Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Administration of the Pharmaceutical Benefits Scheme

Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- The Pharmaceutical Benefits Scheme (PBS) is an Australian Government scheme that subsidises the cost of medicines for Australian residents and eligible overseas visitors.
- This audit examined the effectiveness of the administration of the PBS by the Department of Health and Aged Care (Health) and Services Australia.
Key facts
- As at 30 June 2023, there were 928 medicines (across 5,261 brands) listed on the Schedule of Pharmaceutical Benefits.
- Budgeted expenditure for the PBS for 2024–25 was $19.5 billion (excluding recovery revenue).
- In 2022–23, there were 223.1 million over co-payment PBS prescriptions (67.9 per cent) and 105.6 million under co-payment PBS prescriptions (32.1 per cent) dispensed in Australia.
What did we find?
- The administration of the PBS is partly effective.
- Health’s governance and oversight arrangements for the PBS are partly appropriate. Deficiencies were identified with delegation instruments and performance, risk and stakeholder management arrangements.
- Arrangements to manage the cost of the PBS are largely appropriate. Arrangements are in place to manage individual medicine costs, pharmacy remuneration and patient out-of-pocket costs. Health does not undertake horizon scanning to anticipate future costs.
- Arrangements to manage the delivery of PBS services and payments are partly effective. Deficiencies related to ensuring legislative requirements for certifying claims are met and performance reporting.
What did we recommend?
- There were seven recommendations to improve the administration of the PBS — three to Health, two to Services Australia and two to both entities. Services Australia did not agree to one recommendation.
640,000
patients were estimated in 2021 to be eligible for the PBS Safety Net but did not apply for a PBS Safety Net card.
$1.514 bn
of claims have not been certified by PBS suppliers within 65 days.
29.4%
of PBS medicines require prescribers to gain authority approval from Services Australia prior to prescribing.
Summary and recommendations
Background
1. The Pharmaceutical Benefits Scheme (PBS) is an Australian Government scheme that subsidises the cost of a wide range of medicines for Australian residents and eligible overseas visitors. The PBS is enabled by the National Health Act 1953 (NHA) which regulates the listing, prescribing, pricing, charging and payment of subsidies for the supply of medicines and medicinal preparations as pharmaceutical benefits. The PBS Schedule, made under the National Health (Listing of Pharmaceutical Benefits) Instrument 2024, lists medicines subsidised under the PBS and outlines requirements for the provision of these medicines.
2. The objective of the PBS is to provide Australians with timely, reliable and affordable access to necessary and cost-effective medicines. The Department of Health and Aged Care (Health) is responsible for PBS policy and has a bilateral agreement with Services Australia to deliver PBS-related services and payments.
Rationale for undertaking the audit
3. The PBS is intended to ensure that Australians have timely, reliable and affordable access to medicines. The budgeted expenditure for the PBS for the 2024–25 financial year is $19.5 billion. This performance audit was conducted to provide assurance to Parliament that the PBS is being administered effectively.
Audit objective and criteria
4. The objective of the audit was to assess the effectiveness of the administration of the PBS.
5. To form a conclusion against the audit objective, the following high-level criteria were adopted:
- Has Health established appropriate governance and oversight arrangements for the PBS?
- Has Health established appropriate arrangements to manage the cost of the PBS?
- Have Health and Services Australia established effective arrangements to manage the delivery of PBS services and payments?
Conclusion
6. Health’s and Services Australia’s administration of the PBS is partly effective. While arrangements for managing the cost of the PBS are largely effective, there were deficiencies in arrangements for whole-of-program management and administering the delivery of PBS services and payments.
7. Health’s governance and oversight arrangements for the PBS are partly appropriate. Instruments for delegating statutory powers for administering the PBS have irregularities and anomalies. Health’s PBS Program Management Plan could be improved by including more detail on Health’s management arrangements for the PBS. Health has a largely appropriate bilateral arrangement with Services Australia to oversee its delivery of PBS services and payments. Health’s performance measurement framework for the PBS does not adequately measure and report on program outcomes. Health’s risk management focuses on shared administration risks with Services Australia and has not considered broader strategic risks to the PBS. While mechanisms are in place for stakeholder engagement on the PBS, Health has not conducted an analysis of stakeholder engagement needs or developed an overarching stakeholder engagement plan.
8. Health’s arrangements to manage the cost of the PBS are largely appropriate. Arrangements were in place to assess the cost-effectiveness of individual PBS medicines and manage the cost of listed medicines. Arrangements have been established to manage pharmacy remuneration through successive Community Pharmacy Agreements (CPAs), negotiated with the pharmacy industry, which Health supported through impact analysis for the eighth CPA signed in June 2024. Health has established processes for managing patient out-of-pocket costs and monitoring and forecasting the overall cost of the PBS. Health has not established arrangements to automate patient access to the Safety Net or engaged in horizon scanning analysis to anticipate potential future costs of new and novel medicines.
