to financially commit the Commonwealth that the spending proposal represented a ‘proper use’. The relevant regulation (FMA Regulation 9) provided that ‘an approver must not approve a spending proposal unless the approver is satisfied, after making reasonable inquiries, that giving effect to the spending proposal would be a proper use of Commonwealth resources’. ‘Proper use’ was defined to mean: ‘efficient, effective, economical and ethical use that is not inconsistent with the policies of the Commonwealth’ (Section 44 (3) of the FMA Act). The FMA Act was replaced on 1 July 2014, by the Public Governance, Performance and Accountability Act 2013 (Cth).
188 MBS-eligibility was subject to the applicant entering into a deed of undertaking with Health.
189 The specific index used was the 2006 Index of Relative Socio-economic Advantage and Disadvantage (IRSAD) with decile scores for each statistical area for the ABS’s Census of Population and Housing. Each statistical area has an allocated score in the range 1 to 10, with low scores representing areas of greater disadvantage, and high scores representing areas of greater advantage, available from <http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/2033.0.55.001main+features100042011> [accessed 22 August 2014]).
190 Paragraph 4.64 outlines the approval process in the assessment plan, identifying the department’s delegate, rather than the Minister, as approver.
191 See Footnote 187.
192 Department of Finance and Deregulation (2011), Commitments to spend public money (FMA Regulations 7 to 12): Finance Circular No. 2011/01, p 21.
193 FMA Regulation 12 required that the terms of an approval be recorded in writing as soon as practicable after giving the approval. While Regulation 12 did not require an approver to record the basis of their approval, the Department of Finance advised agencies that: ‘In considering the terms to be recorded, the approver should consider who is going to rely on the record and ensure that the record is proportionate to the significance, value, level of risk and sensitivity associated with the spending proposal.’ ibid., para. 2, p. 34.
194 Where the electorate was held by ALP or Coalition members at the 2010 Federal Election, the electorate type was based on the level of margin with which the seat was held. A marginal seat is one held by less than 56 per cent on a two-party preferred basis, consistent with the Australian Electoral Commission’s definition.
195 This is estimated on the basis of 2014–15 Budget costings and estimated full-year expenditure, shown in Table 4.3.
196 See paragraph 4.35.
197 See paragraph 4.44, noting that the last round of MBS-eligible machines was announced as recently as June 2014.
198 To determine the number and cost of CT scans, the ANAO drew on publicly available information from Medicare Australia. This data groups the age of patients, providing information for under 15 year olds, but not for under 16 year olds.
199 Health advice to ANAO, 15 October 2014.
200 This rate is based on the Australian Bureau of Statistics population estimate of 23.3 million at December 2013, available from <http://www.abs.gov.au/ausstats/abs@.nsf/mf/3101.0> [accessed 29 August 2014].
201 OECD (2013) Health at a Glance – OECD, p 24.
202 There were 829 000 MBS-eligible MRI services undertaken in 2013–14.
203 See paragraph 4.92.
204 Department of Human Services, Budget 2013–14: Medicare Benefits Schedule – introducing patient contributions for general practitioner, pathology and diagnostic imaging services, available from <http://www.humanservices.gov.au/corporate/publications-and-resources/budget/1415/measures /health-matters-and-health-professionals/34-90188> [accessed 17 September 2014].
205 In 2009–10, the number of bulk-billed MRI services was 317 000, rising to 622 000 by 2013–14 – an increase of 96 per cent over the period.
206 There was a sharp increase in MRI services after GP-requesting rights commenced for patients over 16 years on 1 November 2013. The number of services grew by 30 per cent in 2013–14 compared with the previous year.
207 Health’s heightened awareness of the need for Budget confidentiality, and the importance of not giving stakeholders access to financially advantageous information through the consultation process, reflected learnings from an earlier MRI expansion process examined in ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation. See footnote 11.
208 Ministers had originally agreed that ‘current’ (operational) MRI machines be granted MBS-eligibility under the reform package. Following the Budget announcement, the Minister for Health agreed to include ‘planned’ as well as operational MRI machines and to extend the cut-off date for applications from 1 January 2011 to 10 May 2011, following the normal practice of seeking the agreement of senior ministers or the Prime Minister when varying significant budget or policy parameters.
