Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Defence’s Management of Sustainment Products—Health Materiel and Combat Rations

Please direct enquiries relating to reports through our contact page.
The audit objective was to review the effectiveness of the Department of Defence’s (Defence) arrangements for delivering selected non-platform sustainment.
Summary and recommendations
Background
1. Non-platform products are items and supplies that do not represent weapons platforms, but are required to maintain the capability and operation of the Australian Defence Force. These can include clothing items, small firearms, health and dental equipment, and other consumables. The procurement, management and supply of these capabilities is conducted by Systems Program Offices within the Department of Defence (Defence).
2. The Health Systems Program Office (Health SPO) is responsible for the procurement and sustainment of pharmaceuticals, medical and dental equipment and consumables, and combat rations. Health SPO’s budget for sustainment in 2017–18 was $78 million.
3. In 2017, Health SPO undertook procurements for health materiel and combat rations with the resultant contracts having an estimated annual expenditure of $24 million and $26 million respectively. The approved estimated expenditure of the pharmaceuticals and combat rations contracts over a five year period is $120 million and $133 million respectively.
4. Defence’s effectiveness in delivering health materiel and combat rations was selected for audit to provide assurance over significant Commonwealth expenditure not previously subject to audit coverage as well as to provide transparency and assurance to the Parliament with regards to: the operation of sustainment Systems Program Offices; value for money in Defence’s sustainment of non-platform products; and compliance with the Commonwealth procurement rules for the areas under audit. This audit is part of the ANAO’s program of audits relating to Defence sustainment, which has included the recent ANAO Audit Report No.2 2017–18 Defence’s Management of Materiel Sustainment1—which focused on Defence wide governance arrangements for sustainment, including the strategic review of the Systems Program Offices.
Audit objective and criteria
5. The objective of the audit was to assess the effectiveness of Defence’s arrangements for delivering selected non-platform sustainment. To form a conclusion against the objective, the ANAO adopted the following high level audit criteria:
- Defence has implemented effective governance arrangements for the selected Systems Program Office; and
- Defence has appropriate procurement and contract management arrangements for the selected non-platform sustainment products.
Conclusion
6. Defence’s arrangements for delivering health materiel and combat rations through the Health Systems Program Office are effective other than in the areas outlined below.
7. Defence has put in place appropriate governance, reporting and accountability arrangements for the Health Systems Program Office. Effectiveness could be improved through increased IT systems integration and revising the use of internal key performance indicators.
8. Defence’s 2017 procurement and contract management arrangements for the supply and delivery of health systems products were appropriate except Defence did not:
- meet the risk policy of the Department, comply with the Commonwealth Procurement Rules in relation to records management, or implement arrangements for risk and probity management consistent with the intent of the Commonwealth Procurement Rules;
- seek to negotiate a reduction in tendered prices during contract negotiations; or
- plan effectively for the transition to the new contractual arrangements.
9. Defence’s 2017 procurement arrangements for the supply and delivery of combat rations were appropriate except Defence did not:
- meet the risk policy of the Department, comply with the Commonwealth Procurement Rules in relation to records management, or implement arrangements for risk and probity management consistent with the intent of the Commonwealth Procurement Rules; or
- implement a performance-based contract.
10. Defence’s decision to supply freeze-dried meal components as Government Furnished Material rather than through the combat rations contract may have limited the market and impacted the achievement of value for money for the Commonwealth.
Supporting findings
Governance Arrangements and Performance Reporting
11. Defence has established appropriate reporting and accountability mechanisms for the sustainment of health materiel and combat rations. However, the reporting under these arrangements is not fully effective as not all data requirements are being met.
12. Defence has in place appropriate policies to manage the sustainment of the selected products, including a specific Health Materiel Manual. Effective implementation of these policies is hindered by Defence’s monitoring of multiple IT systems that are not linked, leading to complex workarounds and instances of duplication, redundancies or out of date data.
13. Defence has a fit for purpose framework for performance reporting and monitoring within the Health SPO but its implementation is not fully effective. Key performance indicators in the Sustainment Performance Management System are: relevant and reliable, but not complete; linkages between Defence’s internal key performance indicators and those included in the audited prime vendor contracts are limited for the new pharmaceutical contract and there are no linkages with the new combat rations contract; and the Sustainment Performance Management System does not include all indicators used to monitor health materiel. The Sustainment Performance Management System allows for performance monitoring, trend analysis and cross-product comparison, however Land Systems Division only uses the System to report on key performance indicators. Two of the five key performance indicators for health materiel reported in the Sustainment Performance Management System are consistently not met, indicating Defence should take action to remedy performance shortfalls or reconsider the indicators.
14. Risks pertaining to the sustainment of health materiel and combat rations are reported on at key committees and meetings by Health SPO. Defence has not provided evidence that key operational and change management risks faced by Health SPO have been documented in risk management or business plans or that they are being managed.
Health Systems Fleet
15. In Defence’s procurement for pharmaceuticals, medical and dental equipment and medical and dental consumables, Defence largely complied with the Commonwealth Procurement Rules and most of its internal policies; however, it did not meet the risk policy of the Department, records management was not compliant with the Commonwealth Procurement Rules, and Defence’s arrangements for risk and probity management were not consistent with the intent of the Commonwealth Procurement Rules.
16. Defence records indicate that tender information was removed from Defence’s secure system during the procurement evaluation.
17. The 2017 tender and evaluation process for pharmaceuticals, medical and dental consumables and medical and dental equipment was designed to produce a value for money outcome, including the use of an open tender process as a basis for introducing competition. Defence negotiated with the preferred tenderer on a number of issues which improved the value for money outcome for the Commonwealth but did not seek to negotiate a reduction in tendered prices.
18. Defence implemented a performance based contract, which is supported by appropriate reporting procedures and management plans. The contract provides for scheduled reviews of the prime vendor’s performance, with the first review due in early 2018. Defence did not plan effectively for the transition to the new contractual arrangements.
Combat Rations
19. In Defence’s procurement for combat rations, Defence largely complied with the Commonwealth Procurement Rules and most of its internal policies; however, it did not meet the risk policy of the Department, records management was not compliant with the Commonwealth Procurement Rules, and Defence’s arrangements for risk and probity management were not consistent with the intent of the Commonwealth Procurement Rules.
20. Defence records indicate that tender information was removed from Defence’s secure system during the procurement evaluation.
21. The 2017 tender and evaluation process for combat rations was designed to produce a value for money outcome. Defence undertook a two stage, open tender process and conducted detailed evaluation of tenders. Defence negotiated with the preferred tenderer on a number of issues, including actively negotiating a reduction in distribution costs. Defence decided to supply freeze-dried meal components itself as Government Furnished Material rather than having those components supplied under the contract as initially indicated in tender documentation. This may have limited the market and, as Defence did not negotiate a reduction in tendered prices for the relevant ration pack, impacted on the achievement of value for money for the Commonwealth.
22. Whilst the contract for the supply of combat rations sets out the requirements and standards of the products to be delivered and contains some individual delivery payment controls, Defence has not implemented a performance-based contract. The contract does not specify how performance issues will be managed, or link key performance indicators to payments.
Recommendations
Recommendation no.1
Paragraph 2.27
That Defence refines its performance reporting and management arrangements for health materiel and combat rations by:
- aligning key performance indicators reported on in the Sustainment Performance Management System to the prime vendor contracts; and
- making use of the full reporting functionality of the Sustainment Performance Management System.
Department of Defence response: Defence accepts the recommendation.
Recommendation no.2
Paragraph 3.63
That for future procurements which involve a new service provider, Defence develops adequate phase-in plans.
Department of Defence response: Defence accepts the recommendation.
Summary of entity response
Defence acknowledges the observations contained in the audit report on Defence’s Management of Sustainment Products – Health Materiel and Combat Rations; and agrees to the two recommendations made by the ANAO
Key learnings for all Australian Government entities
Below is a summary of key learnings and areas for improvement identified in this audit report that may be considered by entities when managing procurements.
Governance and risk management
Procurement
Transition to new contracting arrangements
Performance monitoring and reporting
1. Background
Introduction
1.1 Non-platform products are items and supplies that do not represent weapons platforms, but are required to maintain the capability and operation of the Australian Defence Force. These can include clothing items, small firearms, health and dental equipment, and other consumables. In 2017–18, the Department of Defence’s (Defence) total budget for its capability sustainment program (including platform and non-platform products) is $9 474 million.
1.2 The procurement, management and supply of these capabilities is conducted by Systems Program Offices. As at December 2017, there were 62 Systems Program Offices within the Capability Acquisition and Sustainment Group of Defence responsible for managing the sustainment2 of 112 fleets of equipment, supplies or services, through a combination of internal work and commercial contracts. Systems Program Offices may be involved in acquiring new Defence capability, sustaining existing capability, disposing of or withdrawing capability, or all of these.
1.3 The Health Systems Program Office (Health SPO) is within the Integrated Soldier Systems Branch of Land Systems Division in Capability Acquisition and Sustainment Group. The Health SPO is responsible for the procurement and sustainment of pharmaceuticals, medical and dental equipment and consumables, and combat rations. The Health SPO is also responsible for the acquisition and through life support of the Australian Defence Force replacement Deployable Health Capability through Joint Project 2060 Phase 3.3
1.4 The Health SPO’s sustainment budget for 2017–18 is: $52.7 million for medical and dental equipment, pharmaceuticals, medical and dental consumables; and $25.3 million for combat rations packs. The Health SPO manages the sustainment of over 15 000 individual items of health materiel and combat rations (referred to as ‘line items’). The breakdown of the Health SPO’s budget for 2017–18 and line items is in Table 1.1.
Table 1.1: 2017–18 budget and number of line items for the Health SPO by sustainment product
|
Category |
Budget 2017–18 ($million)a |
Number of line items |
|
Medical and dental equipmentb |
18.5 |
4 539 |
|
Medical and dental consumables |
15.6 |
9 644 |
|
Pharmaceuticals, medical gases and pathology |
18.5 |
1 165 |
|
Total for health materiel (JHC01) |
52.7c |
15 348 |
|
Combat rations (CA50) |
25.3 |
90 |
|
Total |
78.0 |
15 438 |
Note a: This figure does not include any costs incurred by Defence in undertaking the management of sustainment, for example: staffing, accommodation, and other overhead costs.
Note b: This includes $6.6 million in funding for the Defence Services Agreement with Joint Logistics Command for equipment maintenance.
Note c: Total may differ due to rounding.
Source: ANAO analysis of Defence documentation.
1.5 The provision of health materiel and combat rations in Defence is managed primarily though Materiel Sustainment Agreements. These are contract‐like arrangements that set out the level of performance and support required by the Defence Capability Manager from the Capability Acquisition and Sustainment Group, within an agreed price, as well as the key performance indicators by which service delivery will be measured. Through the agreements, the Defence Capability Manager undertakes to supply funding and the Systems Program Office within the Capability Acquisition and Sustainment Group undertakes the sustainment of a specific platform, product, commodity or service.
1.6 The lead Capability Managers for health materiel and combat rations in Defence are Joint Health Command and Army Headquarters respectively.
Review and reform in Systems Program Offices
1.7 The 2015 First Principles Review4,5 recommended that each of the Systems Program Offices be examined to determine the most appropriate procurement model for delivering capability and achieving value for money.
1.8 A review of the Health SPO was conducted by an external consultant in April 2017. The review made nine recommendations. Key recommendations related to: the SPO changing its supplier engagement model to a single prime vendor arrangement6 for combat rations and multiple prime vendors for health materiel; the SPO developing a workforce planning program to increase its contract management skills; investigating the opportunity to consolidate health services; and addressing issues with existing information technology systems. The Head of Land Systems Division agreed to two recommendations, and conditionally agreed with the remaining seven.7
1.9 The majority of the recommendations are due for implementation in 2019 and 2020. The Health SPO has begun to implement one of the agreed recommendations (which is due to be implemented by mid-2018) relating to moving supplier engagement models to a single prime vendor for combat rations and to multiple prime vendors for health materiel. The transition to a new supplier engagement model is reflected in two recent prime vendor contracts Health SPO has negotiated, which are for the supply and delivery of pharmaceuticals and medical and dental consumables (discussed in Chapter 3), and the supply of combat rations and ancillary items (discussed in Chapter 4).
Audit approach
1.10 The objective of the audit was to assess the effectiveness of Defence’s arrangements for delivering selected non-platform sustainment. To form a conclusion against the objective, the ANAO adopted the following high level audit criteria:
- Defence has implemented effective governance arrangements for the selected Systems Program Office; and
- Defence has appropriate procurement and contract management arrangements for the selected non-platform sustainment products.
1.11 In undertaking the audit, the ANAO:
- reviewed relevant Defence files and documentation;
- collected and analysed data relating to the contract for the provision and delivery of pharmaceuticals and medical and dental consumables, and the current retender for the combat rations pack contract; and
- interviewed key Defence personnel including: staff from the Health Systems Program Office; Joint Health Command; and Army Headquarters.
1.12 The scope of this audit includes the management of sustainment of health materiel and combat rations undertaken by Health SPO. The audit examined the following two procurements in greater detail:
- the contract for the provision and delivery of pharmaceuticals and medical and dental consumables (Chapter 3); and
- the retender for the combat rations packs and ancillaries (Chapter 4).
1.13 The audit was conducted in accordance with the ANAO auditing standards at a cost to the ANAO of approximately $353 898.
1.14 The team members for this audit were Natalie Whiteley, Megan Beven, Sophie Gan and David Brunoro.
2. Governance Arrangements and Performance Reporting
Areas examined
The ANAO examined the effectiveness of governance arrangements for the Health Systems Program Office, focusing on: reporting and accountability mechanisms to senior management outside the Health Systems Program Office; policies, practices, and systems within the Health Systems Program Office; and performance reporting and risk management.
Conclusion
The Department of Defence (Defence) has put in place appropriate governance, reporting and accountability arrangements for the Health Systems Program Office. Effectiveness could be improved through increased IT systems integration and revising the use of internal key performance indicators.
Areas for improvement
The ANAO has made one recommendation aimed at the Defence refining its key performance indicators in relation to health materiel and combat rations, through ensuring clear linkages to the prime vendor contracts, and making use of the full reporting functionality of the Sustainment Performance Management System.
Has Defence established effective reporting and accountability mechanisms to senior management?
Defence has established appropriate reporting and accountability mechanisms for the sustainment of health materiel and combat rations. However, the reporting under these arrangements is not fully effective as not all data requirements are being met.
2.1 The Health Systems Program Office (Health SPO) interacts with a number of key areas in the Department of Defence (Defence) including: Joint Health Command for the health systems fleet and deployed and garrison health support8; Army for the provision of combat ration packs; and all Services for delivery and support of specific single service health requirements (for example, the incorporation of medical facilities and equipment into ships and aircraft). Figure 2.1 outlines the key areas in Defence involved in the sustainment of pharmaceuticals and combat rations.
Figure 2.1: Key areas in sustainment of pharmaceuticals and combat rations in Defence
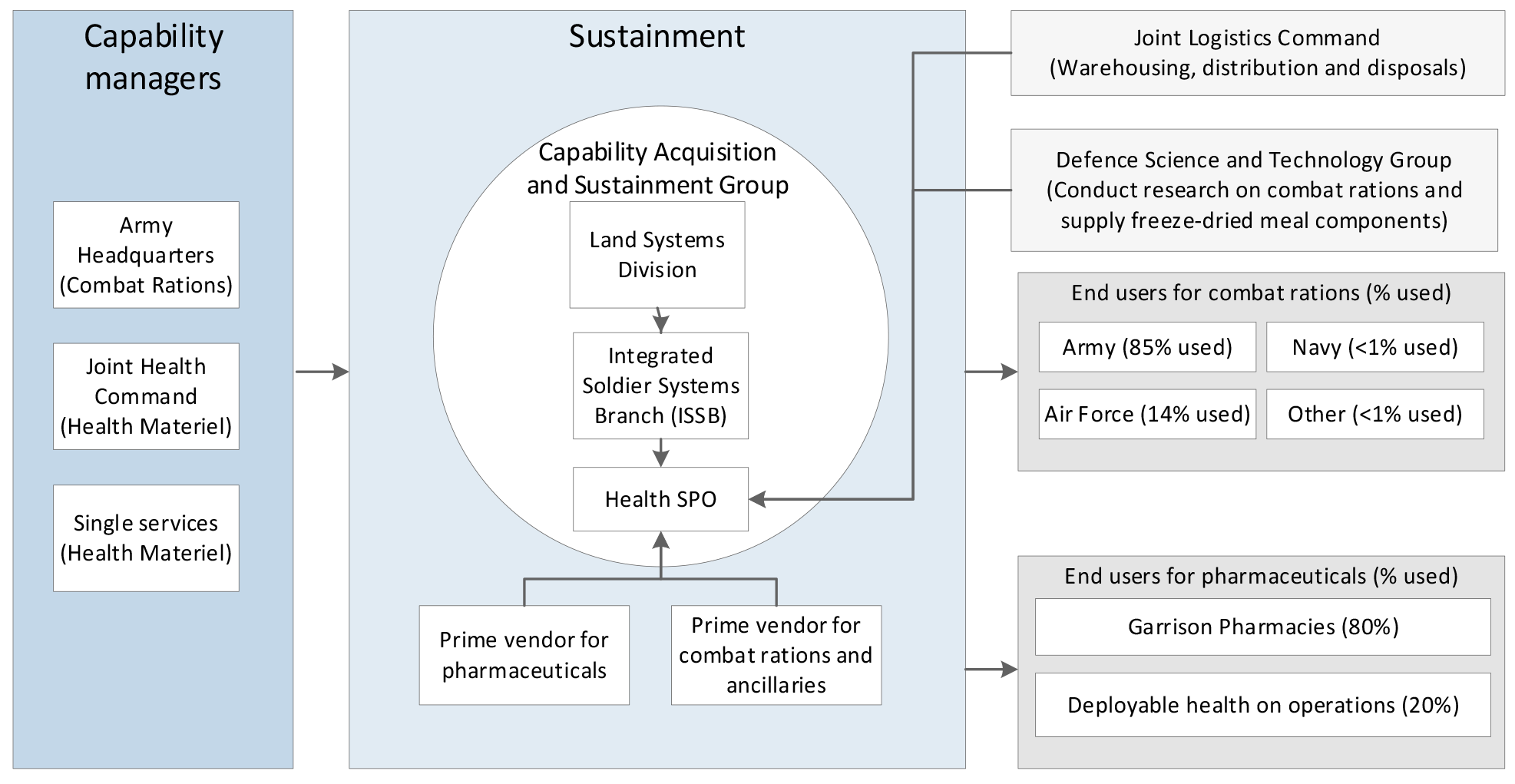
Source: ANAO analysis of Defence documentation.