9. Health and Services Australia’s arrangements to manage the delivery of PBS services and payments are partly effective. Processes and systems for PBS claims processing are not fully effective at ensuring that legislative requirements for PBS claims are met, as Services Australia is not ensuring that PBS suppliers certify claims in accordance with legislative timeframes. While payment integrity is reviewed, it is not subject to performance monitoring or reporting. Payment timeliness is monitored, and targets are regularly met. The results are not included in Services Australia’s Annual Performance Statement. The provision of authority approvals is based on an automated system. There were differences in approval rates between authority applications made online and by phone, and Services Australia’s performance target for reporting on answering authority calls in its Annual Performance Statements does not align with the performance target agreed with Health in bilateral agreements. PBS Safety Net card claims and patient refunds are reliant on manual processes and timeliness performance measures have not been consistently met.
Supporting findings
Governance and oversight
10. Instruments that delegate powers and functions for administering the PBS have irregularities and anomalies. While Health has developed a Program Management Plan for the PBS, it does not adequately cover arrangements for managing PBS costs, stakeholder engagement and whole-of-program performance measurement. Health’s support to independent statutory bodies with responsibilities for the PBS could be improved by developing governance documentation for the Pharmaceutical Benefits Advisory Committee. (See paragraphs 2.3 to 2.24)
11. Health and Services Australia have established a Bilateral Management Arrangement, which includes bilateral agreements and bilateral governance arrangements that relate to the delivery of PBS services and payments.
- PBS-related program agreements were fit for purpose, with clear objectives and defined roles and responsibilities. All protocols supporting the bilateral arrangement were reviewed and updated between November 2023 and September 2024.
- While bilateral governance meetings have not occurred at the most senior levels, there has been regular engagement between the two entities at lower levels. Governance committees relevant to the PBS began considering risk, performance reporting, and updates to bilateral agreements in late 2023. (See paragraphs 2.25 to 2.35)
12. Health has one external performance measure for the PBS, which is not outcome focused and does not provide meaningful performance information to the Parliament or the public. Health receives monthly reporting from Services Australia on bilateral performance measures. It has not used this data to oversee Services Australia’s service delivery. Health does not provide any regular performance reporting on the PBS to the minister or its executive committee. (See paragraphs 2.36 to 2.54)
13. Health has not undertaken appropriate risk assessments or developed appropriate risk management plans for the PBS at the divisional or program level. Its risk assessments and plans do not adequately cover key program activities for which Health is responsible. Health’s shared risk management plan with Services Australia covers risks relating to the services and payments Services Australia delivers for the PBS. From late 2023, bilateral governance bodies began discussing operational risks relevant to the PBS. (See paragraphs 2.55 to 2.69)
14. Health’s arrangements for stakeholder engagement for the PBS include the provision of information through websites, invitation of written submissions from stakeholders on specific PBS issues, agreement-making with industry bodies, and hosting regular stakeholder engagement forums. These arrangements have not been informed by a systematic analysis of stakeholder engagement needs or an overarching stakeholder engagement plan or strategy. (See paragraphs 2.70 to 2.83)
Managing the cost of the PBS
15. Arrangements for assessing medicine cost-effectiveness outlined in the Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee have been followed. Health has complied with administrative procedures for listing medicines on the Schedule and agreeing medicine prices with sponsors. Health has negotiated deeds of agreement with medicine sponsors (covering special pricing arrangements and risk-sharing agreements) to minimise the cost of PBS medicines to government. Statutory price reductions are in place to decrease the cost of listed medicines. Medicines are delisted from the Schedule by medicine sponsors with no regular delisting process performed by Health. (See paragraphs 3.3 to 3.53)
16. The Australian Government has negotiated Community Pharmacy Agreements (CPAs) with the pharmacy sector to determine pharmacy remuneration for dispensing PBS medicines since 1990. CPAs offer flexibility to include terms such as the remuneration adjustment mechanism to mitigate unexpected expenditure for the Australian Government. The choice to negotiate a CPA rather than allowing remuneration to be set by an independent tribunal was not supported by adequate impact analysis for the seventh CPA. Health prepared an Impact Analysis for the eighth CPA, signed in June 2024, which supported continuation of pharmacy remuneration setting through a CPA. (See paragraphs 3.54 to 3.74)
17. Health has used monitoring data to model the impact of proposed changes to patient co-payment amounts and Safety Net thresholds on patient out-of-pocket costs. Based on this modelling, Health has provided advice to government on proposals to help patients achieve greater cost-savings through these mechanisms. Health has not established arrangements to automatically determine eligibility for the Safety Net. Health has estimated that 640,000 patients become eligible for the Safety Net each year but do not apply, foregoing $100 million in medicine subsidies. (See paragraphs 3.75 to 3.95)
18. Health has established arrangements for modelling the overall cost of the PBS and the impact of new medicine listings, and it provides advice to the government and Parliament through the annual Budget processes.
- Health has established a system to model PBS expenditure based on the current legislative requirements, which it uses to model the impact of new and amended medicine listings.
- Reporting on PBS expenditure is available through an annual report and reporting on Services Australia’s website.
