Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Administration of the Pathology Quality and Outlays Memorandum of Understanding
The objective of the audit was to determine the effectiveness of DoHA's administration of the MoU between the Government and the pathology profession, including monitoring whether the MoU is achieving its objectives
Summary
Introduction
Pathology is the scientific study of disease and, as such, underpins most of medicine. Pathology tests are now a common part of modern medical practice and are used to screen for, confirm, exclude and monitor disease.
Under Medicare, many pathology services are eligible for subsidies. Through the Medicare Benefits Schedule (MBS), the Australian Government provides approximately $1.7 billion each year to support private patient pathology services in the Australian community and in hospitals.
Pathology services are funded through a combination of government and private payments. Patients who are bulk billed by a pathology service provider do not incur any out-of-pocket expense for the pathology service. For patients who are not bulk billed, the fee is determined at the provider's discretion, with the patient responsible for paying the difference between that fee and the appropriate Medicare rebate.
Pathology stands out within the medical profession for its consistently high rates of bulk billing and observance of the MBS schedule fee – 87 per cent and 92 per cent of services respectively.1
In recent years there has been a strong growth in the number of Medicare-funded pathology services. The growth can be attributed to an increase in the use of medical services and in the average number of pathology tests per medical service. Factors that may be contributing to an increase in the volume of pathology tests include an expansion in the range of tests available, doctors practising defensive medicine, and the ageing of the population. Government policy can also increase the demand for pathology services, for example, by adding new subsidised items to the MBS or through specific initiatives and incentives designed to improve patient access to medical services and to better manage illnesses and diseases (such as diabetes).
Section 16A of the Health Insurance Act 1973 provides for the payment of Medicare benefits for pathology services. The Health Insurance Act 1973 also provides for the Health Insurance (Pathology Services Table) Regulations 2007 that prescribe a Pathology Services Table (PST) that sets out the items of pathology services, the amount of fees applicable for each item, and rules for interpretation. The Health Insurance Act 1973 requires an annual review process for the Table.
The Pathology Services Table Committee (PSTC)2 manages the PST. This entails drafting new item descriptors and related rules of interpretation, and revising existing ones. The committee also advises on the interpretation of item descriptors, rules and fee levels.
Determining appropriate fee levels for pathology items (fee setting) is a negotiated process between the PSTC and the Department of Health and Ageing (DoHA). Fees are based on an assessment of the similarity in complexity and/or method and associated costs between the service being considered and a comparable item on the table. Implicit in this approach is that schedule fees are based on actual cost structures, plus a profit margin. In the last few years there has been an emphasis on redressing any under-remunerated services on the table, that is, the more complex and labour-intensive services.
A significant objective of the Health Insurance Act 1973, including the enforcement and offence provisions relating to pathology, is to prevent over-servicing. As a complementary strategy, the Government has entered into a Memorandum of Understanding (MoU) with the pathology profession to manage outlays in pathology services.
The Pathology Quality and Outlays MoU
Through Medicare, pathology practitioners are reimbursed by the Australian Government on a fee-for-service basis for many of the pathology services they provide. Most government expenditure on pathology is managed through the use of the Pathology Quality and Outlays MoU between the Australian Government and the pathology profession. The Pathology Quality and Outlays MoU 2004–2009 is the third MoU. It is intended to promote:
- access to quality, affordable pathology services;
- effective management of government outlays relating to the services described in the Pathology Services Table of the MBS;
- improved patient care by enhancing the quality of pathology services and the appropriate use of services; and
- cooperative strategies that promote affordability of services for patients.
The ‘parties' to the MoU include the Australian Government (as represented by the Minister for Health and Ageing), the Australian Association of Pathology Practices (AAPP), the Royal College of Pathologists of Australasia (RCPA) and the National Coalition of Public Pathology (NCOPP).
A Pathology Consultative Committee (PCC) is responsible for managing the MoU, including having principal responsibility for managing pathology outlays within agreed parameters.3 DoHA represents the Minister for Health and Ageing as a signatory to the MoU, and also provides secretariat and project support to the PCC and its various sub-committees.
The current MoU was designed to provide for stable growth in pathology outlays at an average of 5.3 per cent per annum growth between 2004 and 2009. The Government initially committed $8.034 billion for pathology outlays over the period of the agreement and provided an extra $3.75 million to help train pathologists to meet a recognised shortfall.