2.2 The relationship between the Health SPO and the relevant capability manager is set out in Materiel Sustainment Agreements. The agreements outline the level of performance and support required by the capability manager in the sustainment of health materiel and combat rations. The Agreement includes an agreed price for the sustainment work and performance indicators by which the Health SPO’s service delivery is measured and reported.
2.3 Materiel Sustainment Agreements are divided into two sections: a Heads of Agreement which details the high level overarching agreement; and the product schedules that detail the specific agreement for the sustainment of various products. The product schedule sections of the Materiel Sustainment Agreements are reviewed annually by Defence.
2.4 To support the delivery of capability relating to health materiel and combat rations required under the Materiel Sustainment Agreements, there are a number of expert and coordinating committees in Defence (see Box 1).
|
Box 1: Committees |
|
2.5 The management of health materiel and combat rations is also considered in weekly Health SPO reports to Joint Health Command, Director General Senior Leadership Team meetings, and weekly briefing reports and talking points to the Head of Land Systems Division.9
2.6 The Health SPO provides representatives and contributes data to the above committees and reporting processes. The ANAO’s analysis of reporting by the Health SPO indicates that not all data requirements are being met, for example:
- The Pharmacy and Therapeutics Committee requested data from the Health SPO to inform decisions on the management of pharmaceuticals in the Australian Defence Force. The committee’s minutes indicate that Joint Health Command and the Health SPO have had ongoing discussions over the last two years on how the committee’s data requests can be met using available resources and IT systems.
- There is partial reporting against the performance measures specified in relevant Materiel Sustainment Agreements at the Health Materiel Working Group (see paragraphs 2.15 to 2.22 for further discussion on the adequacy of performance reporting and measures for health materiel and combat rations).
2.7 Further to the above reporting, following a projected overspend in the health materiel budget (see Box 2), Health SPO began providing additional weekly reports to Joint Health Command on financial information by commodity (pharmaceuticals, consumables, medical and dental equipment) in June 2017.
Has Defence implemented appropriate policies and systems within the Health SPO?
Defence has in place appropriate policies to manage the sustainment of the selected products, including a specific Health Materiel Manual. Effective implementation of these policies is hindered by Defence’s monitoring of multiple IT systems that are not linked, leading to complex workarounds and instances of duplication, redundancies or out of date data.
2.8 The Health SPO is required to manage the sustainment of health materiel and combat rations in accordance with key Defence manuals, namely:
- The Electronic Supply Chain Manual—this manual provides an overview of the Defence supply chain including inventory management, supply purchasing, financial management and performance reporting; and
- The Defence Logistics Manual, Part 2, Volume 5—Defence Inventory and Assets—this manual outlines key roles and responsibilities in the management of Defence inventory including around logistics, funding agreements, performance management and stocktaking.
2.9 In addition, the Health Materiel Manual outlines the roles and responsibilities, processes and procedures for the management of health materiel in Defence. This comprehensive manual is reviewed every three years and includes references to relevant legislation, standards and practices for the management of health materiel.
2.10 The key IT systems used by the Health SPO in the management of health materiel and combat rations are:
- Military Integrated Logistics Information System (MILIS)—As the primary Defence logistics system, MILIS is intended to provide visibility and management of every catalogued item of supply. Deployable and deployed health facilities use MILIS to order all health materiel.
- Advanced Inventory Management System (AIMS)—AIMS is used to forecast the demand for products based on historical data from MILIS.
- Army Capability Management System (ACMS)—Army uses this system for forecasting requirements for its product schedules including combat rations.
- Pharmacy Integrated Logistics System (PILS)—Garrison pharmacy staff and authorised deployed elements use PILS to order non-exclusion list health materiel from a prime vendor. PILS can be used to: demand and receipt health materiel; support clinical functions; and provide patient medication profiles.
- Resource and Output Management and Accounting Network (ROMAN)—ROMAN is Defence’s core financial transaction system.
2.11 The prime vendor for the provision and delivery of pharmaceuticals also has an online portal, allowing Defence staff to view available stock and place online orders with the prime vendor.
2.12 The key limitations with IT systems identified during the course of the audit relate to:
- The multiple IT systems used in the sustainment of health materiel and combat rations are often not linked, creating data duplication, complex workarounds and redundant and out of date data.
- Frequent oversight is required to ensure the accuracy of data used to forecast requirements when manually transferring data from Defence’s logistic management system (MILIS) to Defence’s forecasting system (AIMS). For example, data from MILIS that is used to forecast requirements in AIMS needs to be reviewed for spikes in usage, otherwise a single order could become an annual order.
- The prime vendor’s online portal does not interface with Defence’s other system for managing pharmaceutical inventory—PILS. As a result, pharmacists at Garrison Health Centres are manually entering data from the portal to PILS. This issue is discussed further in Chapter 3 of this audit report.
2.13 The box below provides an example of the impact of IT system limitations.
|
Box 2: Example of limitations in IT systems used in Health SPO |
|
In early 2017, a projected overspend of the health materiel budget of around $4.5 million triggered an immediate reduction in activity and consumption of stock. The overspend related to the purchase of medical consumable items. Defence’s internal advice noted the overspend was in part caused by limitations in key IT systems and a lack of monitoring of these systems. In particular, a lack of appropriate oversight of relevant IT systems resulted in a purchase being made through Defence’s inventory management system based on incorrect forecasting data from AIMS, in isolation of other funding requirements. As a result of the overspend, Joint Health Command sought to reduce budget expenditure in health materiel through introducing control measures for Garrison Health Centres including filling only critical pharmacy scripts, cross levelling of consumable materiel, and drawing against stocks of consumables held in Defence warehouses. Additionally, maintenance funding for hardware equipment was restricted to essential repair for essential medical hardware and the purchase of the flu vaccine for the Australian Defence Force was staged. The minute detailing the overspend noted that:
In response to the budget overspend Health SPO and Joint Health Command agreed to a set of financial and inventory management reporting including weekly financial reporting by commodity type (pharmaceuticals, medical and dental consumables, medical and dental equipment). |
Has Defence implemented an effective performance reporting and monitoring framework within the Health SPO?
Defence has a fit for purpose framework for performance reporting and monitoring within the Health SPO but its implementation is not fully effective. Key performance indicators in the Sustainment Performance Management System are: relevant and reliable, but not complete; linkages between Defence’s internal key performance indicators and those included in the audited prime vendor contracts are limited for the new pharmaceutical contract and there are no linkages with the new combat rations contract; and the Sustainment Performance Management System does not include all indicators used to monitor health materiel. The Sustainment Performance Management System allows for performance monitoring, trend analysis and cross-product comparison, however Land Systems Division only uses the System to report on key performance indicators. Two of the five key performance indicators for health materiel reported in the Sustainment Performance Management System are consistently not met, indicating Defence should take action to remedy performance shortfalls or reconsider the indicators.
2.14 The performance reporting and monitoring framework stems from the Materiel Sustainment Agreements for health materiel and combat rations. These agreements outline the key performance indicators as well as a number of other performance measures for each area.
2.15 The key performance indicators are reported on through the Sustainment Performance Management System (SPMS) and are outlined in Table 2.1.
Table 2.1: Key performance indicators for health materiel and combat rations
|
Key Performance Indicators |
Green |
Amber |
Red |
Measurement source |
|
Health materiel |
||||
|
Inventory and asset demand satisfaction rate |
≥ 90 per cent |
≥ 80 per cent to < 90 per cent |
< 80 per cent |
Calculated by Inventory Measurement and Analysis Tool.a |
|
Equipment (date equipment is required) |
≤ 30 days |
30 to 45 days |
≥ 45 days |
MILIS (run by Health SPO). |
|
Availability of operational items (A) |
= 100 per cent |
≥ 90 per cent to < 100 per cent |
< 89 per cent |
Operational availability technical state as averaged each month in SPMS. |
|
Availability of operational items (B) |
≥ 95 per cent |
≥ 85 per cent to < 95 per cent |
< 85 per cent |
Operational availability technical state as averaged each month in SPMS. |
|
Percentage inside lead time for operational itemsb |
= 100 per cent |
≥ 90 per cent to < 100 per cent |
< 89 per cent |
MILIS (run by Health SPO). |
|
Combat rations |
||||
|
Demand satisfaction rate for operationsc |
100 per cent |
< 100 to 85 per cent |
< 85 per cent |
Calculated by Inventory Measurement and Analysis Tool. |
|
Demand satisfaction rate for points of entry for Army training centresd |
> 80 per cent |
< 80 to 70 per cent |
< 70 per cent |
Calculated by Inventory Measurement and Analysis Tool. |
|
Demand satisfaction rate for raise, train, sustain |
> 80 per cent |
< 80 to 70 per cent |
< 70 per cent |
Calculated by Inventory Measurement and Analysis Tool. |
|
Maintain contingency stock levels |
100 per cent |
80 to < 100 per cent |
< 80 per cent |
Stock on hand at the MILIS district as a snapshot at the time of SPMS reporting. |
|
Compliance with delivery schedule |
< 14 days |
14 to 30 days |
> 30 days |
MILIS (run by Health SPO). |
Note a: The Land Sustainment Management Directorate within the Capability Acquisition and Sustainment Group provides all Systems Program Offices across Land Systems Division with monthly reports from the Inventory Measurement and Analysis Tool (IMAT). The IMAT reports are a suite of AIMS inventory key health indicators including: demand satisfaction rate, inventory balance trend, requirements determination workload trend, stock on hand and excess stock trends, AIMS replenishment recommendation trend, recommended orders, purchase orders profile, and overdue redistributions.
Note b: This key performance indicator has been removed from the 2018–19 of the Materiel Sustainment Agreement following the annual review in late April 2018.
Note c: Prior to July 2017, the target for this key performance indicator was 95 per cent.
Note d: Prior to July 2017, the target for this key performance indicator was 100 per cent.
Source: Materiel Sustainment Agreement JHC01 Sustainment of Health Capability 2017–18, Module B–Capability Requirements and Measures of Success, pp. 3–4; Materiel Sustainment Agreement CA50 2017–18, Module B—Capability Requirements and Performance Indicators, p. 6.
2.16 The other, non-key performance indicator, performance measures outlined in the Materiel Sustainment Agreement are largely reported on in a variety of other forums rather than through the Sustainment Performance Management System. These forums include the monthly meeting between Joint Health Command and Health SPO, and the Health Materiel Working Group. The performance measures include:
- achievement against planned and unplanned maintenance of medical equipment managed by Joint Logistics Command under the Defence Supplier Agreement;
- planned and phased commitment and expenditure of health materiel funding for the current financial year;
- codification of health equipment10;
- timely approval, publishing, amendments and review of key documents including for end users of medical equipment, equipment schedules, engineering and maintenance plans;
- planned and phased execution of health materiel procurement plan for the current financial year; and
- the delivery of health materiel on time, in full, and in serviceable condition in response to valid demands placed by customer units, organisations and agencies.11
2.17 In addition, the Business Plan for Land Systems Division 2016–18 identifies the measures of effectiveness for the Materiel Sustainment Agreements. These measures include:
- Achieving green traffic lights against key performance indicators on the Sustainment Performance Management System.12
- Achieving within a five per cent variance on the expenditure versus the budget for the product schedule.
- Delivering 100 per cent availability for all critical platforms in accordance with the Materiel Sustainment Agreement.
2.18 Figure 2.2 provides a summary of performance against key performance indicators as recorded in the Sustainment Performance Management System over a twelve month period.
Figure 2.2: Performance against key performance indicators as recorded in the Sustainment Performance Management System November 2016 to October 2017
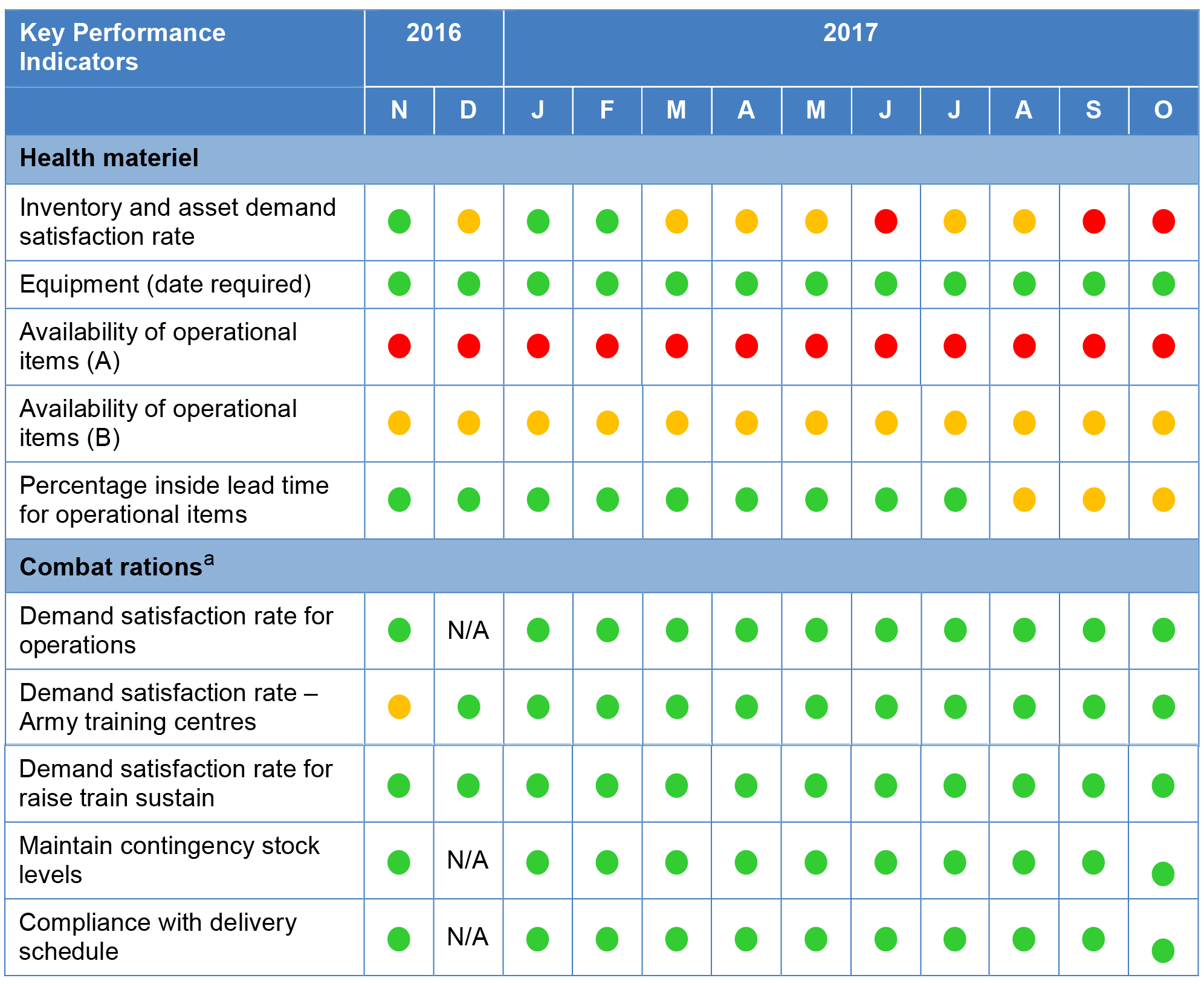
Note a: Performance data for demand satisfaction rate for operations, maintaining contingency stock and complying with delivery schedules was not recorded in the Sustainment Performance Management System for combat rations for the month of December 2016.