- Health has not performed horizon scanning analysis to forecast PBS expenditure and identify potential policy changes. (See paragraphs 3.96 to 3.113)
Delivery of services and payments
19. Almost all claims (99.9 per cent) made by PBS suppliers are submitted through Services Australia’s Online Claiming for PBS system, which automatically assesses claims against legislative rules before processing advance payments. Due to an absence of controls to ensure advance payments to PBS suppliers are certified within statutory timeframes, over one-third of approved PBS suppliers have uncertified claims totalling $1.514 billion (as at 30 June 2024). Payment integrity is reviewed but is not subject to performance monitoring or reporting. Payment timeliness is monitored, and targets are regularly reported as met, but it is not included in public reporting. (See paragraphs 4.3 to 4.30)
20. A system to manage authority-required approvals has been established that is consistent with Health and Services Australia’s respective responsibilities under the PBS bilateral agreement. There are differences in approval rates depending on the method used by an applicant to apply for an authority. Reported results for the timeliness of authority approvals against performance measures set out in bilateral arrangements have largely not met targets. Services Australia reports in its Annual Performance Statement on the achievement of a performance measure target of answering authority calls within 15 minutes. This does not align with the target of answering authority calls, on average, in less than 30 seconds. (See paragraphs 4.31 to 4.52)
21. Services Australia has established processes and systems to manage PBS Safety Net and patient refunds. Both systems are reliant on paper-based application forms which are submitted by post and manually processed by Services Australia. The reliance on manual processing means that performance is sensitive to staffing numbers, which has meant timeliness performance measures have not been consistently met. Services Australia’s quality checking process for Safety Net claims does not provide accurate data on the reasons for rejecting Safety Net card applications to inform education or compliance activities. (See paragraphs 4.55 to 4.78)
Recommendations
Recommendation no. 1
Paragraph 2.8
The Department of Health and Aged Care and Services Australia work to review and update relevant delegation instruments to address irregularities and anomalies.
Department of Health and Aged Care response: Agreed.
Services Australia response: Agreed.
Recommendation no. 2
Paragraph 2.46
The Department of Health and Aged Care establish and report against a performance management framework for the Pharmaceutical Benefits Scheme that:
- includes an appropriate mix of output, efficiency and effectiveness performance measures for key program activities, including those of third-party delivery partners; and
- enables the department’s performance in administering the Pharmaceutical Benefits Scheme purposes to be measured and assessed.
Department of Health and Aged Care response: Agreed.
Recommendation no. 3
Paragraph 2.64
The Department of Health and Aged Care undertake a risk assessment for the Pharmaceutical Benefits Scheme program that covers activities for which the department is responsible.
Department of Health and Aged Care response: Agreed.
Recommendation no. 4
Paragraph 2.82
The Department of Health and Aged Care:
- develop a stakeholder plan for the Pharmaceutical Benefits Scheme that identifies all stakeholder groups, consultation objectives and methods of engagement; and
- publish a stakeholder strategy that informs stakeholders of Health’s planned approach to engaging with stakeholders on the Pharmaceutical Benefits Scheme, including where written agreements or partnerships may be used.
Department of Health and Aged Care response: Agreed.
Recommendation no. 5
Paragraph 4.19
The Department of Health and Aged Care and Services Australia document and implement a strategy for addressing the backlog of uncertified Pharmaceutical Benefits Scheme claims.
Department of Health and Aged Care response: Agreed.
Services Australia response: Agreed.
Recommendation no. 6
Paragraph 4.29
Services Australia report to the Department of Health and Aged Care on payment accuracy for the Pharmaceutical Benefits Scheme (PBS) in accordance with the PBS Program Agreement, and separately report on the integrity and timeliness of PBS payments in its Annual Performance Statements.
Services Australia response: Agreed.
Recommendation no. 7
Paragraph 4.51
Services Australia align its reporting on the timeliness of issuing authority approvals in its Annual Performance Statement with performance measures and targets agreed in bilateral arrangements.
Services Australia response: Not agreed.
Summary of entity responses
22. The proposed audit report was provided to Health and Services Australia. The entities’ summary responses are provided below, and their full responses are included at Appendix 1. Improvements observed by the ANAO during the course of this audit are listed in Appendix 2.
Department of Health and Aged Care
The Department of Health and Aged Care (the Department) welcomes the findings in the report. The Department notes the overall finding by the ANAO that the Department’s and Services Australia’s administration of the Pharmaceutical Benefits Scheme (PBS) is partly effective. The Department is committed to working towards implementing the recommendations in the report as a priority and is already taking steps to address key findings identified in the audit. The Department has also commenced engagement with its partner agency, Services Australia, to address key recommendations in relation to the delivery of the PBS payment arrangement.
The ANAO found that the department has largely appropriate arrangements to manage the cost of the PBS. The Department welcomes the finding that appropriate arrangements have been established for managing patient out-of-pocket costs for Australians and monitoring the overall cost of the PBS. The Department acknowledges the findings that arrangements have been implemented to assess and manage the cost of listed medicines and to manage pharmacy remuneration through successive Community Pharmacy Agreements, and that the bilateral arrangements with Services Australia to oversee delivery of Pharmaceutical Benefits Scheme services and payments are also largely appropriate.
Services Australia
Services Australia (the Agency) notes the findings of the report that the Agency’s arrangements to manage the delivery of the PBS services and payments are partly effective, having regard to certification of claims, reporting differences at the bilateral level compared to Annual Performance Statements, delegation instruments and PBS Safety Net.