The MoU has thresholds and measures designed to limit growth in pathology outlays and was designed to constrain outlays by regulating the price of the Medicare rebates paid to pathologists for items performed when projections suggest the overall level of outlays will exceed established targets.
Audit scope and objective
The objective of the audit was to determine the effectiveness of DoHA's administration of the MoU between the Government and the pathology profession, including monitoring whether the MoU is achieving its objectives.
The audit examined the controls DoHA has implemented to manage the MoU and government outlays for pathology services. Other objectives of the MoU, such as promoting access to quality, affordable pathology services, were examined from the perspective of their contribution as complementary strategies and initiatives to broader requirements, including the Health Insurance Act 1973 and accreditation processes.
The audit did not examine the controls for processing claims by Medicare Australia.
Conclusion
A key objective of the current Pathology Quality and Outlays MoU is to manage Australian Government outlays for pathology services over the period 2004 to 2009. The MoU brings together the Government and the other signatories to the MoU: the Australian Association of Pathology Practices; the National Coalition of Public Pathology; and the Royal College of Pathologists of Australasia to allow the parties to consider possible strategies to ensure that pathology outlays remain within the established targets.
The Government initially committed $8.034 billion over the period of the agreement to fund pathology outlays and provided an extra $3.75 million to help train pathologists to meet a recognised shortfall in the specialisation. The MoU is based on an average annual rate of growth in pathology outlays of 5.3 per cent between 2004 and 2009.
DoHA's monitoring of pathology outlays is comprehensive and consists of a suite of reports for the Pathology Consultative Committee (PCC) members to consider. These reports provide a thorough analysis of outlays, projected trends and estimated variances.
Over the first three years of the MoU, the number of Medicare funded pathology services increased from 77.7 million per year to 87.5 million per year and the average annual rate of growth of actual pathology outlays was 7 per cent. As at October 2007, adjustments to allowable Medicare outlays/benefits under the Pathology MoU had been increased by $530.57 million, bringing the revised total to $8.564 billion.
In examining DoHA's administration of the Pathology MoU the ANAO focused on the MoU objective of most concern to the department, namely, to manage pathology outlays. Based on this analysis, areas for consideration by DoHA include:
- better managing the risks related to increasing pathology outlays;
- improving the timeliness in assessing claims submitted by the pathology profession; and
- reviewing the effectiveness of the MoU.
A precursor in managing pathology outlays is having an understanding of the drivers behind the growth in pathology services. This information is essential for being able to implement strategies to effectively manage pathology outlays in the long term. Views about the key factors driving the high growth in pathology requests include: an ageing population; changes in health care delivery practices by medical practitioners; the changing profile of medical practitioners and their ordering patterns; increases in the demand for pathology services by consumers; and increases in the demand for pathology services as a result of government health policy initiatives. The ANAO has made a recommendation that DoHA develops a better understanding of the impact of the drivers of growth for pathology services to inform policy decisions and management strategies for outlays in future years, given the magnitude of pathology outlays.
Under the terms of the MoU, the Government is responsible for meeting increases in pathology outlays that can be demonstrated to have been caused by government policy. Over the period 2004 to 2007, DoHA and the pathology profession considered the effect of significant policy decisions influencing the demand for pathology services and, hence, pathology outlays. These included the extended Medicare safety Net (introduced in March 2004), the Strengthening Medicare package (introduced in January 2005), and the flow-on to Specialist services. Accurately determining the effects of these policy decisions on pathology outlays has been a lengthy and difficult process and involved considerable debate over the appropriateness of methodologies and the level of economic evidence required to prove claims.
These factors, especially the magnitude of the Strengthening Medicare package, have made managing this MoU considerably more complex than previous MoUs. In this environment, establishing clear evaluation criteria (including the level of economic evidence required) and clarifying the economic modelling methodologies to be used to assess claims to adjust pathology outlay targets, is likely to have assisted the resolution of funding adjustments.
The Pathology MoU has been in operation since 2004 and is scheduled to end in 2009. In considering the design of any of any future program to provide for stable growth in pathology outlays, it is timely for DoHA to review the effectiveness of the current MoU and the lessons learned from its operation during the past three years. A review would require DoHA to establish a framework to monitor the extent to which the pathology outlay objectives of the arrangement have been met.