Source: Sustainment Performance Management System.
2.19 The ANAO’s assessment of the appropriateness of key performance indicators for health materiel and combat rations found that they were relevant and reliable but not complete—as summarised in Table 2.2.
Table 2.2: ANAO assessment of key performance indicatorsa for health materiel and combat rations against key characteristics
|
Characteristicb |
Health materiel assessment |
Combat rations assessment |
|
Relevant |
Yes. Each of the measures is designed to measure relevant outcomes for the sustainment of health materiel in Defence. |
Yes. Each of the measures is designed to measure relevant outcomes for the sustainment of combat rations in Defence. |
|
Reliable |
Yes. Each of the measures is reliable in that they can be objectively and readily measured, and performance can be tracked over time. |
Yes. Each of the measures is reliable in that they can be objectively and readily measured, and performance can be tracked over time. |
|
Complete |
No, because:
|
No, because:
|
Note a: The assessment relates to the key performance indicators in Figure 2.2.
Note b: These characteristics are based on the criteria developed to evaluate the appropriateness of an entity’s key performance indicators contained in Audit Report No.58, 2015–16, Implementation of the Annual Performance Statement Requirements 2015–16.
Source: ANAO analysis.
2.20 The key performance indicators identified in the Materiel Sustainment Agreements, and reported on in the Sustainment Performance Management System (listed in Table 2.1), are not aligned with the performance indicators included in the prime vendor contracts for pharmaceuticals and combat rations. For example:
- Both the pharmaceuticals contract and the Sustainment Performance Management System reporting on health materiel have key performance indicators relating to satisfying demands for inventory. However, they have different targets. The key performance indicator reported on in the Sustainment Performance Management System has a demand satisfaction target of greater than or equal to 90 per cent, while the key performance indicator in the pharmaceuticals contract has a target of 100 per cent. In addition, the key performance indicator in the Materiel Sustainment Agreement does not specify the need for ‘delivery in full’—which means the product meets specific quality standards (see Table 3.2 in Chapter 3).
- The pharmaceuticals contract includes a strategic performance measure of cost effectiveness which is a measure of the prime vendor’s ability to provide services at the best possible cost. A performance indicator for cost is not included in the health materiel key performance indicators in the Sustainment Performance Management System (see Table 3.2 in Chapter 3).
- There are no key performance indicators in the combat rations contract. There is also no explicit section in the contract regarding performance management (see paragraph 4.87 in Chapter 4).
2.21 As indicated in Figure 2.2, Health SPO has reported in the Sustainment Performance Management System that the key performance indicators for combat rations are, with one exception in the 12 month reporting period, consistently met. In contrast, the two key performance indicators regarding operational items for the Health SPO are either consistently reported as ‘red’ or ‘amber’ in the Sustainment Performance Management System. The reported underperformance is due to an inability to procure certain operational items at the 100 per cent stock level required under the key performance indicator. Defence advised that some of these items are subject to a worldwide supply shortage.
2.22 Defence informed the ANAO that there are ongoing discussions between Joint Health Command, Health SPO, and the Services regarding the key performance indicators, and how they impact on the Australian Defence Force’s ability to meet training, operational requirements, and current threat protection rules. Defence informed the ANAO in February 2018 that key performance indicators for Health Materiel will be discussed at the February 2018 Health Materiel Working Group meeting with the aim of incorporating any agreed amendments into the 2018–19 Materiel Sustainment Agreement. Defence’s review process for the 2018–19 Materiel Sustainment Agreement was completed in April 2018. ANAO’s review of the 2018–19 Materiel Sustainment Agreement found one key performance indicator—percentage inside lead time for operational items—had been removed.
Defence’s use of the Sustainment Performance Management System
2.23 The Sustainment Performance Management System is Defence’s primary sustainment reporting and performance management system.13 It is a web-based system designed to provide performance reports for Capability Acquisition and Sustainment Group and Capability Managers. Data is entered monthly, usually by officers in the relevant Systems Program Office. The Systems Program Office Director reviews the data and comments for each measure and provides additional comments. Further comments can be added up the hierarchy to the relevant Capability Acquisition and Sustainment Group division head.
2.24 The System can include key performance indicators and key health indicators (as defined by the Services), and also strategic sustainment analytics, or high level health indicators used for cross-platform performance analysis.
2.25 The ANAO found that the Sustainment Performance Management System was viewed favourably by Health SPO and Army and Joint Health Command as capability managers.
2.26 However, the ANAO also found that the Health SPO and relevant capability managers were not making use of the full reporting functionality of the System and that ongoing review was needed. For example:
- The System has been used inconsistently across the Systems Program Offices and across the Services. While the System allows for performance monitoring, trend analysis and cross-product comparison, some of the Services only use a limited range of the System’s functions. For example, Maritime Division (Navy) undertakes more detailed reporting than Land Systems Division, which does not report on indicators that monitor ongoing trends or allow comparison across products.
- There has been an instance where information recorded in the System was not reviewed and necessary action not taken. A contributing factor to the budget overspend noted in Box 2 was that advice to the capability manager on the System regarding the management of the budget for medical hardware was not reviewed and investigated.
Recommendation no.1
2.27 That Defence refines its performance reporting and management arrangements for health materiel and combat rations by:
- aligning key performance indicators reported on in the Sustainment Performance Management System to the prime vendor contracts; and
- making use of the full reporting functionality of the Sustainment Performance Management System.
Department of Defence response: Defence accepts the recommendation.
Has Defence undertaken appropriate risk assessment and management within Health SPO?
Risks pertaining to the sustainment of health materiel and combat rations are reported on at key committees and meetings by Health SPO. Defence has not provided evidence that key operational and change management risks faced by Health SPO have been documented in risk management or business plans or that they are being managed.
2.28 Health SPO manages two types of risks:
- risks pertaining to the sustainment of health materiel and combat rations; and
- operational risks associated with conducting its business, including changes to the Supplier Engagement Model, workforce planning and meeting Defence policy requirements.
2.29 The Materiel Sustainment Agreement product schedules identify the risks and constraints14 for health materiel and combat rations, as shown in Table 2.3.
Table 2.3: Risks and constraints for Health Materiel and Combat Rations as identified in the Materiel Sustainment Agreements
|
Risks and constraints |
Mitigation strategies |
|
Health Materiel |
|
|
Extant inventory and procurement procedures incur delay to the acquisition and provision of stock. |
|
|
Capability Acquisition and Sustainment Group may be unable to accommodate variation and surge due to increased workload associated with unconstrained demand due to resource limitations or reductions. |
|
|
Commitment and expenditure schedule variation due to extended lead times associated with procurement. |
|
|
Technical failure of equipment, system or deficiency in compliance with technical regulatory framework. |
|
|
Long procurement lead times for medical and dental equipment and limited capacity for commercial marketplace to supply within short timeframes. |
|
|
Technology advances and changes in clinical practice contribute to short life of type for many health systems fleet items. |
|
|
Requirement to integrate equipment with ancillary components and delivery of platforms. |
|
|
Combat Rations |
|
|
Insufficient contingency holdings. |
|
|
Surge above contracted annual order. |
|
Source: Materiel Sustainment Agreement JHC01 2017–18, Module E—Product Issues, Risks and Constraints; and Materiel Sustainment Agreement CA50 2017–18, Module E—Product Issues, Risks and Constraints
2.30 These risks and mitigation strategies are discussed regularly at key committees and meetings including the Health Materiel Working Group and Health SPO Monthly Performance Meeting.
2.31 The significant change occurring across all Systems Program Offices in the Capability Acquisition and Sustainment Group raises some potential operational challenges and risks for Health SPO. These primarily relate to: changes to the Supplier Engagement Model—with an increase in the use of prime vendor contracts; and workforce planning—including ensuring staff are reskilled to move from transactional based work to undertaking more complex contract management roles.15 In addition, the Health SPO has also concurrently undertaken a number of large procurements, including for pharmaceuticals and medical consumables (Chapter 3), medical and dental equipment (paragraph 3.12), and combat rations (Chapter 4).
2.32 Health SPO does not have its own risk management or business plans to document and help manage its specific operational risks, as risk management in the Capability Acquisition and Sustainment Group is undertaken at the divisional level.16 However, the specific key Health SPO risks such as those in paragraph 2.31 are not documented in the Land Systems Division Risk Management Implementation Plan.
2.33 The management of risk relating to the procurement process for the health materiel and combat rations contracts is discussed in Chapters 3 and 4 respectively.
3. Health Systems Fleet
Areas examined
The ANAO examined whether the Department of Defence’s (Defence) 2017 procurement and contract management arrangements for the supply and delivery of pharmaceuticals, and medical and dental consumables, were appropriate—focusing on compliance with procurement requirements, procurement design to achieve value for money, and contract deliverable outcomes.
Conclusion
Defence’s 2017 procurement and contract management arrangements for the supply and delivery of health systems products were appropriate except Defence did not:
- meet the risk policy of the Department, comply with the Commonwealth Procurement Rules in relation to records management, or implement arrangements for risk and probity management consistent with the intent of the Commonwealth Procurement Rules;
- seek to negotiate a reduction in tendered prices during contract negotiations; or
- plan effectively for the transition to the new contractual arrangements.
Area for improvement
The ANAO has made one recommendation aimed at improving Defence’s planning processes when new contracts are introduced.
Overview of the Health Systems Fleet
3.1 The Health Systems Fleet supports medical, dental, veterinary and ancillary health care in the Department of Defence (Defence) for both garrison and deployable environments.17 The fleet has three broad commodity groupings:
- pharmaceuticals18—items which require specialised management and handling to maximise product security, efficacy and shelf-life (for example, paracetamol, adrenaline, vaccines and anti-venoms);
- medical and dental consumables—items which are expendable and consumable (for example, surgical dressing materials, syringes and needles, diagnostic kits); and
- medical and dental equipment—items used for the diagnosis, prevention, monitoring and/or treatment of a medical condition (for example, aeromedical evacuation equipment and radiology diagnostic equipment).
New support contract for the provision of pharmaceuticals
3.2 In early 2016, Health Systems Program Office (Health SPO) undertook a procurement process for a single prime vendor to supply and deliver pharmaceuticals, medical and dental consumables and selected medical and dental equipment.
3.3 Prior to this, Defence procured pharmaceuticals and medical and dental consumables through two separate prime vendor contracts (both contracts were with the same supplier); and procured the selected medical and dental equipment as required through either a Standing Offer Panel or Request for Quotes. Warehousing, distribution and maintenance of the selected medical and dental equipment were managed through a Defence Services Agreement between Joint Logistics Command and Capability Acquisition and Sustainment Group worth $6.6 million.
3.4 The extant contracts and/or standing offers for the three commodity groupings (pharmaceuticals, medical and dental consumables, medical and dental equipment) had different expiry dates. Consequently, Defence planned a staged approach to the implementation of the three separate work packages, as shown in Figure 3.1.
Figure 3.1: Key dates in the new prime vendor contract

Source: ANAO analysis of Defence documentation.
3.5 On 16 June 2017, Defence entered into a contract with a new prime vendor, Central Healthcare Services, to manage the supply, warehousing and distribution of pharmaceuticals.19
3.6 The inclusion of medical and dental consumables was scheduled for introduction under the new contract for November 2017, but has been delayed until May 2018. In the interim the extant provider will continue to deliver medical and dental consumables to Defence.
3.7 The approved estimated expenditure under the contract was $120.64 million across the initial five year period for the pharmaceutical component only. A significant proportion of the approved estimated expenditure under the contract is for non-fixed pricing and task-based services.20
Did Defence comply with the Commonwealth Procurement Rules and relevant Defence policies?
In Defence’s procurement for pharmaceuticals, medical and dental equipment and medical and dental consumables, Defence largely complied with the Commonwealth Procurement Rules and most of its internal policies; however, it did not meet the risk policy of the Department, records management was not compliant with the Commonwealth Procurement Rules, and Defence’s arrangements for risk and probity management were not consistent with the intent of the Commonwealth Procurement Rules.
Defence records indicate that tender information was removed from Defence’s secure system during the procurement evaluation.
3.8 The Commonwealth Procurement Rules21 are the basic rule set for Australian Government procurements and govern the way in which entities undertake their procurement processes.22 To support the application of procurement related legislation and policy, Defence has established a single overarching procurement policy framework, managed by Capability Acquisition and Sustainment Group.23 This includes a number of compulsory activities Defence officials were required to carry out when undertaking procurement.
Planning the procurement
3.9 Defence undertook key planning activities. These included: engagement with the capability manager on key requirements; development of a procurement schedule; endorsements to proceed; and a tender evaluation plan.
Procurement strategy
3.10 Defence’s procurement strategy consisted of the Endorsement to Proceed24 which provided a high-level overview of Defence’s consideration of whether the procurement would deliver value for money. In August 2015 two work packages—pharmaceuticals (Work Package One), and medical and dental consumables (Work Package Two)—were endorsed for inclusion in the procurement. In March 2016, several days prior to the release of the request for tender, an additional work package for selected medical and dental equipment (Work Package Three) was included in the tender.25 The selected medical and dental equipment is only a small proportion of the overall medical and dental equipment procured by Defence.
3.11 The inclusion of the third work package was to allow Defence to:
assess the option to utilise the resultant contract, with the existing contractor, without the need to conduct a separate open approach to market for all or any of the products described in [Work Package Three].26
3.12 There is a separate concurrent tender activity underway by Health SPO for the procurement of the remainder of the medical and dental equipment and services Defence requires. The introduction of Work Package Three will be dependent on the outcome of this activity and decisions regarding Defence’s Randwick facility (see paragraphs 3.75 to 3.76). The relevant Endorsement to Proceed and other planning documentation (such as the Tender Evaluation Plan) contained limited information on how these two procurement processes would interact.
Risk management
3.13 The Commonwealth Procurement Rules state that:
Relevant entities must establish processes for the identification, analysis, allocation and treatment of risk when conducting a procurement. The effort directed to risk assessment and management should be commensurate with the scale, scope and risk of the procurement. Relevant entities should consider risks and their potential impact when making decisions relating to value for money assessments, approvals of proposals to spend relevant money and the terms of the contract.27
3.14 Defence’s internal Project Risk Management Manual also requires that all project risks be documented in a risk register and that staff are to update the risk assessment for a project at key decision points and milestones.
3.15 There was no documented risk management register for the procurement and Defence was unable to provide evidence to the ANAO that it established processes for the identification, analysis, allocation and treatment of risk when conducting the procurement.
Probity and conflict of interest
3.16 The Commonwealth Procurement Rules require that ‘officials undertaking procurement must act ethically throughout the procurement’, including ‘recognising and dealing with actual, potential and perceived conflicts of interest’ and the equitable treatment of participants.28
3.17 Defence also has specific instructions on managing potential conflicts of interest, which apply to Defence personnel and external service providers under contract to Defence.
3.18 In the absence of a risk management plan for the procurement, there is no evidence of specific consideration of or actions taken to ensure officials undertaking the procurement acted ethically. Defence was unable to demonstrate to the ANAO that it established processes to recognise and deal with actual, potential or perceived conflicts of interest. For example, staff involved in the procurement process, including the tender evaluation, were not required to complete conflict of interest declarations.
3.19 Additionally, Defence was unable to demonstrate to the ANAO that it established processes to ensure the equitable treatment of participants. For example, Defence did not develop a probity plan for the procurement which addressed arrangements for the provision of probity advice.29 In April 2018, Defence advised the ANAO that:
Probity advice was available throughout the procurement process from the Integrated Soldier Systems Branch Chief Contracting Officer. Ultimately, no formal request for probity advice was required. The validity of using an internal source of probity advice was confirmed orally by the Branch Chief Contracting Office in 2016, and is consistent with Defence procurement policy that states ‘there is no requirement for Defence officials to engage an external probity or process adviser’ and that ‘internal personnel (for example, contracting officers or Defence Legal officers) can potentially perform the role of a probity adviser for a Defence procurement’.
Security of tender information
3.20 The Commonwealth Procurement Rules provide that: ‘when conducting a procurement … entities should take appropriate steps to protect the Commonwealth’s confidential information’.30 The Tender Evaluation Plan stated that all tendered material should be kept secure.
3.21 Defence records indicate that tender information was removed from Defence’s secure system, and information was sent from Defence email accounts to personal email accounts of Defence staff.31 Documents potentially containing information of a sensitive nature relating to the tender and Health SPO activities that were emailed to personal accounts included:
- internal briefing material on certain vaccines;
- internal briefing material on health materiel budget concerns; and
- internal briefing material on the closure of a Defence facility responsible for warehousing, delivering and maintaining pharmaceuticals, medical and dental consumables, and medical and dental equipment (see paragraph 3.76).