209 Prior to introducing the GP-requested items, only medical specialists and consultant physicians could request MBS-funded MRIs. By allowing GPs to request MRI services, the potential requestor numbers more than doubled. In 2011, there were 25 056 general practitioners and 24 415 medical specialists working in Australia, noting that not all medical specialists would use MRI scans to assist their diagnosis. See Australian Institute of Health and Welfare (2013) Medical Workforce 2011, p 14.
210 ANAO Audit Report No. 42 1999–2000, Magnetic Resonance Imaging Services – effectiveness and probity of the policy development processes and implementation.
211 In that audit report, assessments for planned machines to be MBS-eligible were based on signed statutory declarations and signed contracts with machine suppliers. The audit found that ‘some of the contracts for these ordered machines were apparently backdated’ to meet the eligibility criteria.
212 Health did not confirm agreements between hospitals and private practice providers in the first of its four assessment rounds, and subsequently, the risk that an approved applicant did not meet the MBS-eligibility criteria materialised. Health’s assessment process improved over subsequent rounds. In particular, the department recognised that a supplier’s quote is not in itself sufficient evidence on which to determine compliance with the eligibility criteria. In the last two assessment rounds, Health documented and separately assessed each piece of evidence against the eligibility criteria, outlining the reasons why it might meet or not meet the criteria. This more structured process provided greater assurance that the application was accurately assessed against the eligibility criteria, and that the evidence base was adequate and documented.
213 The ANAO examined all available departmental documentation for a sample of 73 approved applications from the original assessment round, comprising 19 applications randomly selected from the 66 operational machines and all 54 planned machines approved in that round.
214 Applicants were required to demonstrate a financial investment in the planned MRI machine prior to the Budget announcement (10 May 2011) by providing statutory declarations and other relevant documentation, such as receipts for purchase, evidence of capital works or a signed purchase order with a financial penalty for cancellation.
215 See footnote 26 and from paragraph 4.59 onward.
216 Department of Health and Ageing, Diagnostic Imaging Accreditation Scheme: User Guide for Practices Applying for Accreditation, February 2012, p. 1.
217 Regulation of quality and safety in publically-funded healthcare services in Australia takes place across Federal, State and Territory jurisdictions and is administered by multiple government agencies. For diagnostic imaging services, these agencies include not only Health with the DIAS, but also state and territory agencies responsible for radiation control, health complaints and hospital services. ARPANSA, whose work in relation to CT risk is discussed in this chapter, promotes national uniformity and implementation of radiation best practice across all jurisdictions, and the Therapeutic Goods Administration (TGA) regulates the supply of equipment used in diagnostic imaging. The most significant change to the Australian healthcare regulatory environment in recent years is the development by the Australian Commission on Safety and Quality in Healthcare in 2011 of National Safety and Quality Health Service (NSQHS) Standards. The NSQHS Standards represent the first time a national and systematic ‘board down to bedside’ approach to quality and safety in healthcare has been implemented in Australia. The NSQHS Standards are now mandatory for acute health services (for example hospitals, including their in-house diagnostic imaging departments, and day procedure clinics). In addition, the NSQHS Standards have been adopted voluntarily by approximately 1200 other health service providers including dental practices, prison and community health services. Further information is available from <http://www.safetyandquality.gov.au/> [accessed 20 October 2014].
218 Amendments to the Health Insurance Act 1973 introducing the DIAS were passed in 2007 and the scheme commenced on 1 July 2008. The scheme was introduced as a result of a commitment by the Australian Government as part of the 2002–03 Budget, through the Radiology Quality and Outlays Memorandum of Understanding, established between the Royal Australian and New Zealand College of Radiologists (RANZCR) and the Australian Diagnostic Imaging Association (ADIA).
219 The DIAS was introduced initially with three entry-level standards, applying to practices covering radiology services (Stage 1, from 1 July 2008) and then expanded in Stage 2 on 1 July 2010 to cover all medical practices (for example, also cardiology and gynaecology and obstetric practices) claiming rebates for MBS items in the DIST.
220 Department of Health and Ageing, The Stage Two Diagnostic Accreditation Scheme, 1 July 2010, p. 2.
221 Approved accreditors are designated by the Minister for Health under the Health Insurance (Diagnostic Imaging Accreditation—Approved Accreditors) Instrument 2010.