The MoU has other objectives, including promoting access to quality, affordable pathology services. DoHA sees these objectives as complementary initiatives to broader requirements, including the Health Insurance Act 1973 and accreditation processes. The contribution of the MoU to these broader strategies is difficult to measure. DoHA requires more evidence to demonstrate how the MoU has promoted access to quality, affordable pathology services, or improved patient care by enhancing the quality of pathology services.
Key findings by chapter
Management of Government Outlays for Pathology Services (Chapter 2)
In examining DoHA's administration of the Pathology MoU the ANAO focused on the MoU objective of most concern to the department, namely, to manage pathology outlays. This involved examining DoHA's approach to:
- managing the risks related to increasing pathology outlays;
- timeliness in assessing claims submitted by the pathology profession;
- monitoring actual pathology outlays; and
- performance monitoring and reporting.
Managing the risks related to increasing pathology outlays
DoHA's administration of this third Pathology MoU has not involved any formal assessment of risks or the creation of a risk management plan. Instead, risks have been managed on an ad-hoc basis. Without a documented risk management framework it is not possible to conclude on the extent to which risks have been identified, analysed, treated and monitored by the department. Significantly, it does not allow risk mitigation measures to be considered in a holistic and structured manner, commonly accepted practice in relation to significant agreements and projects.
A key risk in managing pathology outlays is not fully understanding the drivers behind the growth in pathology services. In May 2001, during the term of a previous MoU, the Australian Association of Pathology Practices (AAPP), Royal College of Pathologists of Australasia (RCPA), DoHA and the Health Insurance Commission (HIC) participated in a workshop to discuss drivers and structural issues impacting on pathology funding. In August 2001, the AAPP issued a paper entitled Pathology Funding Agreement Issues to be Addressed by PCC, which was a product of these discussions. This document is essentially a risk assessment that: ‘documents a wide range of drivers and structural factors that are impacting on the ability of all parties to effectively manage the pathology agreement'.4
The AAPP paper identifies numerous issues along with recommended actions that have the potential to form the basis of a risk management plan which would clearly identify the links between the activities of the Pathology Consultative Committee (PCC) and the risks identified. Because of its age this particular document would require updating before it could be used as a component of a broader risk management framework for the current MoU.
Timeliness in assessing claims submitted by the pathology profession
Under the terms of the MoU, the Government is responsible for meeting increases in pathology outlays that can be demonstrated to have been caused by government policy. The approach taken under the MoU is for the pathology profession (the Australian Association of Pathology Practices, the Royal College of Pathologists of Australasia, and the National Coalition of Public Pathology) to put forward claims to adjust pathology outlay targets following the introduction of new health policies. To determine the veracity of these claims, DoHA undertakes its own analysis and calculations.
The appropriateness of adjustments stemming from government health policies, particularly the Strengthening Medicare package introduced in January 2005 (the GP claim), and the flow-on to Specialist services (the Specialist claim) was the subject of extensive negotiations.
Following the pathology profession's October 2005 and March 2006 claims, DoHA and the profession agreed on an adjustment to the 2004–05 pathology outlay target arising from the effect of government policy increasing access to GP services (the GP claim). Adjustments for subsequent MoU outlay targets for 2005–06, 2006–07, 2007–08, and 2008–09 were considered more fully by DoHA following the profession's January 2007 claim.
In DoHA's view, the Specialist claims put forward by the profession in October 2005 and March 2006 were unproven. Following the profession's January 2007 claim, the merits behind the Specialist claim were partially accepted by DoHA for MoU outlay targets for 2005–06 through to 2008–09.5
Timeliness in resolving claims is a significant issue for the pathology profession and DoHA. In the past, the pathology profession has been critical of the department acting too soon to adjust fees, only to find that it has over-corrected and further action to restore the balance is then needed. On the other hand, the lengthy processes for considering outlay target adjustments can result in a limited window of opportunity before the conclusion of the MoU to make any fee adjustments, should they be necessary.
DoHA's approach to managing adjustments resulting from new health policy measures has been to consider claims for adjustment as they have been received and to assess whether the case for an adjustment has been satisfactorily established. DoHA advised the ANAO that the terms of the MoU dictate that adjustments to outlays are considered retrospectively, once data showing the actual outlays becomes available. There is no provision in the MoU for DoHA to act before any evidence of outlays exceeding targets becomes available.