3.22 In the course of the audit the ANAO advised Defence of this issue. Defence informed the ANAO in March 2018 that it had undertaken an independent32 assessment of the issue. The security incident was documented in a security report and individuals involved received formal correspondence from their senior executive officer as well as a verbal debriefing on the outcomes of the investigation.33 Defence advised the ANAO in April 2018 that: ‘the issues regarding security of tender information did not affect the conduct or outcome of the tender assessments. Tenders were not altered, as the tender had been closed.’
Approach to market
3.23 Defence adopted an open request for tender process for the procurement. The ANAO’s review of the Request for Tender documentation found that it met the requirements of the Commonwealth Procurement Rules.34
3.24 Tenderers were required to tender for Work Packages One and Two, and provide a response to demonstrate their capacity and capability to provide Work Package Three.
3.25 The tender was open for applications for 13 weeks (from March 2016) to ensure ‘tenderers had adequate time to address the tender requirements and form any business arrangements that they require to ensure the submission of one tender to address all requirements sought’.35 This was compliant with the minimum time limits of the Commonwealth Procurement Rules.36 Defence held an industry briefing for potential suppliers in April 2016, with the opportunity for organisations to request individual meetings with Defence.
Evaluation
3.26 Defence developed a Tender Evaluation Plan outlining the evaluation process for the procurement.
3.27 A Tender Evaluation Board was formed to conduct the evaluation of the tenders received. The Board consisted solely of Defence officials from within Health SPO, including those staff involved in the management of the extant contract. As discussed earlier in paragraphs 3.17 to 3.19, Defence was unable to provide evidence that there were probity plans or conflict of interest arrangements in place for the procurement.
3.28 The evaluation process and outcomes are summarised in Table 3.1 below. The Tender Evaluation Board considered only two of the seven tenders received to be competitive. Of the five tenders considered to be non-competitive37:
- one was not accepted due to late submission;
- one was excluded for not containing sufficient information to enable a competitive assessment and did not contain a number of key deliverables; and
- three were considered non-competitive or not exhibiting value for money and were set aside by the Tender Evaluation Board.38
Table 3.1: Tender evaluation process and outcomes
|
Process |
Description |
Outcome |
|
Registration and late tenders |
Register the receipt of all tenders, with late tenders excluded.a |
Seven tenders received, with one tender not accepted as it was received after the tender period closed. |
|
Screening |
Screen tenders, excluding tenders which did not satisfy the minimum content and format requirements from detailed evaluation. |
Six tenders screened and shortlisted:
|
|
Shortlisting |
Identify non-competitive tenders that had no reasonable prospect of exhibiting the best value for money in comparison to other tenders received. Tenders that were identified as non-competitive were not considered for detailed evaluation. |
|
|
Detailed evaluation |
Detailed evaluation to assess tender deliverables against the requirements of the request documentation, producing analysis and comparative assessment of each tender against the evaluation criteria. Each tender would also undergo a value for money assessment. Tenders were assessed as preferred and non-preferred. |
Five tenders underwent detailed evaluation, with:
|
Note a: The Commonwealth Procurement Rules specify late tender submissions must not be accepted, unless the submission was late as a consequence of mishandling by the relevant entity.
Source: ANAO analysis of Defence documentation.
3.29 The detailed evaluation against the evaluation criteria39 was performed by each of the Tender Evaluation Working Groups and reported to the Tender Evaluation Board. The Tender Evaluation Board then assessed the tenderers’ overall compliance and the level of risk of each tender against the requirements and ranked them accordingly.40 Tenders were provided with an overall ranking based on these assessments.
3.30 According to the Tender Evaluation Plan the evaluation of tenders was anticipated to have been completed by 15 August 2016 and approved by the delegate by 1 September 2016. The evaluation of tenders was completed on 30 November 2016 and approved by the delegate in December 2016.
Evaluating prices and pricing structure
3.31 As a requirement of the tender, in addition to providing recurring service fee costs, tenderers were asked to provide information on how they would price product and delivery costs for both Work Package One and Work Package Two. Defence did not require a tendered price for Work Package Three, as noted in the Endorsement to Proceed:
Due to the limited information that will be provided in the release documentation on [Work Package Three] content, the Commonwealth will not seek or evaluate tenderers’ offers regarding price or delivery rates. Detailed evaluation will be limited to an assessment of tenderers ability and managerial capability to supply products within the categories identified in [Work Package Three] and associated risk assessment.
3.32 To ‘obtain consistency across responses to facilitate evaluation’, tenderers provided cost information for 605 pharmaceutical items and 670 medical and dental consumable items against Defence’s estimated requirements.41
3.33 In evaluating prices and pricing structure, Defence undertook a compliance and risk rating for each tender. Defence also assessed and compared each tender on:
- the pricing structure and tendered prices for a sample of 475 pharmaceutical items42;
- the pricing structure for medical and dental consumables;
- recurring service fee costs; and
- delivery costs.
3.34 There was no assessment or comparison between tenderers for the tendered prices for medical and dental consumables (Work Package Two), with the Tender Evaluation Working Group advising that, whilst ‘the evaluation of pharmaceuticals was achievable due to the use of industry based identification numbers’, there were ‘significant levels of complexity to the evaluation of the [medical and dental consumables] items due to the inability to match like-for-like items due to varying descriptions’.
3.35 In the assessment of the pricing structures, Defence assessed that pricing structures which fixed prices annually and were required to be re-negotiated each year presented limited flexibility and increased risk to the Department. This is because pricing reductions or increases would not be passed on in real time, and imposed ‘an administrative task on the parties which is unlikely to provide the Commonwealth with cost comparative saving’. Defence instead opted for the preferred tenderer’s cost mark-up model, which Defence assessed as ‘consistent with the [Request for Tender] pricing model, which seeks to take advantage of market fluctuating pricing’.
3.36 The preferred tenderer did not have the lowest tendered price compared to other tenderers. The preferred tenderer was ranked higher as it was considered to have a lower risk pricing structure as well as lower recurring service fees. Additionally, advice to the delegate noted that a comparison with the preferred tenderer’s tendered prices and the current general product price list with Defence offered an average cost saving of 1.5 per cent.
Australian Industry Capability Plans
3.37 Consistent with Defence policy, Australian Industry Capability43 plans were required to be submitted as part of tender responses.
3.38 In evaluating tenders responses the Tender Evaluation Board found all responses were ‘deficient to various degrees’ with the relevant Tender Evaluation Working Group, noting that ‘they will take a lot of work to reach compliance, even the marginal ones’.
3.39 The Tender Evaluation Board agreed that ‘no tender response would be ranked’ and that ‘every tender was assessed as similar … due to the restrictions placed by legislation and the tender process did not identify any significant [Australian Industry Capability] within any offer’. The Tender Evaluation Board noted ‘that the [Australian Industry Capability Plan] would need to be developed as part of the negotiation stage with the preferred tenderer’.
3.40 During negotiations Defence and the preferred tenderer reached agreement that the preferred tenderer would amend its plan by the contract effective date and that it would continue to evolve for the duration of the contract. An Australian Industry Capability Plan was included in the resultant contract.
Records management
3.41 The Commonwealth Procurement Rules require that ‘officials must maintain for each procurement a level of documentation commensurate with the scale, scope and risk of the procurement’.44 The Commonwealth Procurement Rules note this should include, for example, the process that was followed, how value for money was achieved and relevant decisions and the basis for those decisions.
3.42 Defence maintained records of key documentation for the procurement; however, there are instances where there was insufficient documentation to assess some of the processes that were followed, some of the relevant decisions made, and/or the basis for some of the decisions, namely:
- correspondence and/or communication with potential suppliers, tenderers and suppliers;
- no record of any directive from the delegate on the inclusion of the medical and dental equipment in the procurement; and
- minutes, directives and working documents for meetings of the Tender Evaluation Board and some relevant Tender Evaluation Working Group reports.
Were Defence’s procurement arrangements designed to achieve value for money for the Commonwealth?
The 2017 tender and evaluation process for pharmaceuticals, medical and dental consumables and medical and dental equipment was designed to produce a value for money outcome, including the use of an open tender process as a basis for introducing competition. Defence negotiated with the preferred tenderer on a number of issues which improved the value for money outcome for the Commonwealth but did not seek to negotiate a reduction in tendered prices.
3.43 Achieving value for money is the core rule of the Commonwealth Procurement Rules.45
3.44 Defence adopted an open tender process for the procurement, as a basis for introducing competition and contributing to value for money outcomes.
3.45 In advice to the ANAO, Defence commented that ‘despite the large annual budget, Defence is considered by industry to be a relatively small customer with a consumption rate for health products similar to a large city based hospital.’ Defence considered that the option to consolidate the services and items contained in the three work packages under one contract would increase the commercial attractiveness of supplying these services and items, ‘potentially increasing cost savings and streamlining the requirement’. Defence advised the ANAO that, as a result of the consolidation, ‘a number of major providers to the healthcare industry responded [to the request for tender], which has not been the case for previous [tenders] of this nature’.
3.46 Consistent with the First Principles Review, Defence also aimed to reduce transactional and administrative overheads by reducing its involvement in the supply process. For example, Defence sought direct order placement by its pharmacists with the prime vendor. Defence did not estimate or undertake analysis of the potential savings that may be delivered to government as a result of these changes.
3.47 Defence also sought a value for money solution by broadening the pharmaceuticals that the prime vendor would be required to supply to a ‘full range of pharmaceutical[s] and medicines’.46 The previous contract was limited to the current, approved formulary list47 which Defence identified as contributing to ‘large administrative overheads’ when changes to the formulary were made.48
3.48 As noted in paragraph 3.35, Defence considered the tenderers’ pricing structures in the tender evaluation. Consistent with broadening the items to be supplied, for task-based services, Defence gave preference to a formula based costing method applied across the full range of requested items, over a fixed-price model negotiated annually.
3.49 In evaluating price tenders, Defence did not explicitly consider the pricing model for the supply of Pharmaceutical Benefits Scheme items. Defence informed the ANAO in February 2018 that the open tender ensured the best price possible and that a ‘desktop scan only of [Pharmaceutical Benefits Scheme] pricing was taken from the Department of Health [Pharmaceutical Benefits Scheme] Website … to provide a high level comparison’.
3.50 The resultant contract includes arrangements for the pricing of Pharmaceutical Benefits Scheme items. Items with a cost price to the provider of $930 or greater will have a flat rate mark-up of $69.94 (before applying any supplier funded discounts).49 Defence informed the ANAO in February 2018 that the $930 threshold resulted from the preferred tenderer’s offer and contract negotiation. These amounts are set under the Pharmaceuticals Benefits Scheme as listed on the Department of Human Services website.50
Contract negotiations
3.51 In January 2017, around four months behind the original schedule, Defence commenced negotiations with the preferred tenderer. Defence’s negotiation strategy identified several issues to be resolved during negotiations, but Defence did not plan to negotiate a reduction in tendered prices during negotiations phase. Even relatively small price savings achieved through negotiation could have delivered overall savings to the Commonwealth.51
3.52 Advice to the delegate noted that, in all issues negotiated, Defence achieved its preferred or minimum fall-back position.
3.53 The delegate approved the provision of the contract to the preferred tenderer in May 2017. The contract was signed on 16 June 2017, approximately six months after the original planned date. As a result of the delay, the extant provider—whose contract expired in December 2016—continued to supply pharmaceuticals to Defence as per their contractual requirements.52
3.54 The contract notice was published on AusTender on 1 August 2017 (46 days after contract signature), which is above the Commonwealth Procurement Rules requirement of 42 days.53
3.55 Regarding possible savings from the contract, advice to the delegate noted that:
Compared to the extant Contract costs of $18.3 [million] annually, subsequent information provided through the tender evaluation and negotiation process has borne possible savings of $2.9 [million] equating to a potential 15.94 [per cent] savings of contracted costs.
3.56 Defence documentation indicates that the calculation of the $2.9 million in savings was based on a comparison of the extant contractor’s fixed-price contract costs (such as management fees, delivery charges, progress meetings, and warehousing costs) and that proposed by the preferred tenderer for the first year only.
Are Defence’s contract deliverables provided to the required standard, within the agreed budget and timeframes?
Defence implemented a performance based contract, which is supported by appropriate reporting procedures and management plans. The contract provides for scheduled reviews of the prime vendor’s performance, with the first review due in early 2018. Defence did not plan effectively for the transition to the new contractual arrangements.
Management of transition arrangements
3.57 The contract was phased in with around two weeks between contract signature and the contract’s operative date, 3 July 2017. The contract with the new prime vendor, Central Healthcare Services, included information on the requirements and responsibilities of the transition as well as the implementation of subsequent work packages. Defence did not seek or receive a documented phase out plan as required under the extant contract. Additionally, Defence did not develop its own broader plan to manage the transition to the new contract, nor did it conduct a risk assessment to identify and mitigate risk.54
3.58 Defence pharmacists received three working days’ notice of the transition to the new contract, receiving advice from Health SPO on Thursday 29 June 2017 that the ‘go live’ date for the new contract and online ordering was Monday 3 July 2017.55 In addition, limited information was provided to pharmacists on the arrangement for placing orders (pharmacists were required to have additional software installed on their computers to enable them to order stock through the new prime vendor’s portal).
3.59 There were several issues identified by Defence with the phase in of the contract56, including:
- the duplication of processes for ordering pharmaceuticals as relevant IT systems were not integrated57;
- insufficient stocks of common medication (for example, paracetamol);
- problems with the delivery of medication (for example, couriers not having access to closed Defence bases);
- unclear procedures on returning medication supplied by the previous prime vendor; and
- problems with the ordering of pharmaceuticals by generic names rather than brand names and consequently receiving unintended products (for example, variations in the number of items per packet; different expiry dates; constantly switching the brands of pharmaceuticals a single patient uses, potentially introducing the risk of reduced medication compliance).
3.60 Defence advised the ANAO in November 2017 that:
Defence is meeting with (the prime vendor) on a weekly basis to resolve minor teething issues identified in the implementation. Defence is expected to meet with (the prime vendor) and [Joint Health Command] in (early 2018) to go over a lessons learnt activity to ensure we have a smooth transition of medical and dental consumables in May (2018).
Transition to subsequent Work Packages
3.61 As specified in the contract, the planned commencement date for Work Package Two—the medical and dental consumables component—was 14 November 2017 when the extant provider’s contract expired. In late 2017, Defence delayed the introduction of the work package until May 2018. This was to ensure the new prime vendor is ‘able to deliver [Work Package Two] without compromising [Work Package One]’.
3.62 In the interim, the extant prime vendor will continue to deliver medical and dental consumables through a six month contract extension worth up to $7.39 million.58 There is an additional cost to Defence for this extension, as the fixed-price component under the extant provider is higher than under the new contractual arrangements. This extension was approved by the relevant delegate.
Recommendation no.2
3.63 That for future procurements which involve a new service provider, Defence develops adequate phase-in plans.
Department of Defence response: Defence accepts the recommendation.
Management of budget
3.64 The new support contract had an estimated average annual expenditure of $24.13 million per year. Only 1.7 per cent of this estimated expenditure is classified by Defence as having certainty in terms of scope and cost. For example, while monthly administrative fees are known, the consumption of health items can only be estimated.
3.65 Following recent issues with the management of the budget for health materiel (discussed in Box 2) Defence has implemented additional weekly reporting by Health SPO to Joint Health Command to monitor actual expenditure against the allocated budget.
3.66 Defence documentation indicates that the delay in the introduction of medical and dental consumables (Work Package Two) component of the contract has increased pressure on the annual budget and will reduce the funds available for task-based services. Defence advised the ANAO that ‘any pressure on the annual budget is being managed within the governance framework established for [health materiel]’.
Management of contract deliverables
3.67 Health SPO is responsible for contract management and ensuring that the provider meets the contractual obligations, including ensuring that contract deliverables are provided to the required standard, within the agreed budget and timeframes.
3.68 Defence developed a statement of work which set out the requirements of the work to be carried out under the contract and the allocation of responsibilities between the Commonwealth and the new prime vendor. The statement of work was included in both the request for tender documentation and in the resultant contract with the new prime vendor.
Contract deliverables
3.69 The contract set out the requirements and standards of the products to be delivered. There were some inconsistencies with the requirements outlined in the contract and Defence policy, as discussed below.
3.70 The conditions of contract outlined the process for managing products which failed to meet: the specified requirements and standards; the specified timeframes; and/or delivery to the appropriate delivery points. The management process would be at Defence’s discretion according to the extent and type of failure and could include, for example: the prime vendor providing replacement products; correcting rejected products; and/or repossessing the rejected products. Defence can determine a reduction in payment or refuse payment if the contractor does not meet required standards.