The Agency welcomes the findings of the report and is committed to delivering the payments and services related to the PBS, which subsidises the cost of medicines for Australian residents and eligible overseas visitors. The Agency administers the PBS in accordance with the policy and legislation for which the Department of Health and Aged Care (Health) has responsibility. The Agency continues to work with Health to address the issue of uncertified claims and changes to delegation instruments in addition to expanding the work types to include PBS in its Annual Performance Statements for 2024-25.
The Agency agrees with the finding that the performance targets for answering authority calls is different for bilateral agreement and Annual Performance Statement purposes. Due to the expansive nature of the services it provides, reporting is done on a tiered basis for different purposes. The Agency continues to focus on reducing reliance on the PBS Authorities telephone line and increasing digital PBS authorities.
Key messages from this audit for all Australian Government entities
23. Below is a summary of key messages, including instances of good practice, which have been identified in this audit and may be relevant for the operations of other Australian Government entities.
Administration of long-term programs
Delegations of authority
1. Background
Introduction
1.1 The Pharmaceutical Benefits Scheme (PBS) is an Australian Government program that subsidises the cost of medicines. Australian residents who hold a current Medicare card are eligible to receive subsidised medicines under the PBS, as are some overseas visitors through reciprocal healthcare agreements.
1.2 Medicines that are subsidised through the PBS are listed on the Schedule of Pharmaceutical Benefits (the Schedule), which is available online and updated each month.1 As of 30 June 2023, there were 928 medicines (across 5,261 brands) listed on the Schedule. The budgeted expenditure for the PBS for the 2024–25 financial year was $19.5 billion (excluding recovery revenue), placing it among the top 10 Australian Government programs by estimated expenditure (see Figure 1.1).
Figure 1.1: Top 10 Australian Government programs by estimated expenditure, 2024–25a
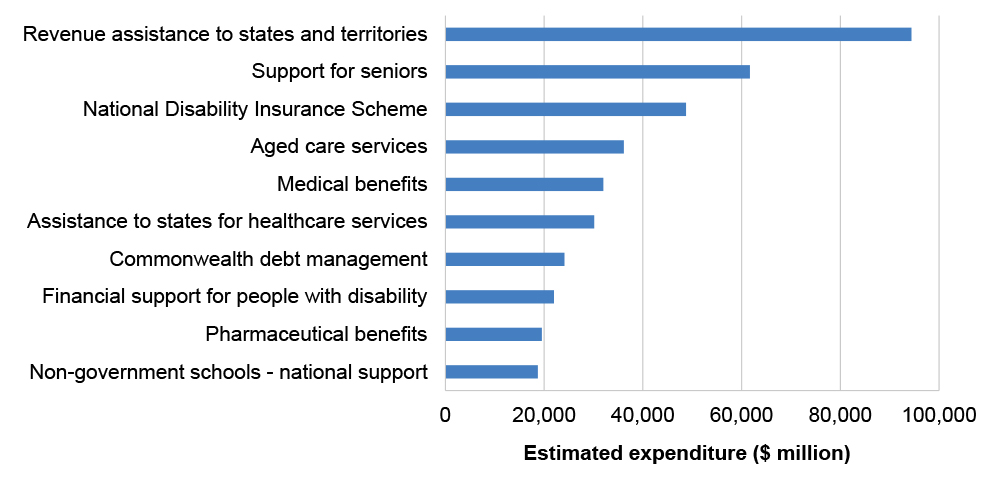
Note a: Estimated expenditure for each program includes eliminations for inter-agency transactions within that program.
The estimated expenditure for the National Disability Insurance Scheme is a combination of agency costs, support for participants and administered expenses.
Source: Australian Government, Budget Paper No. 1: Budget Strategy and Outlook 2024-25, Commonwealth of Australia, Canberra, 2024, available from https://budget.gov.au/content/bp1/index.htm [accessed 26 July 2024].
Patient out-of-pocket costs
1.3 The cost of a PBS-listed medicine at point of sale comprises three components:
- the approved PBS subsidy (paid by the Australian Government);
- a co-payment determined by the Australian Government (paid by the patient); and
- certain discretionary charges (if any) charged by the pharmacist (paid by the patient).
1.4 Co-payments are capped at amounts that are adjusted annually in line with the Consumer Price Index. As of 1 January 2024, the ordinary co-payment amounts were $31.60 for general patients or $7.70 for patients with a concession card. The Australian Government subsidy covers the difference (if any) between the co-payment and the full cost of the medicine but does not cover any manufacturer premiums charged by the pharmacist. In 2022–23, there were 223.1 million over co-payment PBS prescriptions (67.9 per cent) and 105.6 million under co-payment PBS prescriptions (32.1 per cent) dispensed in Australia.2
1.5 Patients who purchase a large number of PBS-subsidised medicines in a calendar year may take advantage of an arrangement called the PBS Safety Net, which is intended to reduce out-of-pocket costs. When an individual’s or a family’s out-of-pocket costs for PBS medicines reaches a specified threshold in a calendar year they can apply for a Safety Net card, which reduces the co-payment charged for each medicine for the remainder of the relevant calendar year to either: the concessional co-payment (if they are general patients); or zero dollars (if they are concession card holders). The Safety Net thresholds are also adjusted annually in line with the Consumer Price Index. As of 1 January 2024, the PBS Safety Net thresholds were $1,647.90 for general patients and $277.20 for concession card holders.