The difficulties experienced in the resolution of funding adjustments has been further complicated by:
- the MOU describing the circumstances in which outlay targets can be adjusted, but lacking any specified mechanism for determining and agreeing the value of those adjustments;
- the number and unprecedented magnitude of new health policy initiatives experienced during the term of the current MoU; and
- a lack of agreed methodologies for economic modelling undertaken separately by DoHA and the pathology profession and clarity over the level of proof required to advance claims to increase outlays.
The ANAO notes that PCC members have also expressed concern that there has been a lack of clarity about the level of proof required to successfully argue for outlay targets to be adjusted.
Monitoring actual pathology outlays
From discussions with stakeholders and a review of the minutes of PCC meetings, it was apparent that the monitoring of outlays to ensure that outlay targets are not exceeded has been the predominant focus of the PCC during the term of the third Pathology MoU.
DoHA takes the lead role in facilitating the monitoring of outlays. It has a small team focusing on pathology outlays, and sources information for monitoring purposes predominantly from Medicare data.
Overall, DoHA's monitoring of pathology outlays is comprehensive and consists of a suite of reports for PCC members to consider. These reports provide a thorough analysis of outlays, projected trends and estimated variances. PCC members provide regular feedback on the suitability of reports received and where additional information might prove useful. They considered that DoHA was generally responsive to such requests and, overall, were satisfied with the monitoring undertaken on their behalf.
DoHA also monitors and reports on movements in the pathology cost index and the underlying year on year growth in outlays. This is necessary as excessive movements in these measures could trigger an adjustment to outlay targets in accordance with clauses 5.11 and 5.14 of the MoU. DoHA's monitoring of actual outlay expenditure to date compared with outlay targets is shown in Appendix 4.
Performance monitoring and reporting
DoHA is required to prepare biannual reports to the Minister for Health and Ageing about the activities of the PCC and the operation of the MoU as a whole. These reports have focused predominantly on pathology outlays, but also contain information about affordability measures in the form of bulk billing rates, percentage of schedule fee observance, the average patient gap and contribution percentage.
Biannual reporting to the Minister has not been occurring as required, with the last report provided for the period January to June 2005. At the time of the audit fieldwork, DoHA advised that the report for the period January to June 2007 was being prepared. The reason attributed to three biannual reports not being provided was uncertainty over the target outlay figures arising from then unresolved claims by the pathology profession stemming from new government policies.
During the 18-month period when the Minister did not receive biannual reports, some information was provided by way of correspondence accompanying the request to adjust targets following the March 2006 claim from the pathology profession. Similarly, correspondence to the Minister in September 2007 relating to subsequent claims from the pathology profession for target adjustments for GP and Specialist claims provided an update of the progress of the MoU with regards to outlays.
Reviewing the effectiveness of the MoU
Review and evaluation is an important part of managing government programs. Large programs, especially, should be evaluated on a regular and systematic basis to:
- gauge the continued relevance and priority of program objectives in the light of current circumstances;
- assess whether the program is achieving its stated objectives; and
- ascertain whether more efficient ways of achieving these objectives.
The Pathology MoU was signed in September 2004. It was based on total Australian Government funding outlays/benefits of $8.034 billion dollars from 2004–05 to 2008–09, incorporating an average of 5.3 per cent annual growth in outlays over the life of the MoU.
Clauses in the MoU provide for adjustments to pathology outlay targets. As at October 2007, allowable Medicare outlays/benefits under the Pathology MoU had been increased by:
- $11.5 million in 2004–05 for fee adjustments foregone in the previous MoU;
- $8.0 million in 2004–05 and 2005–06 (and provisionally for 2006–07, 2007–08 and 2008–09) when patient contributions were below identified Patient Affordability targets;
- $45.11 million in 2004–05 arising from the pathology profession's claim that government policy decisions increased pathology referrals from General Practitioners. This included the extended Medicare Safety Net ($5.89 million), the Round the Clock Medicare/Strengthening Medicare package (38.95 million), and Aboriginal and Torres Strait Islander pap smears (0.27 million);
- $9.1 million in 2004–05 arising from the pathology profession's claim that government policy decisions had increased pathology referrals from Specialist practitioners;
- $442.0 million for the years 2005–06 to 2008–09, comprising $379.7 for flow-ons arising from the extended Medicare Safety Net and the Strengthening Medicare package, and $62.3 million for flow-ons arising from Specialist services.