Management and accounting for Health Materiel in Defence
3.71 The Defence Health Manual outlines how pharmaceuticals are to be managed and accounted for in Defence. These procedures include:
- Accounting for pharmaceuticals, including for controlled drugs, on Defence’s inventory management systems, in accordance with the Electronic Supply Chain Manual and the Defence Health Manual.59
- Undertaking a number of stocktake and assurance activities for scheduled substances including quarterly stocktakes and independent yearly checks of all Schedule 8 substances.
- Having in place controls for the process of disposal of pharmaceuticals, including: using clinical waste bins; and ensuring disposals are recorded in accordance with the Electronic Supply Chain Manual. Additionally, controlled drugs must be destroyed by pharmacists in the presence of an authorised officer60 or a Member of the Service Police.
3.72 Joint Health Command also has in place an audit program at health facilities. Information on the management and accounting procedures for health materiel in Defence is included at Appendix 2. An examination of these procedures was not included as part of the audit scope.
Defence policy requirements for management of health materiel
3.73 Defence policy has a number of key requirements for the management of health materiel, which sometimes exceed what is required in a commercial environment.
3.74 The cold chain requirements61 specified in the contract were not compliant with Defence policy. Defence advised the ANAO in February 2018 that ‘temperature data loggers have been procured and provided to the contractor. The contractor is amending its internal procedures to maintain compliance with the relevant standard’.62 Defence also advised the ANAO that a Contract Change Proposal has been drafted to enact the cold chain requirements and is expected to be implemented in the first quarter of 2018.
Closure of Defence’s Randwick facility
3.75 Defence’s Randwick facility provides warehousing, equipment maintenance and distribution services of medical and dental equipment, pharmaceuticals and medical and dental consumables (referred to as Class 8 Support).63
3.76 Defence is planning to close the Randwick facility in June 2018 and move to new arrangements in July 2018. Prior to this, Defence is reviewing the requirements currently undertaken at the facility, with some potentially to be included in the new support contract, namely the assembly and refill of first aid kits. There is no evidence the potential inclusion of these requirements in the support contract was appropriately planned for in the procurement and may have cost implications for Defence.
Management plans
3.77 The contract includes a management plan, known as the Support Services Management Plan, which is used to specify how a range of deliverables and requirements under the contract would be delivered. This is the primary plan for the contract and includes information on, for example, the prime vendor’s: risk management processes; quality management; and health, safety and environmental management.
3.78 To assist in the management of contract deliverables during the contract term, the prime vendor is also required under the contract to develop and deliver several plans and reports (for example, contract status reports) to Defence.
Performance measures
3.79 Defence set out to implement an ‘outcomes focussed performance based contract’. Table 3.2 outlines the one key performance indicator and the three strategic performance measures under the contract. As at February 2018, no performance reporting had occurred.
Table 3.2: Contract performance measures
|
Performance measure |
Description |
Assessment period, basis and outcome |
|
Key Performance Indicator |
||
|
Delivery in full on time |
Contractor’s performance in satisfying orders for products covered under the contract within the review period. |
Quarterly assessment. Assessment based on the number of satisfied ordersa as a proportion of total orders that were raised during the review period. Required level of performance is 100 per cent, with marginal or unacceptable performance resulting in impacts to monetary entitlementsb and/or performance management. |
|
Strategic Performance Measures |
||
|
Cost effectiveness |
A subjective measure of the contractor’s ability to provide services at the best possible cost within the review period.c |
Annual assessment, aligned with the contract status reportd and results provided at the annual performance review. Assessment based on the following:
|
|
Operational health |
The contractor’s performance in maintaining the required operational stock levels within the review period. |
Annual assessment, aligned with the contract status report. Assessment based on a fortnightly report that outlines: the latest stock levels for operational items; any incidents of deviations from the required stock level; and any Commonwealth approval for the deviation. |
|
Relationship |
The contractor’s ability to demonstrate positive relationships with the Commonwealth and other relevant third parties. |
Annual assessment, aligned with the contract status report. Assessment based on the contractor’s performance against specific relationship performance attributes. For example: resolves disputes at the lowest possible level; approaches problem solving in a joint manner; and displays a willingness to share critical information. |
Note a: A delivery is considered satisfied if: the correct product is delivered; the product is delivered to the correct location; the product is delivered on or before the specified delivery date; and the product meets the quality standards (if applicable). All of the conditions must be met, otherwise the delivery will be considered unsatisfied.
Note b: Defence is entitled to a refund based on an agreed formula for each review period if the prime vendor’s performance does not achieve the required level.
Note c: The Contract Performance Review is to be conducted at intervals no greater than 12 months and Service Performance Reviews at intervals no greater than six months.
Note d: The contract status reports are to be provided to Defence by the prime vendor ahead of contract performance reviews. For the new support contract, contract performance reviews occur every 12 months.
Note e: Defence advised the ANAO that the generic product report is provided to Defence by the contractor and is annually aligned with the contract status report.
Source: Defence documentation.
3.80 The key performance indicator used in the previous contract did not accurately measure the quality of the products being delivered (for example, whether upon receipt they have the required shelf-life and are in the right condition). The new key performance indicator focuses on performance and provides a basis for seeking a refund under the contract.
3.81 The cost-effectiveness strategic performance measure was introduced as a key means for the Commonwealth to ‘drive the Contractor to focus on realising cost savings for the Commonwealth by effectively aligning stock turnover rate with the perishability of the items and by purchasing generic products when they become available’. Defence estimated that effectively aligning stock management and moving to generic brands would provide a saving to the Commonwealth over the life of the contract. However there was no analysis underpinning this or dollar value estimated to help assess performance. The first review of the prime vendor’s performance is due in early 2018.64
4. Combat rations
Areas examined
The ANAO examined whether the Department of Defence’s (Defence) 2017 procurement and contract management arrangements for the supply of combat ration packs were appropriate, focusing on: compliance with procurement requirements; procurement design to achieve value for money; and contract deliverable outcomes.
Conclusion
Defence’s 2017 procurement arrangements for the supply and delivery of combat rations were appropriate except Defence did not:
- meet the risk policy of the Department, comply with the Commonwealth Procurement Rules in relation to records management, or implement arrangements for risk and probity management consistent with the intent of the Commonwealth Procurement Rules; or
- implement a performance-based contract.
Defence’s decision to supply freeze-dried meal components as Government Furnished Material rather than through the combat rations contract may have limited the market and impacted the achievement of value for money for the Commonwealth.
Areas for improvement
Defence has not implemented a performance-based contract. The contract does not specify how performance issues will be managed or link key performance indicators to payments.
Overview of combat rations
4.1 Combat rations are required to sustain Australian Defence Force personnel during operational conditions where suitable fresh rations are unavailable.65 Combat rations are used by the Australian Defence Force for both training and operations within Australia and overseas.
4.2 The Department of Defence (Defence) has specified requirements in regards to: nutritional content; number of personnel to be sustained; packaging; weight; ancillaries; and shelf-life. Defence has several types of combat rations, varying in size and menus66, to meet different operational requirements.67
4.3 In addition to food components, combat ration packs contain ancillary items, including: foot powder; hexamine fuel; compressed hexamine stoves; insect repellent; and water purification tablets.
4.4 The combat rations are made to military specifications and are a combination of Military off the Shelf and Commercial off the Shelf items.
4.5 As noted in Chapter 2, the Health Systems Program Office (Health SPO) is responsible for the sustainment of combat rations. The capability manager is Army.68
4.6 In 2016–17, the combat rations sustainment activity included the procurement of 90 different line items, worth around $30.5 million. Defence procures approximately 400 000 combat ration packs per year. This figure does vary, with over 706 000 procured in 2015–16. Army is the main consumer in the Australian Defence Force, using over 80 per cent of all combat ration packs.
4.7 Combat ration packs may also include Government Furnished Material.69 This included, for example, freeze-dried meal and rice components manufactured and supplied by the Defence Science and Technology Group and some ancillary items.70
4.8 In support of preparedness requirements, contingency holdings are in place for combat rations. Contingency holdings are managed by Joint Logistics Command, ‘ensuring holdings remain within life, fully functional and serviceable to meet…requirements’.71 Health SPO is responsible for ‘verify[ing] stock on hand correlates with directed holdings’ and reporting to the capability manager.
New support contract for the provision of combat rations
4.9 In late 2015, Defence undertook a procurement process to ‘source, construct, distribute, and provide continual design and development of the combat ration packs and ancillaries’. Defence intended to incorporate in the new contract those items which were previously Government Furnished Material.
4.10 Prior to this, Defence procured combat rations and ancillary items through two contractual arrangements and, for most of the ancillary items, through an annual Request for Quote process from a variety of suppliers. The extant contract for combat rations expired in October 2017.
4.11 The original timeframe for the combat rations retender was for the new contract to be signed in mid-2017, with the prime vendor providing combat rations for 2018–19. Defence advised the ANAO that sufficient combat rations were ordered under the extant contract to meet requirements in 2017–18. The new contract was signed with the new prime vendor, Prepack Ltd, on the 29 January 2018.72
4.12 Figure 4.1 provides an overview of the key dates for the combat rations retender.
Figure 4.1: Key dates for the combat rations retender

Source: ANAO analysis of Defence documentation.
4.13 Under the contract, the approved estimated annual expenditure across the initial five year period was $26.66 million (GST included), totalling $133.24 million (GST included).73
Management and accounting for combat rations
4.14 Combat rations are considered ‘mission critical items for combat arms and combat support personnel’.74 The appropriate management and accounting for combat rations ensures: current preparedness obligations are met; future capability needs are realised as planned; realisation of economic benefits75; and Defence works effectively with, and supports, a sustainable Defence industry.
4.15 In addition to managing the procurement arrangements for combat rations, Health SPO is responsible for in-service management.76 Health SPO’s in-service support of combat rations and ancillary items is focussed on: correct storage; periodic inspection and classification; management of stock rotation to ensure consumption before the use-by date; and bulk procurement of replacement combat rations. Defence advised the ANAO that wastage rates for combat rations and ancillaries from 1 January 2017 to 31 December 2017 included over 15 000 items, worth over $1.13 million. Defence advised the ANAO in April 2018 that ‘there is no targeted wastage rate for combat rations’.
4.16 Combat rations are accounted for on Defence’s inventory management systems, in accordance with Defence’s Electronic Supply Chain Manual. The Military Integrated Logistics Information System (see Chapter 2) is the logistics information system used for managing demand and utilisation of combat rations and ancillaries.
4.17 There are a number of audits and compliance activities undertaken in this area by those external to Health SPO, including:
- Army’s compliance and assurance section undertakes compliance activities in units on safety, finance, security, supply chain and technical integrity and maintenance;
- Capability Acquisition and Sustainment Group’s annual assurance program focuses on the identification of systemic issues to drive continuous improvement; and
- Business Process Testing, undertaken by Defence Logistics Compliance and Assurance Network Teams, reviews financial and logistics controls for business units that use the Military Integrated Logistics Information System (MILIS).
4.18 Information on the management and accounting procedures for combat rations in Defence is included at Appendix 3. An examination of these procedures was not included as part of the audit scope.
Did Defence comply with the Commonwealth Procurement Rules and relevant Defence policies?
In Defence’s procurement for combat rations, Defence largely complied with the Commonwealth Procurement Rules and most of its internal policies; however, it did not meet the risk policy of the Department, records management was not compliant with the Commonwealth Procurement Rules, and Defence’s arrangements for risk and probity management were not consistent with the intent of the Commonwealth Procurement Rules.
Defence records indicate that tender information was removed from Defence’s secure system during the procurement evaluation.
4.19 The Commonwealth Procurement Rules77 are the basic rule set for Australian Government procurements and govern the way in which entities undertake their procurement processes.78 To support the application of procurement related legislation and policy, Defence has established a single overarching procurement policy framework, managed by Capability Acquisition and Sustainment Group.79 This included a number of compulsory activities Defence officials were required to carry out when undertaking procurement.
Planning the procurement
4.20 Defence undertook a number of planning activities, which included: engagement with the capability manager on key requirements; development of a procurement schedule; endorsements to proceed; and a tender evaluation plan.
Procurement strategy
4.21 In November 2015 Defence released a Request for Information via AusTender, seeking information from industry to help inform the development of the Request for Tender and provide awareness to industry of the upcoming tender release.80
4.22 As noted in the Endorsement to Proceed81, the Request for Tender tested if the market was able to provide freeze-dried products:
ability for the market to provide [freeze-dried meal products] has not been tested since the last open tender process in 2008. It is intended to incorporate the freeze-dried products as contractor sourced product and establish whether the market is currently able to provide this product.
4.23 Seven responses were received by the 18 December 2015 closure date. Defence concluded that the Request for Information had provided ‘assurance that there are a number of companies who are interested and capable of providing the service we are seeking’.
4.24 The Defence Science and Technology Group supplied freeze-dried meals as Government Furnished Material to the previous extant contractor. There was limited information included in the Endorsement to Proceed on whether this would continue if industry was found not to be able to provide freeze-dried meals.82
Risk management
4.25 The Commonwealth Procurement Rules state that:
Relevant entities must establish processes for the identification, analysis, allocation and treatment of risk when conducting a procurement. The effort directed to risk assessment and management should be commensurate with the scale, scope and risk of the procurement. Relevant entities should consider risks and their potential impact when making decisions relating to value for money assessments, approvals of proposals to spend relevant money and the terms of the contract.83
4.26 Defence’s internal Project Risk Management Manual also requires that all project risks be documented in a risk register and that staff are to update the risk assessment for a project at key decision points and milestones.
4.27 There was no documented risk management register for the procurement and Defence was unable to provide evidence to the ANAO that it established processes for the identification, analysis, allocation and treatment of risk when conducting the procurement.
4.28 The ANAO identified similar shortcomings in the Health SPO’s procurement of Health Systems Fleet items (see paragraph 3.15). Defence should have established processes for the identification, analysis, allocation and treatment of risk when conducting the procurement. This would have also assisted in the Health SPO’s broader planning and management, noting that the combat rations procurement was one of several major procurements84—most estimated to be worth over $100 million—underway within Health SPO during the same time period.
Probity and conflict of interest
4.29 The Commonwealth Procurement Rules require that ‘officials undertaking procurement must act ethically throughout the procurement’, including ‘recognising and dealing with actual, potential and perceived conflicts of interest’ and the equitable treatment of participants.85
4.30 Defence also has specific instructions on managing potential conflicts of interest, which apply to Defence personnel and external service providers under contract to Defence.
4.31 Defence was unable to demonstrate to the ANAO that it established processes to recognise and deal with actual, potential or perceived conflicts of interest. For example, staff involved in the procurement process, including the tender evaluation, were not required to complete conflict of interest declarations.
4.32 Additionally, Defence was unable to demonstrate to the ANAO that it established processes to ensure the equitable treatment of participants. For example, Defence did not develop a probity plan for the procurement nor engage a probity advisor.
4.33 The ANAO identified the same shortcomings in Health SPO’s procurement of Health Systems Fleet items (see paragraphs 3.18 to 3.19).
Security of tender information
4.34 The Commonwealth Procurement Rules provide that: ‘when conducting a procurement … entities should take appropriate steps to protect the Commonwealth’s confidential information’.86 The Tender Evaluation Plan stated that all tendered material should be kept secure.
4.35 Defence records indicate that tender information was removed from Defence’s secure system, and information was sent from Defence email accounts to personal email accounts of Defence staff. Documents potentially containing information of a sensitive nature relevant to the combat rations tender that were emailed to personal accounts included:
- reports from each Tender Evaluation Working Group;
- the Source Evaluation Report; and
- information on Defence’s Scottsdale facility which produces ration pack components.
4.36 In the course of the audit the ANAO advised Defence of this issue. As noted in paragraph 3.22 of this audit report, Defence undertook an investigation, documented the incident in a security report, and debriefed the individuals involved.
Approach to market
4.37 As noted earlier, Defence conducted a Request for Information stage as part of the procurement. Internal advice noted that ‘the Request for Information undertaken in November 2015 has provided assurance that the current industry market is well positioned both within Australia and overseas to meet Defence requirements’.
4.38 Defence adopted an open tender process for the procurement. The ANAO’s review of the Request for Tender documentation found that it met the requirements of the Commonwealth Procurement Rules.87
4.39 The tender was originally open for applications from 14 September 2016 to 21 February 2017; however, this was extended to 28 February 2017.88 Defence had a four month open period to ensure ‘tenderers have adequate time to address the tender requirements’. This was compliant with the minimum time limits of the Commonwealth Procurement Rules.89
Evaluation
4.40 Defence developed a Tender Evaluation Plan outlining the evaluation process for the procurement, and conducted the evaluation in accordance with this plan.
4.41 A Tender Evaluation Board was formed to conduct the evaluation of the tenders received. The Board only included Defence officials from within Health SPO, including those staff involved in the management of the extant contract.
4.42 As noted in Table 4.1 below, the Tender Evaluation Board considered that two of the five tenders received were competitive. The remaining three tenders were considered non-competitive90 and were set aside as they were assessed as high or extreme risk and not exhibiting value for money.