Administrative responsibilities
1.6 The PBS is governed by the National Health Act 1953 (NHA) and is enabled by over 100 individual legislative instruments.3 The NHA confers powers relating to administering the PBS to the Minister for Health and Aged Care (the minister), the Secretary of the Department of Health and Aged Care (Health) and the Chief Executive Officer (CEO) of Services Australia (as the Chief Executive Medicare).
1.7 Health and Services Australia share responsibility for the administration of the PBS. Health is responsible for PBS policy and health provider compliance, supporting the operations of PBS statutory bodies, managing the approval of PBS suppliers and overseeing the delivery of programs relating to pharmacy services and medicine supply. Services Australia delivers PBS-related services and payments.
1.8 Health and Services Australia have established a Bilateral Management Arrangement for the delivery of health programs, including the PBS. Under this arrangement, Services Australia is responsible for delivering PBS services and payments including:
- processing claims for payment for the supply of PBS medicines;
- providing approval to prescribe certain authority-required PBS medicines;
- processing claims for payment for the supply of eligible PBS medicines made under the Remote Area Aboriginal Health Services program;
- processing claims for the issuing of Safety Net cards;
- processing PBS patient refunds;
- facilitating Health’s processes for the approval of PBS suppliers;
- undertaking public compliance functions; and
- supplying official PBS stationery.
Listing medicines on the Schedule
1.9 Before a medicine can be listed on the Schedule, it must first be approved for use in Australia by the Therapeutic Goods Authority (TGA) and included on the Australian Register of Therapeutic Goods. Applications for TGA approval and for listing on the Schedule can occur in parallel.
1.10 A medicine sponsor (generally a pharmaceutical company) initiates the process for listing a medicine on the Schedule by making an application through the Health Products Portal, an online platform run by Health that allows users to track and manage applications for health-related products and services. All applications for listing must be considered by the Pharmaceutical Benefits Advisory Committee (PBAC), an independent expert committee established under the NHA. PBAC is required to consider the effectiveness and cost of the proposed medicine compared with other treatments for the same medical condition. Medicines cannot be listed on the Schedule without a positive recommendation from PBAC to the minister.
1.11 If PBAC makes a positive recommendation on a medicine, Health undertakes a process of negotiation with the sponsor to agree to a pricing structure, and medicine utilisation and costing model. Deeds of agreement concerning risk sharing agreements and special pricing arrangements may also be negotiated as part of the price negotiation process. Once agreed, the submission for listing is submitted for decision to the minister or their delegate or, if the annual outlay by government is anticipated to exceed $20 million, to Cabinet.4 PBAC can review existing PBS listings for cost-effectiveness and medicine utilisation. Formal post-market reviews require agreement from the minister. Medicines can be removed from the Schedule, including at the request of the sponsor. PBAC provides advice on requests for delisting where they would result in the removal of a medicine from the PBS completely.
1.12 All changes to the PBS Schedule are summarised on the PBS website5 and the status of medicines can be monitored as they progress through the PBS listing process on the Medicine Status Website.6
Prescribing and supplying PBS medicines
1.13 PBS medicines can be prescribed by doctors, dentists, optometrists, midwives and nurse practitioners who are authorised to prescribe medicines under the NHA.7 Requirements for PBS prescriptions are set out under the National Health (Pharmaceutical Benefits) Regulations 2017.
1.14 Certain PBS medicines require authority approval from Services Australia to be prescribed. This can apply to a medicine when used for a specific condition, or when approving a medicine for a higher quantity.8 While most authority approvals are processed online or by phone, some PBS authority-required medicines require a written application due to the need for specific evidentiary requirements.
1.15 PBS medicines can be supplied by pharmacists, medical practitioners or hospital authorities who are approved under the NHA.9 An approved pharmacist may only supply PBS medicines at or from premises for which they have been approved. Applications for pharmacists to supply PBS medicines at particular premises are considered by the Australian Community Pharmacy Authority, a statutory body established under the NHA that makes recommendations to the Secretary of Health as to whether a pharmacist should be approved in respect of particular premises.
1.16 Approved pharmacists can claim payment for the Commonwealth price of a PBS medicine, which includes:
- the cost of purchasing the PBS medication for dispensing (the manufacturer’s price plus a wholesale mark-up);
- an administration, handling, and infrastructure fee; and
- dispensing fees.10
1.17 The approved pharmacist may also claim other fees, such as for issuing a Safety Net card. Almost all PBS claims are processed electronically (99.9 per cent), either through the pharmacist’s prescription dispensing software or through Services Australia’s online system for processing claims.