Over the first three years of the MoU, the number of Medicare funded pathology services increased from 77.7 million per year to 87.5 million per year and the average annual rate of growth of actual pathology outlays was 7 per cent. As at October 2007, adjustments to allowable Medicare outlays/benefits under the Pathology MoU had been increased by $530.57 million, bringing the revised total to $8.564 billion.6
The Pathology MoU has been in operation since 2004 and is scheduled to end in 2009. In considering the design of any future program to provide for stable growth in pathology outlays, it is timely for DoHA to review the effectiveness of the current MoU and the lessons learned from its operation during the past three years. A review would require DoHA to establish a framework to monitor the extent to which the objectives of the arrangement have been met.
Strategies that promote access to affordable pathology services (Chapter 3)
The Government's primary tool for managing patient affordability of pathology services is bulk billing, which involves the pathology provider billing Medicare directly and accepting the Medicare benefits attributable as full payment for the service provided.
In addition, the pathology profession has agreed, through the MoU, to consider patient affordability when setting fees. Patient affordability monitors out-of-pocket expenses for patients. The MoU includes provision for annual affordability bonuses to the agreed level of outlays if patient contributions to the cost of pathology services in the given year are less than specified targets (ranging from 9.5 to 11 per cent) over the life of the MoU.
The targets for the pathology profession reflect the current high rates of bulk billing. If the targets are met (that is, actual patient contributions are below 9.5 to 11 per cent), an additional $8 million a year is added to the outlay target. Targets and actual results are shown in Table 1.
Table 1 Patient affordability targets versus actual patient contributions
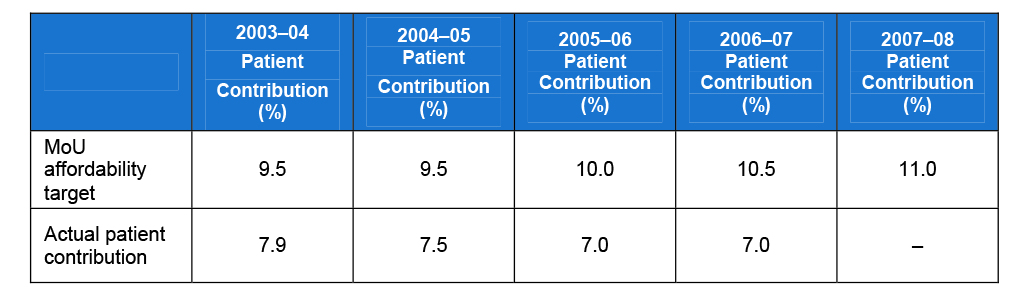
Source: Targets are sourced from the Pathology Quality and Outlays MoU and actual contributions from Medicare data.
Trends in actual patient contributions illustrate that the affordability bonus has been easily achieved and that the percentage targets are generous to the pathology profession. Investigation of the rationale behind these percentages was inconclusive, suggesting the percentages were negotiated between the parties with no evidentiary basis for establishing the thresholds. DoHA indicated that it is unlikely that the affordability targets established in the MoU would be exceeded. This is also reflected in monitoring information provided to the Department of Finance and Deregulation, which has factored in the $8 million affordability bonus for all years of the MoU.
‘Patient affordability' is not defined in the MoU and the predefined thresholds contained in the MoU regarding payment of the affordability bonus are not supported by any detailed rationale. Furthermore, there is no clearly defined strategy addressing the principle and objective of the MoU to promote consumer access and patient affordability. Overall, promoting access to affordable pathology services has not been a focus in managing the MoU as the pathology profession has a high level of bulk billing, which has been a consistent trend during the life of this MoU. DoHA noted, however, that should the situation change during the life of the MoU or in the context of any future agreements, it would consider a broader range of policy options for promoting patient affordability.
Enhancing the quality of pathology services (Chapter 4)
Since 1986, the Health Insurance Act 1973 has required pathology laboratories to be accredited in order to access the MBS. In determining laboratory accreditation status, Medicare Australia relies on assessments conducted by the National Association of Testing Authorities (NATA), in conjunction with the Royal College of Pathologists of Australasia (RCPA), against national standards established by the National Pathology Accreditation Advisory Council (NPAAC).