222 The RANZCR Medical Imaging Accreditation Program (MIAP) was developed over three stages between 1999 and 2005 with funding support provided to RANZCR from the then Department of Health and Ageing. Stage Three of the MIAP was implemented in 2004 in the form of a comprehensive, onsite peer-review based accreditation program focused on continuous quality improvement. MIAP-registered practices represent less than 10 per cent of RANZCR members.
223 Department of Health, Annual Report 2013–14, Volume 1, DOH, Canberra, 2014, p. 70, available from <http://www.health.gov.au/internet/main/publishing.nsf/Content/DC5839D1C54A92C3CA257D50001CB666/$File/2.1%20Outcome%203%20%20Access%20to%20Medical%20Services.pdf> [accessed 3 November 2014].
224 The funding review, p. 20.
225 Unlike CT and X-ray, MRI does not use ionising radiation and can be substituted in some circumstances.
226 The funding review, page. 24.
227 ibid.
228 ibid.
229 Implementation of other initiatives that have patient safety impacts are discussed in other chapters of this report. The initiatives to improve access to MRI in order to address increasing safety concerns about excess radiation exposure from CT scans are discussed in Chapter 4 and implementation of the initiatives to improve ‘appropriate requesting’ of diagnostic imaging are discussed in Chapter 3.
230 The Health Insurance Act (Diagnostic Imaging Services Table) Regulations 2012 included a new section (Division 2.3.1 Group I3 Diagnostic radiology ‘Who must perform diagnostic imaging procedure’) requires that MBS-funded diagnostic radiology procedures be performed by a medical practitioner or a medical radiation practitioner employed or supervised by a medical practitioner. Health’s reporting on this initiative was discussed in paragraphs 2.20 to 2.21.
231 From July 2010, state and territory registers for health professionals, including medical practitioners, were replaced by national registers supported by AHPRA. Registration with the national boards is mandatory for the 14 health professions covered.
232 The Health Insurance Act (Diagnostic Imaging Services Table) Regulations, as remade and amended as required (at least annually).
233 The funding review, p. 20.
234 Health informed the ANAO that any outcomes from the RIS process are unlikely to be implemented before 2016. Further discussion of changes being clarified and proposed to be moved out of the regulations and into the DIAS is at paragraphs 5.34 to 5.35 of this chapter.
235 The funding review, p. 20. ARPANSA undertook the first national survey in August 2011 of radiation doses to the Australian population from the use of CT and other types of diagnostic radiation which showed that there was a large variation in radiation doses across providers. This survey is now repeated every 12 months with results published on ARPANSA’s website, available from <http://www.arpansa.gov.au/Services/NDRL/statistics.cfm> [accessed 19 October 2014].
236 Reference levels have been identified separately for adults and children, available from <http://www.arpansa.gov.au/radiationprotection/FactSheets/is_CTScansForChildren.cfm> and <http://www.arpansa.gov.au/radiationprotection/FactSheets/is_CTScansReferrers.cfm> [accessed 18 September 2014].
237 RPS.14, available from <http://www.arpansa.gov.au/Services/NDRL/ndrlFactsheet.cfm#5> [accessed 18 September 2014].
238 Through its code of practice and related safety guidance, ARPANSA suggests that as part of practice equipment quality assurance programs, patient dose surveys be undertaken by diagnostic imaging practices to periodically establish that the doses are acceptable when compared with published reference levels. ARPANSA further suggests that accrediting bodies should consider including monitoring compliance of a sample of patient radiation doses against reference levels as one element in achieving accreditation to encourage institutions to perform dose surveys. ARPANSA advises that repeatedly and substantially exceeding the reference levels might indicate an underlying fundamental problem that warrants investigation.
239 The ANAO notes that radiation risks from CT scans has been a recognised concern for some years, and implementation of measures to address this risk during the reform period is not an example of a swift policy response. In 2000, a study was published in the American Journal of Radiology that identified paediatric CT was linked to higher radiation risk and cancer mortality, and that lower radiation settings can be used for children without significant loss of information. This study cited earlier research conducted between 1984 and 1999 that measured radiation exposure and risk, see DJ Brenner, CD Elliston, EJ Hall and WE Berdon, ‘Estimated Risks of Radiation-Induced Fatal Cancer from Pediatric CT’, American Journal of Radiology, 176, February 2001, pp. 289–296, available from <http://www.columbia.edu/~djb3/papers/ajr1.pdf> [accessed 11 September 2014].