Agreements with the pharmacy and medicines industries
1.18 The Australian Government has agreements with industry peak bodies to support the administration of the PBS.
- The Eighth Community Pharmacy Agreement with the Pharmacy Guild of Australia (July 2024–June 2029) includes the agreed remuneration for pharmacists for services that support the administration of the PBS.11
- The Strategic Agreement on Pharmacist Professional Practice with the Pharmaceutical Society of Australia (July 2024–June 2029) includes joint commitments to support professional pharmacy practice.12
- Strategic agreements with Medicines Australia and the Generic and Biosimilar Medicines Association (September 2021–June 2027) include commitments to reforms intended to safeguard the supply of medicines to Australia (including implementation of a minimum stockholding obligation on Australian medicines manufacturers for medicines most at risk of shortages and delivery of an independent review of health technology assessment methods and policies).13
Rationale for undertaking the audit
1.19 The PBS is intended to ensure that Australians have timely, reliable and affordable access to medicines. In 2024–25 estimated expenditure on the PBS and associated programs represented 2.7 per cent of all Australian Government expenditure. This performance audit was conducted to provide assurance to Parliament that the PBS is being administered effectively.
Audit approach
Audit objective, criteria and scope
1.20 The objective of the audit was to assess the effectiveness of the administration of the PBS.
1.21 To form a conclusion against the audit objective, the following high-level criteria were adopted:
- Has Health established appropriate governance and oversight arrangements for the PBS?
- Has Health established appropriate arrangements to manage the cost of the PBS?
- Have Health and Services Australia established effective arrangements to manage the delivery of PBS services and payments?
1.22 The audit scope included an examination of governance and oversight arrangements for the PBS, the arrangements to manage PBS costs and the delivery of key PBS services and payments. The audit scope did not include Health’s management of programs under Community Pharmacy Agreements or its management of health provider compliance.
Audit methodology
1.23 The audit methodology involved:
- examining documentation held by Health and Services Australia, including bilateral agreements, meeting papers and minutes, policies, procedure and guidance documents, delegation instruments, and internal and external reporting;
- conducting walkthroughs and undertaking targeted testing to assess key PBS processes and systems;
- extracting and analysing PBS administrative data held by Health and Services Australia;
- meetings with Health and Services Australia staff; and
- considering 32 public contributions to the audit received from 21 organisations and six individuals (five contributors provided two contributions).
1.24 Australian Government entities largely give the ANAO electronic access to records by consent, in a form useful for audit purposes. For the purposes of this audit, Health advised the ANAO that it would not voluntarily provide certain information requested by the ANAO due to concerns about its obligations under the Privacy Act 1988, secrecy provisions in Health and Aged Care portfolio legislation, confidentiality provisions in contracts and the Public Interest Disclosure Act 2013. Health advised that this type of information largely was not segregated in Health’s record keeping systems and Health could not be certain, in providing documents through electronic means, that documents containing this type of information were excluded. To provide comfort to the secretary regarding Health’s obligations under portfolio legislation, on 8 August 2023 the Auditor-General issued the secretary of Health with a notice directing the secretary to provide information and produce documents pursuant to section 32 of the Auditor-General Act 1997. Under this notice, Health agreed to provide the information and documents requested through electronic means.
1.25 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $1,102,000.
1.26 The team members for this audit were Magdalena Carrasco, Dr Vivian Turner, Ewan McPherson, Alex Soundias, Dale Todd, Grace Sixsmith, Michael McGillion, Alexandra Collins and Daniel Whyte.
2. Governance and oversight
Areas examined
This chapter examines the appropriateness of the Department of Health and Aged Care’s (Health) governance and oversight arrangements for the Pharmaceutical Benefits Scheme (PBS).
Conclusion
Health’s governance and oversight arrangements for the PBS are partly appropriate. Instruments for delegating statutory powers for administering the PBS have irregularities and anomalies. Health’s PBS Program Management Plan could be improved by including more detail on Health’s management arrangements for the PBS. Health has a largely appropriate bilateral arrangement with Services Australia to oversee its delivery of PBS services and payments. Health’s performance measurement framework for the PBS does not adequately measure and report on program outcomes. Health’s risk management focuses on shared administration risks with Services Australia and has not considered broader strategic risks to the PBS. While mechanisms are in place for stakeholder engagement on the PBS, Health has not conducted an analysis of stakeholder engagement needs or developed an overarching stakeholder engagement plan.
Areas for improvement
The ANAO made four recommendations to ensure Health maintains an appropriate whole-of-program governance framework that incorporates appropriate delegations, performance management, risk management and stakeholder consultation.
The ANAO also identified three opportunities for improvement relating to improving the PBS Program Management Plan developing documentation to guide the operations of the Pharmaceutical Benefits Advisory Committee, and documenting consideration of critical elements from Services Australia’s Bilateral Agreement Framework when negotiating bilateral agreements.
2.1 Where multiple parties administer different aspects of a program, roles and responsibilities should be clear, appropriately documented and understood. To support effective oversight of delivery partners, robust governance arrangements need to be established with clear objectives and effective processes for issue identification and dispute resolution.
2.2 Appropriate governance and oversight arrangements include frameworks for performance monitoring and measurement, risk management and stakeholder engagement. Effective performance measurement and reporting supports effective program management by enabling entities to assess whether programs are achieving their purposes; it also enables the Parliament and the public to assess whether entities are delivering the outcomes for which they are funded. Risk management frameworks should support effective oversight and management of program-level risks and shared risks, in accordance with the requirements of the Public Governance, Performance and Accountability Act 2013 (PGPA Act) and the Commonwealth Risk Management Policy.