The MoU was designed to complement these accreditation requirements. DoHA states that ‘Improving the quality use of pathology in patient care is an important element of the third Pathology Quality and Outlays Memorandum of Understanding (MoU) between the Australian Government and the pathology profession.'7
Section 9 of the MoU focuses on ‘Quality Initiatives'. Subject to the appropriate agreements relating to the funds being entered into, the Government will make funding available for a Quality Use of Pathology Program (QUPP), up to a maximum of $2 million for each year of the MoU.
The MoU states that the objectives of the QUPP are to improve patient care through enhancing the quality of pathology services and the appropriate use of services. This may include support for research into consumer needs; development of education and information programs for consumers, requestors and/or providers of pathology services; utilisation of infomatics and the application of information technology; and quality assurance programs.
DoHA commissioned consultants to map a strategy to ensure QUPP-funded projects are effectively implemented in order to realise potential benefits. The consultancy was a positive initiative, providing a more robust management framework. Notwithstanding, the strategic plan for the QUPP, the MoU and the terms of reference for the PCC and the Quality Use of Pathology Committee (QUPC) do not align, and there is a risk that the newly established direction for the QUPC potentially overlaps with other established structures such as National Pathology Accreditation Advisory Council (with regard to standard setting) and the RCPA (with regard to addressing quality in pathology practice). The existing framework does not specify how the interrelationships with these bodies should be conducted.
While there have been a number of QUPP initiatives, there is no defined measure to determine whether the MoU objectives of quality pathology services ?{ improved patient care and the appropriate use of services ?{- have actually been enhanced by the MoU. A central aspect of quality pathology use is excessive or unnecessary referrals, commonly believed to be a key contributor to increasing outlays. While this relationship is generally appreciated, there is no strategy in the MoU that aligns the objectives of the QUPP with containing outlays in overall growth.
Workforce development and support (Chapter 5)
The MoU is not a major mechanism for supporting pathology training, but was designed to make a modest contribution to it.
The primary providers of pathology training positions in Australia are the States and Territories, in line with their public hospital service delivery employment functions. The Australian Government provides funding for public hospital based training of medical specialists through the Australian Health Care Agreements. States and Territories are required under the Agreements to continue to provide support for medical specialist training positions and determine the level of funding to be allocated within the individual hospital budgets for this purpose. States and Territories also determine the number and type of accredited training places to be provided.
In addition, through the MoU, the Australian Government has contributed $3.75 million towards the cost of training new pathologists in the private sector. Funding for up to 10 pathology training places in the private sector began in January 2005.
Summary of agency response
The Department of Health and Ageing was supportive of the report and agreed with the recommendation.
Recommendation
The Pathology Quality and Outlays MoU is the third in a series of MoUs designed to provide for stable growth in pathology outlays. It is due to end in 2009. The ANAO's recommendation is framed to assist DoHA in the work it is already undertaking to ensure that any future arrangement incorporates the lessons learned.
Footnotes
1 DoHA releases quarterly statistics on Medicare bull billing and schedule fee observance rates for broad types of services with the latest report available from its website at www.health.gov.au for the December quarter 2007. The latest rates for pathology are 86.6 per cent bulk billing rate and 92.3 per cent schedule fee observance rate.
2 The PSTC consists of members from the Australian Association of Pathology Practices (AAPP), the Royal College of Pathologists of Australasia (RCPA), the National Coalition of Public Pathology (NCOPP), the Australian Medical Association (AMA), Medicare Australia and DoHA.
3 The Pathology Consultative Committee's membership includes up to three representatives each from the Australian Government; the Australian Association of Pathology Practices (AAPP); and the Royal College of Pathologists of Australasia (RCPA) and one representative from the National Coalition of Public Pathology (NCOPP). The Prime Minister's authorisation is required for adjustments to pathology outlay targets in the MoU that exceed $10 million.
4 Australian Association of Pathology Practices, Pathology Funding Agreement Issues to be Addressed by PCC, 2001, p. 17.
5 On 16 October 2007, the Government agreed to increase allowable outlays under the Pathology MoU by $102.4 million for 2005–06, totalling $442 million over the remainder of the MoU period. An amount of $9.1 million for flow-ons from Specialists was agreed for 2004–05 pathology outlays.
6 There are minor deductions for the P9 group of services - see Appendix 3 and Appendix 4, Table A 5 for details.
7 See DoHA, Quality Use of Pathology homepage: <http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pa…;