Table 4.1: Tender evaluation process and outcomes
|
Process |
Description |
Outcome |
|
Registration and late tenders |
Registration of receipt of all tenders, with late tenders excluded. |
Seven complete tenders received, with two tenders excluded as they were identified as duplicates. No late or part tenders were received. |
|
Screening |
To exclude from detailed evaluation tenders which did not satisfy the minimum content, format requirement or essential requirements specified in the request documentation. |
All tenderers determined to have responded sufficiently to the requirements of the tender were recommended for detailed evaluation. |
|
Shortlisting |
Identified non-competitive tenders that had no reasonable prospect of exhibiting the best value for money in comparison to other tenders received. Tenders identified as non-competitive were not considered for detailed evaluation. |
|
|
Detailed evaluation |
Detailed evaluation to assess tender deliverables against the requirements of the request documentation, producing analysis and comparative assessment of each tender against the evaluation criteria. Each tender also underwent a value for money assessment. Tenders were assessed as preferred and non-preferred. |
Five tenders were subject to detailed evaluation, with:
|
Source: ANAO analysis of Defence documentation.
4.43 An evaluation against the evaluation criteria91 was performed by each of the Tender Evaluation Working Groups92 and reported to the Tender Evaluation Board. Each tender was assessed against the seven evaluation criteria. Tenderers were provided rankings for six of the seven criteria. Though financial viability was assessed, tenders were not provided a ranking.
4.44 According to the Tender Evaluation Plan the evaluation of tenders was anticipated to have been approved by the delegate by 26 May 2017. The delegate approved tender evaluation outcomes on 28 September 2017.
Evaluating prices and pricing structures
4.45 In the Request for Tender documentation, tenderers were required to provide pricing information for several components, namely recurring services and task-based services (that is, combat rations, ancillaries and transport costs). In evaluating prices and pricing structure, Defence assessed and compared each tender on:
- pricing structure and tendered prices for recurring services;
- pricing structure and tendered prices for task-based services (including for each of the five types combat ration pack, ancillary items, and delivery); and
- mark-up on prices for materials, subcontracts and associated survey and quote services.
4.46 In its advice to the delegate the Tender Evaluation Board noted the differences in pricing structures, for example that some: tenderers had no recurring service fees; transport costs were a significant proportion of the tendered price; and recurring service fees were not linked to delivery of combat ration packs.
4.47 Tenders were compared on their cost competitiveness, with some tenders considered ‘not price competitive’ based on their overall tendered price. Whilst the preferred tender was considered price competitive—delivering savings of $1.7 million per annum compared to the 2016–17 expenditure—it did not have the lowest tendered price compared to other tenderers.
Consideration of economic benefit
4.48 In November 2016, the government announced changes to the Commonwealth Procurement Rules, which included that ‘for a procurement above $4 million, there will be a requirement to consider the economic benefit of the procurement to the Australian economy’.93 This requirement was effective from 1 March 2017.
4.49 The Tender Evaluation Board commented in advice to the delegate that ‘economic benefit against each of the bids has not formed any part of the assessment due to the tender being released under the previous Commonwealth Procurement Rules’. Noting the timing of the tender and evaluation period94, Defence should have sought advice from the Department of Finance on the application of the new requirements.
Australian Industry Capability Plans
4.50 Consistent with Defence policy, Australian Industry Capability Plans were required to be submitted as part of tender responses. In evaluating the tender responses, the Tender Evaluation Board assessed all five tenders as having significant or critical deficiencies in their Australian Industry Capability Plans. Defence advised the ANAO that the ‘Deficiencies in the preferred tenderer’s Australian Industry Capability plan were addressed in contract negotiations in accordance with Contract Negotiation Directive.’
4.51 As noted in Chapter 3, the quality of the Australian Industry Capability plans submitted was also an issue in the recent procurement for Health Fleet items. There would be benefit in Defence reviewing the information and guidance provided to industry in these procurements to help establish the reasons for the identified deficiencies.
Records management
4.52 The Commonwealth Procurement Rules require entities to maintain a level of documentation commensurate with the scale, scope and risk of the procurement.95 The Rules note this should include, for example, the process that was followed, how value for money was achieved, relevant decisions and the basis for those decisions.
4.53 Defence maintained records of key documentation for the procurement; however, there were instances where there was insufficient documentation to assess some of the processes that were followed, some of the relevant decisions made, and/or the basis for some of the decisions, namely:
- correspondence and/or communication with potential suppliers, tenderers and suppliers; and
- minutes, directives and working documents for meetings of the Tender Evaluation Board and some relevant Tender Evaluation Working Groups.
4.54 Similar record keeping issues were identified by the ANAO in respect to the procurement of Health Fleet items (see paragraph 3.42).
Were Defence’s procurement arrangements designed to achieve value for money for the Commonwealth?
The 2017 tender and evaluation process for combat rations was designed to produce a value for money outcome. Defence undertook a two stage, open tender process and conducted detailed evaluation of tenders. Defence negotiated with the preferred tenderer on a number of issues, including actively negotiating a reduction in distribution costs. Defence decided to supply freeze-dried meal components itself as Government Furnished Material rather than having those components supplied under the contract as initially indicated in tender documentation. This may have limited the market and, as Defence did not negotiate a reduction in tendered prices for the relevant ration pack, impacted on the achievement of value for money for the Commonwealth.
4.55 Achieving value for money is the core rule of the Commonwealth Procurement Rules.96 Defence’s procurement process and arrangements under the new contract aimed to enhance value for money to the Commonwealth.
4.56 Defence adopted a two-staged, open tender process for the procurement as a basis for introducing competition and contributing to value for money outcomes.
4.57 Consistent with the First Principles Review, Defence also aimed ‘to continue to reduce the involvement of Defence in the supply process of [Combat Ration Packs] therefore reducing transactional and administrative overheads’.97 Defence advised the ANAO in February 2018 that this approach included, for example, increased contractor responsibility for warehousing and ancillaries. Defence further advised the ANAO in February 2018 that it ‘has not yet undertaken analysis of potential savings’.
4.58 As noted in paragraphs 4.45 to 4.47, the Tender Evaluation Board’s advice to the delegate noted the differences in pricing structures and also compared tenders on their cost competitiveness—with some tenders considered ‘not price competitive’ based on their overall tendered price.
4.59 The Tender Evaluation Board estimated, and advised the delegate, that the three lowest tendered prices represented a real cost saving against the 2016–17 spend of between $1.7 million and $4.4 million per annum. Defence advised the ANAO that these estimated savings were ‘based on the tender submission from [the preferred tenderer] and the current price sourced through [Military Integrated Logistics Information System] for the same pack menus’.98 Defence could not provide the ANAO with the supporting analysis which underpinned these savings.
4.60 Defence did not only consider the price in its value for money assessment, but also considered the risks and quality of the solutions offered by the tenderers.
Contract Negotiations and Government Furnished Material
4.61 Defence commenced negotiations with the preferred tenderer in December 2017.
4.62 Defence’s negotiation strategy identified several issues to be resolved during negotiations. This included negotiating a reduction in the tendered distribution costs. Advice to the delegate noted that ‘the reassessment of the transportation component will produce possible savings of approximately $200 [000] per annum.’99
4.63 Defence also negotiated for quarterly meetings to discuss opportunities for innovation and increased Australian industry content, and for the preferred tenderer to hold a minimum number of samples for quality control purposes.
4.64 The delegate approved the provision of the contract to the preferred tenderer in January 2018. Defence signed a contract with the preferred tenderer on 29 January 2018. As the extant contract had expired in October 2017, ‘to ensure continuity of supply purchase orders were raised before contract expiration’.
Government Furnished Material
4.65 As noted in the Endorsement to Proceed, Defence intended to incorporate in the new contract those items which were previously Government Furnished Material. Defence advised this to potential suppliers in an addendum to the Request for Tender (released on AusTender in November 2017), as well as in responses to individual potential supplier’s questions.
4.66 The resultant contract noted that the Commonwealth would continue to provide Government Furnished Material as part of the contract and ‘will advise in advance the cessation of Commonwealth Supply’. The Government Furnished Material included:
- freeze-dried meals (estimated quantity 40 000 items);
- emergency flying tins (estimated quantity 3000 items); and
- water purification tablets (estimated quantity 3000 items).100
4.67 Defence advised the ANAO in March 2018 that the supply of emergency flying tins and water purification tablets as Government Furnished Material was temporary and would transition to the prime vendor following the drawdown of existing stock held by Defence. Freeze-dried meals, however, will continue to be supplied as Government Furnished Material. Defence advised this was because the Defence freeze-dried meals manufactured by the Defence Science and Technology Group are of a higher standard than industry freeze-dried meals.
4.68 The decision to retain freeze-dried meals as Government Furnished Material is not recorded in key tender evaluation documents or in advice to the delegate. The tender was conducted on the basis that industry would furnish certain items previously supplied by government. This may have limited the market. Had the market been informed that government would continue to supply certain items, additional tenders may have been received.
4.69 Government Furnished Material in the form of freeze-dried meal components is included in one of the five types of ration packs procured by Defence.101 The new contract sets out the price for each type of combat ration pack, by menu option, and the price of the individual components which make up each type of combat ration pack. ANAO analysis indicates that approximately 45 per cent of the contracted price for the ration pack containing Government Furnished Material is for the freeze-dried components.102 Based on the estimated quantities of this type of ration pack to be procured, as identified in the contract, estimated expenditure for the Government Furnished Material under the contract equates to approximately $850 000 per year or $4.26 million103 over the initial five year contract term.
4.70 The contract does not explicitly make provision for the cost of rations packs containing Government Furnished Material to be reduced by an amount equivalent to the cost of the Government Furnished Material.104 In the absence of such a contract provision or other Defence documentation specifically addressing this issue (such as a Defence contract management plan), the ANAO asked Defence how it would manage the risk that the Commonwealth may pay twice for Government Furnished Material if it continues to be manufactured and supplied (to the contractor, Prepack) by the Defence Science and Technology Group.
4.71 Defence advised the ANAO in May 2018 that it is ‘unable to identify any specific clause in the contract that specifies that Defence will only pay for those components we order’. Defence further advised that:
There is no risk that the Commonwealth will pay twice. Government Furnished Material is provided to PrePack (against the provisions detailed in the Statement of Work) in order to assemble complete Ration Packs as ordered. Any payment will only be for those components procured from PrePack. The Purchase Order will not include any purchase line for Government Furnished Material. The payment will only be made when proof of goods received is provided and the Invoice matches the Purchase Order and Goods Received.
The concept of a contract is that the payment is only made for those goods ordered via a Purchase Order for the price specified in the contract. There is no obligation to order all or any components specified in a contract. The Statement of Work makes provision for variations to menu build components including such clauses as “Defence define the requirements of each CRP [Combat Ration Pack] variant and require the flexibility to change the composition of the CRP as operational demands change. For the purposes of this section Products includes any whole CRP (e.g. one man CRP, etc.), each CRP menu variant (e.g. one man CRP menu A or B etc.), and each menu build component within each CRP menu variant.”
4.72 Defence also advised that to date it has not ordered any of the relevant combat ration packs under the new contract.
Are Defence’s contract deliverables provided to the required standard, within the agreed budget and timeframes?
Whilst the contract for the supply of combat rations sets out the requirements and standards of the products to be delivered and contains some individual delivery payment controls, Defence has not implemented a performance-based contract. The contract does not specify how performance issues will be managed, or link key performance indicators to payments.
Management of the budget
4.73 As noted earlier, the new support contract involves an estimated average annual expenditure of around $26 million per annum. As there are no fixed fees included in the contract, all of this estimated expenditure is classified by Defence as having certainty in terms of scope and cost.
4.74 Health SPO, in consultation with the capability manager, forecasts annually the quantity of combat rations and ancillary items to be procured.105 Where requirements are greater than those approved, Health SPO is required to seek additional financial resources from the capability manager. Variations to the combat ration quantities procured need to be negotiated with the prime vendor.
Contract management
4.75 Health SPO is responsible for the management of the contract and ensuring that the resultant prime vendor meets the contractual obligations, including ensuring that contract deliverables are provided to the required standard, within the agreed budget and timeframes.
4.76 Defence’s Request for Tender documentation106 sets out: the scope, standards and requirements of the work to be carried out under the contract; management plans; and performance arrangements. The documentation also allocated responsibilities between Defence and the resultant prime vendor.
Contract deliverables
4.77 The resultant contract sets out the specified requirements and standards of the products to be delivered. In developing the contract, Health SPO consulted with the capability manager to ensure the specified requirements and standards were consistent with Defence policy. The delivery timeframes were also articulated.
4.78 The contract also outlines the process for the managing instances of non-delivery under the contract relating to: the specified requirements and standards; the specified timeframes; and/or to the appropriate delivery points. The management process can include, for example: Defence withholding payment for individual deliveries; the prime vendor providing replacement products; correcting rejected products; and/or repossessing the rejected products.
4.79 As outlined in the contract, every six months Defence and the resultant prime vendor will meet to review the combat ration packs menu items and composition, including incorporating new or replacement items, and/or changes to Defence’s combat ration requirements and standards.
Management plans
4.80 As outlined in the Request for Tender documentation, the resultant prime vendor would be required to develop a management plan, known as the Support Services Management Plan, which would be used to specify how a range of deliverables and requirements under the contract would be delivered. This would be the primary plan for the contract and would include information on, for example, the resultant prime vendor’s: risk management processes; quality management; and health, safety and environmental management.
4.81 To assist in the management of contract deliverables during the contract term, the prime vendor is also required under the contract to develop and deliver several plans and reports to Defence. For example, contract status reports and the Australian Industry Capability Plan.
Performance-based contracting
4.82 Performance-based contracts are structured to motivate the supplier to achieve the required outcomes. Performance-based contracting has been underway in Defence sustainment for over a decade, with Defence defining performance-based contracting as ‘an outcomes-oriented contracting method that ties a range of monetary and non-monetary consequences to the contractor based on their accomplishment of performance requirements’.107 Defence contracts can use both key performance measures and key performance indicators to assist with performance based contracting.
4.83 As outlined in Defence internal guidance, strategic performance measures are annually assessed performance measures typically used to reflect long term behaviours against key result areas (for example: reliability and quality; safety; cost; supportability; and behaviours).
4.84 As outlined in guidance from the Capability Acquisition and Sustainment Group’s Performance Based Contracting Centre of Excellence, key performance indicators are a critical element in performance management as they:
- communicate the requirements (that is, sets performance expectations); and
- communicate the performance to be delivered (that is, feedback on actual performance).
4.85 Key performance indicators are commonly linked to performance payments as they are able to be objectively measured.108
4.86 The combat rations contract specifies requirements and standards of the products to be delivered (see paragraph 4.77) and outlines the process for the management of products which the contractor has failed to meet (see paragraph 4.78). Three strategic performance measures are included in the contract (see Appendix 5), two of which will be self-assessed by the prime vendor.
4.87 The strategic performance measures included in the combat rations contract are not linked to performance payments. There is no explicit section in the contract regarding performance management. Additionally, there are no key performance indicators.
4.88 Defence’s internal guidance lists performance based contracting sections as ‘optional modules’ of ‘short’ contracts—as used for the both combat rations and heath materiel contracts. The choice not to include the performance based contracting module in the combat rations contract differs from the inclusion of this module in the health materiel contract of similar value.
Appendices
Appendix 1 Entity response


Appendix 2 Defence’s procedures and policies for accounting and management for health materiel
Accounting for health materiel
1. Health materiel management occurs within a regulatory environment with control, authorisation and accountability requirements. The Defence Health Manual outlines how pharmaceuticals are to be managed and accounted for in Defence.
2. Pharmaceuticals are accounted for on Defence’s inventory management systems, in accordance with the Electronic Supply Chain Manual. Garrison pharmacy staff and authorised deployed elements can use the Military Integrated Logistics Information System (MILIS) and/or the Pharmacy Integrated Logistics System (PILS) (see Chapter 2) which includes recording the order, receipt and dispensing of health materiel.
3. A stock controller109 or stock guardian110 of therapeutic substances is responsible for all therapeutic substances held in a garrison, deployable or deployed health facility.
4. Defence has established entitlements and approved stocking levels for health facilities. These, combined with consumption and usage, determine health materiel orders. There are procedures in place for the receipt of pharmaceuticals, including for restricted Schedule 8 substances.111 An Authorised Officer—typically a medical officer or pharmacist—is required to accept custody of pharmaceuticals (in accordance with relevant State and Territory regulatory requirements) and record in the appropriate registers. The Authorised Officer must sign a Confirmation of Receipt of Controlled Substances and mail the original of the form to the supplier. The supplier holds the receipt forms for auditing. Defence also has facility requirements for the pharmacies, dedicated deployable health storage areas, and health facilities that hold pharmaceuticals. This includes, for example, access control, emergency protocols, supervisory and storage requirements.