Has an appropriate governance framework been established with clearly defined roles and responsibilities?
Instruments that delegate powers and functions for administering the PBS have irregularities and anomalies. While Health has developed a Program Management Plan for the PBS, it does not adequately cover arrangements for managing PBS costs, stakeholder engagement and whole-of-program performance measurement. Health’s support to independent statutory bodies with responsibilities for the PBS could be improved by developing governance documentation for the Pharmaceutical Benefits Advisory Committee.
Legislative basis
2.3 The PBS is governed by a legal framework consisting of the National Health Act 1953 (NHA) and over 100 subordinate legal instruments.14 This legal framework includes a range of decision-making powers and functions for persons and bodies to support the administration of the PBS.
2.4 Table 2.1 outlines the key responsibilities relating to the administration of the PBS which are conferred by the NHA on decision-makers and bodies, including the Governor-General, Minister for Health and Aged Care (minister), heads of Australian Government entities, statutory bodies, peak industry bodies and health providers.
Table 2.1: Key PBS statutory responsibilities under the National Health Act 1953
|
Entitya |
Key PBS statutory responsibilities |
|
Governor-General |
|
|
Minister for Health and Aged Care (minister) |
|
|
Secretary of the Department of Health and Aged Care (secretary of Health) |
|
|
Chief Executive Officer (CEO) of Services Australia (as Chief Executive Medicare)b |
|
|
Pharmaceutical Benefits Advisory Committee (PBAC) and its subcommittees |
|
|
Australian Community Pharmacy Authority (ACPA) |
|
|
Pharmaceutical Benefits Remuneration Tribunal (PBRT) |
|
|
Pharmaceutical Services Federal Committee of Inquiry (PSFCI) |
|
|
Pharmacy Guild of Australia |
|
|
Pharmaceutical Society of Australia (PSA) |
|
|
PBS suppliers (pharmacists, medical practitioners and hospital authorities that have been approved under the NHA) |
|
|
PBS prescribers (medical practitioners, dentists, optometrists, midwives and nurse practitioners authorised under the NHA) |
|
Note a: A Pharmaceutical Services State Committee of Inquiry (PSSCI) may be established by the minister under section 115 of the NHA. There is no PSSCI currently established.
Note b: Powers in the NHA are conferred to the Chief Executive Medicare. Section 4 of the Human Services (Medicare) Act 1973 provides for the CEO of Services Australia to be the Chief Executive Medicare.
Source: ANAO analysis of relevant legislation.
2.5 The minister, the secretary of Health and the CEO of Services Australia have delegated specific powers under the NHA and subordinate legal instruments to specified officers in Health and Services Australia through written delegation instruments. The ANAO found irregularities and anomalies in the instruments of delegation from the minister, the secretary of Health and the CEO of Services Australia. These included instances where:
- sub-delegation has occurred without referencing in the delegation instrument the power that allows for the sub-delegation15;
- sub-delegation has occurred where the power was not delegated;
- delegation has occurred where the section in the NHA vests powers in more than one person, and the delegation has been made of the whole section, which is in excess of the powers vested in that one person under the NHA;
- delegation has occurred where there are express provisions against the delegation of a specific power or function; and
- delegation has occurred where powers or functions have been repealed.
2.6 The principal effect of delegations is to confer on specified individuals or bodies the legal capacity to exercise statutory powers and functions to support government administration. Irregularities and anomalies in delegations may create uncertainty in the exercise of delegated powers. Examples of the irregularities and anomalies identified above are at Appendix 3.
2.7 As the primary department administering the PBS, Health is responsible for establishing governance arrangements to support the management of the PBS program. This includes working with Services Australia to ensure that instruments of delegation are complete, accurate and clear.
Recommendation no.1
2.8 The Department of Health and Aged Care and Services Australia work to review and update relevant delegation instruments to address irregularities and anomalies.
Department of Health and Aged Care response: Agreed.
2.9 The Department notes the ANAO report identified examples of irregularities and anomalies in the relevant instruments of delegation (Appendix 3). The Department will conduct a review of the NHA delegations to address irregularities and anomalies to ensure best practice approach is applied to the associated instruments.
Services Australia response: Agreed.
2.10 Services Australia will work with Department of Health and Aged Care to review and update relevant delegation instruments.
Program management
2.11 Two divisions within Health have key responsibilities for PBS program management:
- Technology Assessment and Access Division — which manages PBS policy, listing and pricing; and
- Benefits Integrity Division — which manages pharmacy approvals and PBS provider compliance activities.16
The First Assistant Secretary of Technology Assessment and Access Division is the senior responsible officer for the PBS and is accountable to the secretary of Health through the Deputy Secretary of the Health Resourcing Group.
2.12 In July 2022, Health contracted KPMG to undertake an internal audit of the PBS Program Agreement, a component of its Bilateral Management Arrangement with Services Australia (discussed at paragraphs 2.25 to 2.29). The report was finalised and the findings were agreed by the First Assistant Secretaries of Technology Assessment and Access Division and Benefits Integrity Division in September 2022. The internal audit was reported to Health’s Audit and Risk Committee in September 2022.