240 This initiative followed the publication of recent Australian research, ‘The Mathews Study’: J Mathews et al, Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians, British Medical Journal, 346, 2013.
241 The project involves working with ARPANSA and the Australian Commission on Safety and Quality in Health Care to reduce radiation exposure from CT scans. It was informed by ‘The Mathews Study’ and involves fact finding and data collection, for example, in relation to CT usage patterns. Health is looking to provide this information to professionals using project funding over two years.
242 Refer to footnote 218.
243 Health advice to ANAO, 15 October 2014.
244 Digital imaging standards and protocols aim to ensure the requester receives information and images of a quality that enables diagnosis and treatment, and that information can be stored and shared across practices.
245 The funding review, p. 20.
246 Health has examined and undertaken consultation in relation to digital imaging standards with reference to the broader e-health agenda, identifying that progress will be undertaken with reference to the personally controlled e-health record (PCEHR) initiative.
247 Australian Technical Specification – Digital images for diagnostic and other clinical purposes: Presentation, communication, display and manipulation (ATS5816–2013), published by Standards Australia on 20 November 2013. The technical specifications are the outcome of the work of a Standards Australia working group, consisting of members from across the diagnostic imaging sector.
248 The funding review p. 20. DHS is responsible for compliance monitoring under the Health Insurance Act 1973 and its regulations, including the DIST. This audit did not examine DHS’s compliance activities in relation to diagnostic imaging services. DHS’s broad compliance role was examined as part of a recent ANAO performance audit, ANAO Audit Report No.26 2013–14 Medicare Compliance Audits.
249 The funding review, p. 20.
250 The funding review, p. 24.
251 A desktop accreditation is defined in Health Insurance (Diagnostic Imaging Accreditation) Instrument 2010 as ‘a review by an approved accreditor carried out other than in a diagnostic imaging practice to assess whether the diagnostic imaging practice meets the [standards] or has MIAP approval.
252 Health Insurance (Diagnostic Imaging Accreditation) Instrument 2010, Schedule 1.
253 Department of Health, Diagnostic Imaging Accreditation Scheme Practice Accreditation Standards, [internet], Department of Health, Canberra 2012, available from <http://www.health.gov.au/internet/main/publishing.nsf/Content/diagnosticimaging-accred-2-stand> [accessed 10 September 2014].
254 The frequency with which repeated requests for information occurs, or systemic problems with documentation that is provided for assessment is not required to be recorded by accreditors.
255 Health provides secretariat support for a pathology accreditation scheme which was introduced in 1986 and is compulsory in order to claim Medicare benefits for pathology services. In order to be accredited, a pathology laboratory must meet specified quality standards that are developed by the National Pathology Accreditation Advisory Council (NPAAC). DHS manages the administration of pathology laboratory accreditation including tracking the accreditation status of laboratories and managing the arrangements with the approved accreditor. This long-established scheme, involving on-site inspections by a team that comprises accreditation and expert pathology staff and overseen by a ministerially-appointed advisory council, is considered by Health and accreditors to provide a high level of assurance over compliance with quality and safety standards. Further detail on the accreditation scheme and NPAAC is available from <http://www.health.gov.au/npaac> [accessed 19 September 2014].
256 Any changes to the DIAS can be made by way of a regulatory instrument that may be approved by the delegate of the Minister (a senior Health executive officer) and is disallowable by either house of the Parliament.
257 Diagnostic radiology includes X-ray, angiography and fluoroscopy.
258 These initiatives may result in amendments to the diagnostic imaging regulations to improve supervision of diagnostic imaging services, as well as ultrasound equipment standards, amongst others. Health has also reviewed the wording of the existing DIAS standards and commenced work on aligning DIAS standards with the National Safety and Quality Health Service Standards.
259 Self-assessment processes have inherent limitations with respect to the level of assurance they can provide on adherence to a regulatory framework.