5. Pharmaceuticals are to be dispensed to an individual Defence member, whether the substance is an over-the-counter or prescription medicine, in accordance with policy. PILS contains an electronic history of medicines dispensed to each Defence member. Defence policy requires stock controllers and stock guardians to record and retain physical copies of all transactions, with specific procedures in place for specific pharmaceuticals.112
6. There are a number of stocktake and assurance activities both stock controllers and stock guardians must undertake for scheduled substances. This varies depending on the facility and substance, and can include, for example:
- reporting of lost or stolen substances in accordance with Defence procedures;
- quarterly stocktake of all Schedule 8 drugs, reporting and investigating any discrepancies in accordance with Defence instructions;
- independent check of Schedule 8 drugs at least once every 12 months by a senior medical officer;
- stock controller change over checks;
- monthly stock balance check on Schedule 4 and 8 substances held on emergency trolleys and in kits;
- daily checks of substances held in wards and treatment rooms, with any discrepancies reported to the local therapeutics committee;
- change of shift checks for stock guardians for all Schedule 4 and Schedule 8 substances, recording the check according to relevant procedures, and confirm security seals on specific medial kits, reporting any issues in accordance with Defence procedures;
- pre- and post-deployment checks by stock guardians of first aid and emergency kits containing Schedule 8 substances.
7. Defence also has in place controls around the process of disposal for pharmaceuticals. These include: using clinical waste bins; ensuring disposals are recorded on the electronic register in accordance with the Electronic Supply Chain Manual; and that paper records are completed and retained. Additionally, controlled drugs must be destroyed by pharmacists in the presence of an authorised officer113 or a Member of the Service Police.
Fraud and compliance
8. Joint Health Command had implemented a ‘Governance Audit System’. Auditing and monitoring occurred at three levels: at the health facility level through the internal audit schedule; at the Joint Health Unit level; and at the Joint Health Unit strategic level through Clinical Governance Joint Health Command. Defence informed the ANAO in February 2018 that the internal audit system has been revised and will lead to a cycle of audits with the results consolidated and reported to the Garrison Health Clinical Governance Board.
9. Defence informed the ANAO in February 2018 that in addition to the auditing program, there is also regular ad hoc engagement between Health SPO and Joint Health Command to discuss issues relating to the management and accounting for pharmaceuticals.
Appendix 3 Defence’s procedures and policies for accounting and management for combat rations
Accounting for combat rations
1. Combat rations are accounted for on Defence’s inventory management systems, in accordance with the Department’s Electronic Supply Chain Manual. The Military Integrated Logistics Information System (MILIS) (see Chapter 2) is the logistics information system used for the demand and utilisation of the combat rations and ancillaries. The use of MILIS is subject to internal audit functions.
2. Defence establishes the planned usage for each quarter by unit and location. Combat rations are to be ordered for authorised activities based on this planned usage. Any orders for unauthorised activities or above the requested allocation require additional approvals by Army Headquarters and Command.
3. Defence has additional policies in place for the use of contingency stocks. These include specific authorisations for ordering and holding contingency stocks. Contingency stock is to be held by the supporting Joint Logistics Unit where possible and is subject to regular quality control inspections and rotated with new rations every six months.
4. There are procedures in place for the receipt of combat rations, including signing and approval of a supplies acceptance certificate.114 This certificate is provided to the Fleet Manager within Health SPO.
5. Once received into service, combat rations are managed individually by batch number and date. The shelf life for combat rations is around two years, with some variation depending on whether the stock is stored in temperate or tropical climates. Defence has policies and procedures in place for the storage of combat rations and a stock rotation policy. These are supported by periodic inspections of combat rations, namely:
- Non-technical inspections—surveillance inspections each fortnight in tropical climates or each month in temperate climates, of aspects such as cleanliness of the storage facility, infestation, correct stacking and marking of cartons, accounting documents and compliance with Standard Operating Procedures. These are conducted by Regional Fleet Managers and senior store persons. Results are kept as unit records.
- Technical Inspections—undertaken by Food Technologists and Inspectors Foodstuffs.115
6. Defence has policies in place for what type and number of combat rations can be issued. Once the combat rations have been issued to the individual Australian Defence Force personnel it is their responsibility to manage in accordance with relevant policies and instructions.
7. Defence has policies and procedures in place for: the recall of contaminated foodstuffs; return procedures (including from overseas); and for the disposal of combat rations, including when they are condemned by an Inspector Foodstuff or required to be disposed of overseas. Defence’s disposal policy is outlined in Defence Logistics Manual Part 2, Volume 5, Chapter 10.
Fraud and compliance
8. Army, the capability manager, has a compliance and assurance section which undertakes compliance activities in units on, for example: safety, finance, security, supply chain and technical integrity and maintenance. Some of these compliance activities include a review of those management and accounting activities outlined in Box 3. For instance, the supply chain compliance and assurance activity examines the unit’s application of the Electronic Supply Chain Manual (for example, the disposal register, completed stocktakes, discrepancy reports, and completed MILIS purchase orders). The most recent business process test conducted within Health SPO was in October 2017 (see paragraphs 20 to 22).
9. Capability Acquisition and Sustainment Group also has an annual assurance program in place, which includes: the provision of advice and data analysis to Senior Management and stakeholders; identification of systemic issues to drive continuous improvement; alignment of activities between Centre of Excellence and Functional Leads; and to ensure assurance activities and resources are optimised to provide efficiencies across the Capability Acquisition and Sustainment Group program.
10. This assurance program is supported by the Assurance Management Information System which is used to track and monitor audit and assurance activities and associated remedial actions. Defence informed the ANAO in February 2018 that the Assurance Management Information System was initially provided to quality management systems practitioners across Capability Acquisition and Sustainment Group in December 2017. Training is scheduled to occur in 2018. The Assurance Management Information System will be provided to other areas in Capability Acquisition and Sustainment Group.
Business Process Testing and Monitoring
11. Business Process Testing is an internal audit function, undertaken by Defence Logistics Compliance and Assurance Network Teams116, which reviews financial and logistics controls for business units that use MILIS. It is one of several controls Defence uses to ascertain ‘compliance and assurance with Defence supply chain policies and procedures’. At the minimum, testing is conducted on business units once every three years for units that operate one or more MILIS warehouses. Prior to 2017–18, a Business Process Test was conducted on Health SPO in 2014–15.
12. The results of the Business Process Test conducted on Health SPO in August 2017 scored the Health Systems Program Office with a level of compliance of 33 per cent. While the reviewer noted that the low score was partly due to a smaller number of controls assessed, it also indicated ‘poor adherence to process’, as a result of unfamiliarity with the supply chain process and issues with records management.
13. Health SPO also undertakes its own Business Process Monitoring to gain visibility over transactions that exceed the normal business process timeframes. Transactions are monitored against key performance indicators and reported monthly at the branch level using a traffic light dashboard report which indicates how transactions fare in complying with policy and agreed time frame.
Compliance and assurance meetings
14. Health SPO participates in compliance and assurance meetings relating to inventory assurance, in particular: supply chain Business Process Testing; Stocktaking and Security Assurance Stocktakes; Business Process Monitoring dashboard key performance indicators; price assurance; lessons learnt; and feedback relating to issues experienced by relevant Systems Program Offices or directorate. Meetings are held monthly and are attended by Materiel Compliance and Assurance, Land Engineering Agency, other Systems Program Office Directors (or a representative) from within Integrated Solider Systems Branch, and Land 121. These are coordinated through the Land Materiel Sustainment Directorate in Land Systems Division Headquarters.
Appendix 4 Defence’s definition of pharmaceuticals, medical and dental consumables, and medical and dental equipment for the purposes of the contract
1. Table A.1 provides Defence’s definition of pharmaceuticals, medical and dental consumables, and medical and dental equipment for the purposes of the contract.
Table A.1: Defence’s definition of pharmaceuticals, medical and dental consumables, and medical and dental equipment for the purposes of the contract
|
Term |
Definition |
|
Pharmaceuticals |
|
|
Medical and dental consumables |
Items which can be categorised into the following categories:
|
|
Medical and dental equipment |
Items which can be categorised into the following categories:
|
Source: Defence documentation.
Appendix 5 Strategic Performance measures included in the new combat rations contract.
1. Table A.2 outlines the Strategic Performance measures included in the new combat rations contract.
Table A.2: Strategic Performance measures included in the new combat rations contract
|
Performance measure |
Description |
Review period and assessment basis |
|
Strategic Performance measures |
||
|
Capability Manager Satisfaction |
Measure of the resultant prime vendor’s performance to supply an effective and efficient Combat Ration Pack Capability, to meet Defence Capability requirements during each Review period from the perspective of the Capability Manager. |
Annual Assessment based on the following:
|
|
Innovation |
The resultant prime vendor’s performance in demonstrating consistent innovation by developing and adopting strategies aimed at improving the design, content and quality of the product. |
Annual Self-assessed by the resultant prime vendor using the Innovation Performance Attributes. For example, researchers and develops new ideas for the product that results in realised improvements. |
|
Relationship |
The resultant prime vendor’s ability to demonstrate positive relationships with the Commonwealth and other relevant third parties. |
Annual Self-assessed by the resultant prime vendor using performance against specific relationship performance attributes. For example: resolves disputes at the lowest possible level; approaches problem solving in a joint manner; and displays a willingness to share critical information. |
Source: Defence documentation.
Footnotes
1 The audit report is available from: <https://www.anao.gov.au/work/performance-audit/defence-management-materiel-sustainment> [accessed on 20 December 2017].
2 Defence defines sustainment as involving the provision of in-service support for specialist military equipment, including platforms, fleets and systems operated by Defence. Typically, sustainment entails repair and maintenance, engineering, supply support and disposal of equipment and supporting inventory.
3 Joint Project 2060 is a multi-phase project which identifies and develops the capabilities required to prevent, treat, manage and evacuate casualties in joint operations in the defence of Australia and its interests. Phase 3 aims to provide the required materiel and infrastructure and maximise the use of emerging health technologies. Joint Project 2060 is in acquisition phase and not in the scope of this audit.
4 The First Principles Review was commissioned by the Government in August 2014. The outcomes of the review were released in April 2015 and are available from <http://www.defence.gov.au/Publications/Reviews /Firstprinciples/Docs/FirstPrinciplesReviewB.pdf>. [accessed 20 December 2017]. The ANAO has undertaken a performance audit of Defence’s implementation of the recommendations from the First Principles Review, available from <https://www.anao.gov.au/work/performance-audit/defence-implementation-first-principles-review>.
5 Defence, First Principles Review: Creating One Defence, 1 April 2015, pp. 32–33.
6 Following the First Principles Review, Defence is moving to contracting with single suppliers to deliver capability. This includes outsourcing procurement and logistics roles previously undertaken by Defence to the supplier.
7 Defence informed the ANAO in February 2018 that five of the recommendations require financial and resourcing inputs from outside the Land Systems Division. These include employing additional resources in Health SPO, increasing commercial skills of Health SPO staff, and upgrading ICT systems. As a result, Land Systems Division has conditionally agreed to these five recommendations. The Deputy Secretary of Capability Acquisition and Sustainment Group endorsed the Head of Land Systems Division response in June 2017.
8 The Health Systems Fleet comprises pharmaceuticals, health consumables and health equipment needed to maintain the health and operational fitness of Defence members. Garrison health services are health care services delivered from military bases in Australia. Joint Health Command is responsible for garrison health services.
9 Health materiel and combat rations are not part of Defence’s top 30 sustainment products, and therefore Defence is not required to publicly report on them.
10 Codification refers to the act of establishing and maintaining item identification and related data under the Defence cataloguing system, or the national system of another country participating in the North Atlantic Treaty Organisation (NATO) codification system. Source: Health Manual Volume 24 – Health Materiel Manual, p. i.
11 The partial reporting of performance measures is discussed at paragraph 2.6.
12 The Sustainment Performance Management System is Defence’s primary sustainment reporting and performance management system. It is discussed in paragraphs 2.23 to 2.26.
13 The Sustainment Performance Management System was rolled out across Capability Acquisition and Sustainment Group over a two year period, with Navy coming online in May 2015, Air Force in April 2016, Army in August 2016, and the remaining products by June 2017. The use of the system was examined by the ANAO in ANAO Audit Report No.2 2017–18, Defence’s Management of Materiel Sustainment which discussed issues with the system. The audit report is available from <https://www.anao.gov.au/work/performance-audit/defence-management-materiel-sustainment>, [accessed on 20 December 2017.]
14 Risks are defined in the Materiel Sustainment Agreement as ‘having the potential to impact on the sustainability of the products’. Constraints are defined in the Materiel Sustainment Agreement as having the potential to ‘vary (increase and decrease) capability requirements and performance for all parties that support the product schedule’.
15 The review of Health SPO in April 2017 (discussed in Chapter 1) recommended that the Systems Program Office: ‘undertake a series of workforce planning and human resource initiatives to manage this organisational restructuring and skills transformation.’ Land Systems Division conditionally agreed to this recommendation noting that external support outside its domain was required to train and support staff, and to help transition staff that could not be reskilled.
16 Defence advised the ANAO in December 2017 that risk management in Capability Acquisition and Sustainment Group occurs at the divisional level.
17 The Health Systems Fleet does not include or support: patient-specific items (for example, spectacles, artificial eyes, or orthopaedic and artificial aids); health books and clinical references; work health and safety items; and medical or dental forms.
18 This includes medicines and items regulated under the Therapeutic Goods Act 1989. Defence also uses specified unregistered items to meet operational needs. The Secretary of the Department of Health, through an instrument of delegation, has authorised specified medical officers to import, export, or supply specified unregistered therapeutic goods for use in service. Individual patients may also access unregistered items through the Therapeutic Goods Administration’s Special Access Scheme.
19 The new support contract is for an initial five year term (from 3 July 2017) with options for two by two year extensions. The decision to proceed with the two options of two year extensions would be based on the prime vendor meeting and/or exceeding the contracted performance requirements.
20 Fixed-price/recurring services are predicable costs such as contract management, preventative maintenance and delivery of consumable spares. Non-fixed price/task-based services are services under the contract that have cost certainty only, as the frequency of the tasks involved cannot be forecasted (for example, corrective maintenance). Its inclusion allows for parts of the contract to work as a standing offer.
21 The Commonwealth Procurement Rules are issued by the Minister for Finance under section 105B(1) of the Public Governance, Performance and Accountability Act 2013 (PGPA Act). The Commonwealth Procurement Rules are revised from time to time. In this audit any reference to the Commonwealth Procurement Rules relates to either the Commonwealth Procurement Rules issued in July 2014 or in March 2017, depending on which version applied at the relevant point in time.
22 As the procurement was above the $80 000 threshold and not subject to an exemption, the procurement was required to comply with the additional rules detailed in Division 2 of the Commonwealth Procurement Rules.
23 Defence Procurement Policy Manual, p. 6.
24 In addition to delegations under the PGPA Act, and in accordance with Defence’s Accountable Authority Instruction, Defence officials were required to obtain an ‘Endorsement to Proceed’ prior to approaching the market for all procurements which established a standing offer arrangement and/or for all procurements that are valued at or above $200 000 (including GST). The Endorsement to Proceed provides a control mechanism by which Defence satisfies itself that proceeding with the procurement would be an efficient, effective, economical and ethical use of resources, and that it will not be inconsistent with the policies of the Commonwealth. For this procurement there was an original Endorsement to Proceed and an addendum to the Endorsement to Proceed.
25 See Appendix 4 for Defence’s definition of pharmaceuticals, medical and dental consumables, and medical and dental equipment in the approach to market.
26 Defence, Addendum to Endorsement to Proceed, March 2016.
27 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 8.2.
28 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 6.6.
29 Defence’s internal guidance states that there is no requirement to engage an external probity advisor and that the decision whether to engage an independent probity advisor should be made based on the individual circumstances of the case, and in particular, whether the procurement is likely to be high profile, high value, controversial or sensitive. The Defence Procurement Policy Manual, 4 May 2017, p. 21.
30 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 7.20.
31 The ANAO previously examined security issues relevant to using non-government-agency-sanctioned email services for official Australian Government business in the performance audit on the Moorebank Intermodal Terminal (ANAO Audit Report No.23 2017–18, Delivery of the Moorebank Intermodal Terminal, Appendix 2: Use of non-government email services for official Australian Government business).
32 Internal to Defence but independent from the area involved.
33 Defence defines a security incident as ‘any event that prejudices security and/or breaches security regulations. Such events might be deliberate, negligent or accidental, and are often the result of a failure to comply with security policy as detailed in the Defence Security Manual’.
34 The 2014 Commonwealth Procurement Rules required tender documentation to include a complete description of:
a) the procurement, including the nature, scope and, when known, the quantity of the goods and services to be procured and any requirements to be fulfilled, including any technical specifications, conformity certification, plans, drawings, or instructional materials;
b) any conditions for participation, including any financial guarantees, information and documents that potential suppliers are required to submit;
c) any minimum content and format requirements;
d) evaluation criteria to be considered in assessing submissions; and
e) any other terms or conditions relevant to the evaluation of submissions.
35 Four addendums were released during the 13 weeks to correct information in the original Request for Tender. These were released to the market as a whole via AusTender.
36 The 2014 Commonwealth Procurement Rules required the time limit for potential suppliers to lodge a submission to be at least 25 days from the date and time that a relevant entity publishes an approach to market for an open tender.
37 The Tender Evaluation Plan allowed for tenders to be set aside if it became apparent at any stage of the process that a tender was clearly non-competitive or otherwise had no reasonable prospect of exhibiting value for money.
38 The three tenders were set aside for a variety of reasons, including: critical and significant non-compliance with draft conditions of contract; unacceptable title transfer position; and/or a sub-optimal perceived partnership.
39 This aligned with the evaluation criteria specified in the Request for Tender documentation.
40 Tenders were not ranked for one criterion, see paragraphs 3.37 to 3.40 for further discussion.
41 The estimated requirements were based on the pharmaceuticals and medical and dental consumables ordered by Defence for the previous year. Only those items with a purchase quantity greater than 100 units over the 12 months were included, with quantities ranging from 100 units to over 64 000 units (for a pharmaceutical) and 500 000 units (for a consumable).
42 One tenderer did not provide individual pricing by item for Work Package One and was unable to be assessed on its tendered prices. The remaining four tenderers provided pharmaceutical pricing by item; however, not all of the tenders provided quotes by identifiable International Article Number (EAN). The sample was used for those items the Tender Evaluation Working Group were able to match to an EAN and directly compare the pricing across the tenders. This method was verbally agreed to by the Tender Evaluation Board.
43 The Australian Industry Capability Program requires companies looking to supply and support Defence capability to Defence to submit an Australian Industry Capability Plan. The plans detail: how the company has engaged with Australian industry to identify Australian companies capable of being part of the supply chain; how the competitive source selection decisions were made in relation to the proposed subcontractors; and how the company intends to support the transfer of technology and foster innovation within Australian industry. Plans are sought for all Defence procurements where the value of the tender is expected to exceed $20 million or where the procurement will impact on Sovereign Industrial Capability (previously Priority Industry Capabilities).
44 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 7.2.
45 Department of Finance, Commonwealth Procurement Rules, March 2017, p. 9.
46 Defence requested that the prime vendor provide:
- all available Pharmaceuticals which are included in the Pharmaceutical Benefit Scheme (PBS);
- all available Pharmaceuticals which are not included in the PBS;
- all available Pharmaceuticals which are Therapeutic Goods Administration (TGA) approved; and
- all available Pharmaceuticals which are not TGA approved but required by the Commonwealth.
47 The Australian Defence Force had a single medicines formulary which listed all the medicines approved for use in Defence from the Therapeutic Goods Administration’s Australian Register of Therapeutic Goods or medicines that have other approvals in place. The formulary provided a narrower range of medicines than was commercially available. Requests for changes to the formulary were considered by Defence’s Pharmacy and Therapeutics Committee.
48 Under the previous contract changes in products occurred largely through a Survey and Quote process.
49 Prescription medicines not listed on the PBS and with a cost price to the provider of $930 or greater will have a mark-up of two per cent applied.
50 Under the Pharmaceuticals Benefits Scheme, the $930 threshold and flat rate mark-up of $69.94 is the level of wholesale mark-up based on the cost of the medicine from the manufacturer. The cost to the pharmacist includes the manufacturer’s price plus wholesale mark-up. The cost to the pharmacist is a component of pricing of medication under the Pharmaceuticals Benefit Scheme. Available from <https://www.humanservices.gov.au/organisations/health-professionals/enablers/pricing-pharmaceutical-benefits-scheme-medicine> [accessed 15 March 2018].
51 See also ANAO Audit Report No.28 2017–18 Defence’s Procurement of Fuels, Petroleum, Oils, Lubricants, and Card Services, paragraphs 2.85 to 2.87. In that audit, the ANAO found that Defence’s negotiation strategy for the procurement of fuel and lubricants did not include the negotiation of prices.
52 A clause in the contract required the extant provider to ensure continuity of supplies until the take-over of responsibilities by any incoming prime vendor or the Commonwealth.
53 The Commonwealth Procurement Rules (7.16 to 7.17) require entities to report contracts and amendments on AusTender within 42 days if entering into (or amending) a contract if they are valued at or above the reporting threshold. The reporting threshold for non-corporate Commonwealth entities, such as Defence, is $10 000.
54 The phase in plan included a risk assessment undertaken by the prime vendor and mitigation strategies. All four risks identified were rated as low. Several of the risks identified materialised, for example: interface between prime vendor’s online ordering system and Defence’s nominated system is delayed or not available for operational date, and operation takes longer to reach a stable point.
55 An earlier email had been sent from the prime vendor to pharmacists containing their username and ordering details. This email was deleted by some pharmacists as they were not aware of who the email was from and considered the email spam. In response, Health SPO sent an email on Tuesday 27 June 2017 advising pharmacists of the new prime vendor; however, a ‘go live’ date was still to be confirmed.
56 Internal advice in November 2017 from Health SPO to the capability manager identified that some of the other issues raised were known and had been, or were in the process of being, addressed.
57 Pharmacists were required to enter the pharmaceutical order and patient information into both the Defence Pharmacy Integrated Logistic System (PILS) and the prime vendor’s online portal as the two systems were not integrated. As pharmacists do not know the brand of pharmaceutical they will receive from the prime vendor until it is delivered, they may also then need to adjust the order in PILS (as PILS works on a brand name).
58 Defence was required to provide 120 days’ notice to the extant provider of an extension. The extant provider waived this requirement in its unsolicited offer.
59 Controlled drugs are referred to as Schedule 8 substances, which are substances requiring restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence.
60 An authorised officer is a military officer or civilian (employed or contracted by the Department of Defence) who is registered as a medical practitioner, dental practitioner, nurse practitioner, pharmacist or veterinarian and who is employed in that capacity and is working within their scope of practice. Inside the pharmacy, the authorised officer is the pharmacist. Depending on the health setting, the role can also be conducted by an authorised non-pharmacist.
61 The Health Materiel Manual (paragraph 10.36–10.37) notes that: ‘Some pharmaceuticals must be stored and handled within a closely controlled temperature range to ensure they remain suitable for use … Operational orders will specify the cold chain arrangements for specific operations. When preparing a cold chain shipment, [Joint Logistics Command] is to prepare a container, attach a log sheet, handling instructions, Radio-frequency identification (RFID) tag, waybill and consignment documentation to the exterior of the container. A data logger is placed next to the shipped item inside each container to record temperature and time information.’
62 The cost to Defence for the procurement of the temperature data loggers was $27 747.50 (GST inclusive).
63 The facility is managed by Joint Logistics Command through a third-party contract worth $10.2 million per annum.
64 As at early February 2018, the first review of the prime vendor’s performance is yet to be conducted. The review period commenced on the 3 July 2017 and finished on 3 January 2018.
65 The Department of Defence has separate arrangements in place for the supply of fresh rations and allocated rations (previously known as field fresh rations).
66 The food components which make up the rations packs vary according to these requirements. For example, some ration packs contained freeze-dried components to reduce weight, whilst others contained sugar and carbohydrate rich items to provide increased energy value.
67 There are five types of combat ration packs: Combat Ration One Man; Patrol Ration One Man; Combat Ration Five Man; Emergency Ration and Emergency Flying Ration. Each type of combat ration can have multiple menu builds.
68 Army is responsible for outlining the requirements for combat rations.
69 Government Furnished Material are items provided to the contractor by the Government. It may be incorporated into the end item or may be consumed in the performance of a contract.
70 The Defence Science and Technology Group also provides research, technical and quality assurance support to Health SPO.
71 Defence has procedures in place for the management of contingencies, for example contingencies are to be: released for use only under the authority of the capability manager; fully functional, serviceable and available for issue for support of readiness notices; managed, auditable and accountable; replenished as required; and captured within the appropriate Material Sustainment Agreement Product Schedule.
72 The new prime vendor contract was for an initial five year term with options for two, two year extensions.
73 Defence estimated the average annual expenditure across the initial five year period would increase from $25.10 million in the first year to $28.32 million in the fifth year of the contract. Defence advised the ANAO in April 2018 that the estimated expenditure of $115.4 million in the Endorsement to Proceed was based on previous history and procurement arrangements in place at that time, and the approved estimated annual expenditure of $133.24 million was based on the preferred tenderer’s offer against the tender requirements and the resultant contract negotiations.
74 Defence, Australian Defence Force Ration Scales and Scales of Issue (SUPMAN 4), edition 7, p. 3.
75 For example: reduced wastage; reduced total cost of ownership; reduced underspend or overspend; increased cash savings.
76 In-service management of combat rations encompasses: safety; fitness for service; and environmental compliance with relevant policies and procedures.
77 The Commonwealth Procurement Rules are discussed in footnote 21. In this Chapter, any reference to the Rules relates to either the Rules issued in July 2014 or in March 2017, depending on which version applied at the relevant point in time.
78 As the procurement was above the $80 000 threshold and not subject to an exemption, the procurement was required to comply with the additional rules detailed in Division 2 of the Commonwealth Procurement Rules.
79 Defence Procurement Policy Manual, p. 6.
80 Information was specifically sought in the following areas:
- management—information which evidenced the respondent’s technical ability, track record and experience, and capacity and resources to fulfil the Statement of Defence Needs;
- solution—description of solutions respondents believed would meet the Statement of Defence Needs, including details of any development or formation of partnerships that are required; and
- industry—respondents were to outline Australian Industry opportunities within their solution, including identified further development work that would enable further Australian Industry opportunities.
81 The Endorsement to Proceed provided a high-level overview of Defence’s consideration of whether the procurement would deliver the best value for money.
82 Defence Science and Technology Group had informed the Minister for Defence in April 2016 it was ‘re-evaluating its participation in the manufacture of Defence’s freeze-dried components noting that its current manufacturing capability will reach its life of type at the end of 2017’. In March 2016, the Minister for Defence announced $7.2 million in funding to establish a microwave assisted thermal sterilisation research and development plant within the Defence Food and Nutrition Centre in Scottsdale (Tasmania) along with a production facility in Launceston (Tasmania). Available from <https://www.minister.defence.gov.au/minister/ marise-payne/media-releases/minister-defence-72m-new-food-processing-technology-tasmania> [accessed 17 January 2018].
83 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 8.2.
84 Other major procurements were: pharmaceuticals, medical and dental consumables, and medical and dental equipment (see Chapter 3), estimated to be worth $182.78 million over five years; managed equipment services, estimated to be worth $154.25 million over five years; and medical and dental equipment for the garrison and deployable health capabilities, estimated to be worth $78.39 million over five years.
85 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 6.6.
86 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 7.20.
87 Commonwealth Procurement Rule 10.6 requires tender documentation to include a complete description of the procurement.
88 There were six amendments to the Request for Tender circulated. This included responses to questions from potential tenderers and some amendments to documents.
89 The Rules required the time limit for potential suppliers to lodge a submission to be at least 25 days from the date and time that a relevant entity publishes an approach to market for an open tender.
90 The Tender Evaluation Plan allowed for tenders to be set aside if it became apparent at any stage of the process that a tender was clearly non-competitive or otherwise had no reasonable prospect of exhibiting value for money.
91 This aligned with the evaluation criteria specified in the Request for Tender documentation.
92 The Tender Evaluation Working Groups provided detailed assessments against one or more of the evaluation criteria.
93 This requirement is set out in paragraph 10.30 of the 2017 Commonwealth Procurement Rules. Paragraph 10.31 notes that ‘this policy operates within the context of relevant national and international agreements and procurement policies to which Australia is signatory, including free trade agreements and the Australian and New Zealand Government Procurement Agreement’.
94 The Request for Tender closed on 28 February 2017 and the outcome of the evaluation was approved by the delegate on 28 September 2017.
95 This was a requirement of both the July 2014 and March 2017 Commonwealth Procurement Rules, section 7.2.
96 Commonwealth Procurement Rules, March 2017, p. 9.
97 Defence’s planning documentation did not include any estimates or consideration of these potential savings.
98 See Chapter 2 for information on the Military Integrated Logistics Information System.
99 In April 2018 Defence provided the ANAO with the revised transportation price per ration pack. Based on the combat ration pack type, this represented savings of between $0.09 and $0.68 per ration pack. Defence was unable to demonstrate the calculation which underpinned the $200 000 savings estimates.
100 Defence advised the ANAO in April 2018 that ‘the quantities are the total stock held by the Commonwealth which will be drawn upon until the Commonwealth advises Prepack (the Prime Vendor) of the cessation of supply’.
101 Of the five types of combat ration packs, the Patrol Ration One Man includes freeze-dried meals. There are five menu options for this combat ration pack.
102 The ANAO analysis of contracted prices for combat ration packs with freeze-dried goods indicates that the total contracted unit price is on average $47.17. The ANAO’s analysis also indicates that the contracted cost of the freeze-dried component is on average $21.29, or 45 per cent of the contracted cost.
103 Not adjusted for inflation. The contract makes provision for Defence to purchase a certain number of ration packs per year, with a plus or minus 20 per cent variation to account for demand. The ANAO’s calculation is based on the mid-point. If 20 per cent fewer ration packs were purchased the cost of the Government Furnished Material would be approximately $680 000 per year ($3.4 million over five years). If 20 per cent more ration packs were purchased the cost of the Government Furnished Material would be approximately $1.0 million per year ($5.1 million over five years).
104 Attachment B of the contract describes the amounts payable by the Commonwealth to the Contractor for the provision of Task-Priced Services in accordance with the contract, by item number. Those items are the different types of combat ration packs to be supplied. Table B-1 of Annex B to Attachment B of the contract specifies the unit price for each item number. The unit price excludes GST, customs duty and premiums, but does not specifically exclude Government Furnished Material for any item. Annex D to Attachment B of the contract provides for certain price adjustments for Recurring Services and Task-Priced Services, but makes no provision for price adjustments relating to Government Furnished Material.
105 The procurement of ancillary items is based on previous usage and forecasts, with the forecast for combat rations based on:
- usage forecasts for training and operational requirements, as advised by the capability manager;
- quantities of combat rations that have been disposed of since the last buy; and
- quantities of remaining stock, giving cognisance to their expiry dates.
The forecasts also take into consideration contingency holdings in accordance with Chief of Army directives.
106 Relevant documents in the Request for Tender documentation included: the Conditions of Tender; Annexes to the Conditions of Tender; the Draft Conditions of Contract; Attachments to the Conditions of Contract; Draft Statement of Work; Annexes to the Draft Statement of Works; and Data Item Descriptions.
107 ANAO Audit Report No. 2 2017–18 Defence’s Management of Materiel Sustainment considered Defence’s use of performance-based contracts in the management of sustainment (paragraphs 5.33 to 5.40). The report is available from <https://www.anao.gov.au/work/performance-audit/defence-management-materiel-sustainment> [accessed 20 December 2017].
108 In addition, an external review of the Health SPO in April 2017 noted that under the recommended new contracting arrangements ‘prime suppliers will be managed to provide outcomes against performance based contracts’.
109 A stock controller is responsible for overseeing all transactions involving scheduled and unscheduled medicines, both within their facility and in any supported health facility including those held by a stock guardian. The stock controller also accounts for bulk supplies of medicines. The stock controller is generally the senior pharmacist.
110 A stock guardian is a person who holds scheduled or unscheduled medicines. For example: a pharmacist, medical practitioner, veterinary practitioner, nurse practitioner, dental practitioner; registered nurse; a person responsible for the custody of medicines in field medical kits; or a ship’s commanding officer.
111 Schedule 8 refers to controlled drugs which are substances which require restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence.
112 For example, Schedule 3 (Pharmacist Only Medicine) and Schedule 4 (Prescription Only Medicine) transactions must be retained for two years; Schedule 8 (see definition in footnote 59) transactions must be retained for seven years.
113 An authorised officer is any military officer or civilian (employed or contracted by the Department of Defence) who is registered as a medical practitioner, dental practitioner, nurse practitioner, pharmacist or veterinarian and who is employed in that capacity and is working within their scope of practice. Inside the pharmacy, the authorised officer is the pharmacist. Depending on the health setting, the role can also be conducted by an authorised non pharmacist.
114 Combat rations and ancillary items are delivered to the Defence National Storage and Distribution Centre (the Primary Logistics Unit). From there, they are then transferred to Joint Logistics Units, Combat Service Support Battalions and Force Support Battalions, as required.
115 Inspector Foodstuffs are responsible for the technical inspection of all Class 1 commodities. Class 1 commodities are subsistence items such as foodstuffs, combat rations, packaged water, water purification tablets, hexamine tablets, stoves, foot powder, mosquito repellent and ecclesiastical support stores.
116 Part of Joint Logistics Command, provides a single point for all Defence inventory management governance activities.