2.13 The internal audit noted that Health did not have strategic program level documentation for the PBS, which prevented the internal audit from being able to assess:
- Alignment of roles and responsibilities articulated within the program agreement [with Services Australia], to those required to administer the PBS from the broader Department’s perspective.
- Completeness and alignment of risks articulated within the shared risk management plan [with Services Australia].
2.14 The internal audit recommended that:
The Department should develop a program overview / similar document that outlines how the PBS is administered across the Department and external stakeholders, which can subsequently be used as a reference point to direct / confirm the Department’s needs for the relationship with Services Australia moving forward. This document should also be incorporated into the Department’s PBS program management activities more broadly.
2.15 Health committed to implement the recommendation by March 2023. Health developed an initial draft of a PBS Program Management Plan in May 2023. The plan was approved by the First Assistant Secretary of Technology Assessment and Access Division on 17 August 2023. Health’s Audit and Risk Committee endorsed the closure of the recommendation at its meeting on 27 September 2023.
2.16 The stated purpose of the PBS Program Management Plan is to ‘[define] the program operating context, governance arrangements, roles and responsibilities of key stakeholders, assurance and reporting, risks and benefits of the program’. Table 2.2 outlines content in the plan for each component of its purpose.
Table 2.2: PBS Program Management Plan content
|
Purpose component |
Description of content |
|
Program operating context |
|
|
Governance arrangements |
|
|
Roles and responsibilities of key stakeholders |
|
|
Assurance and reporting |
|
|
Risks |
|
|
Benefits |
|
Source: ANAO assessment of PBS Program Management Plan.
2.17 The PBS Program Management Plan does not:
- identify or define roles and responsibilities for the full range of persons and bodies with statutory decision-making powers;
- identify the funding appropriation for the PBS or the budget and arrangements for managing PBS cost (such as cost-recovery of applications for listing or risk-sharing arrangements with medicine sponsors);
- identify arrangements for managing stakeholder engagement;
- identify performance measures for the PBS (other than bilateral performance measures with Services Australia); or
- include a schedule for reviewing the plan.
2.18 In addition, Services Australia is identified within the plan as administering approval arrangements for pharmacies. This is inconsistent with the NHA which confers the power to approve PBS suppliers, including pharmacies, to the secretary of Health (refer to Table 2.1).17
Opportunity for improvement
2.19 Health could revise its PBS Program Management Plan to incorporate more detail on aspects of PBS program management for which Health is responsible, including arrangements for managing PBS costs, stakeholder engagement and whole-of-program performance measurement.
2.20 The PBS Program Management Plan identifies three ‘key departmental governance forums’ for the PBS: Health’s executive committee, the Data Strategy Working Group and the External Request Evaluation Committee. All three committees meet on a regular basis and two committees (the executive committee and Data Strategy Working Group) regularly discuss PBS matters consistent with the scope of the committees’ roles (see Table 2.3).
Table 2.3: PBS governance committees identified in Program Management Plan
|
Committee |
Role |
Membership |
Meeting frequency |
|
Executive committee |
Responsible for providing strategic direction and leadership relating to departmental performance, risk planning, financial management, culture and capability |
Chair: Health secretary Members: Deputy secretaries (7) |
Weekly |
|
Data Strategy Working Group |
Responsible for coordinating and refining the framework to manage PBS and Repatriation PBSa data across Health, Services Australia and Department of Veterans’ Affairs |
Chair: Nominated Health Executive Level 2 officer Members: Nominated executive level staff from Health, Services Australia and Department of Veterans’ Affairs |
Every 6 weeks |
|
External Request Evaluation Committeeb |
Responsible for considering external requests to access health data for research, health service planning or other purposes |
Chair: Nominated Services Australia Executive Level 2 officer Members: Nominated Executive Level staff from Health and Services Australia |
Fortnightly |
Note a: The Repatriation PBS provides is administered by the Department of Veterans’ Affairs and provides subsidised medicines to eligible veterans.
Note b: Services Australia advised the ANAO in October 2024 that Health took responsibility for managing External Request Evaluation Committee meetings from May 2024, and Services Australia provides research request discussion items and documentation but no longer attends meetings.
Source: ANAO analysis of internal Health records.
PBS statutory bodies
2.21 As outlined in Table 2.1, there are four statutory bodies established under the NHA that support the administration of the PBS: PBAC, ACPA, PBRT and PSFCI. PBAC has two subcommittees: the Economics Subcommittee (ESC) and the Drug Utilisation Subcommittee (DUSC). An overview of PBS statutory bodies is provided in Table 2.4.
Table 2.4: PBS statutory body overview
|
Statutory body |
Statutory responsibilities |
Membership |
Meeting frequency |
|
Pharmaceutical Benefits Advisory Committee (PBAC) and its sub-committees |
Making recommendations to the minister on medicines it considers should be made available as pharmaceutical benefits |
PBAC can have between 12 and 21 members (including the chair). Members forming at least two-thirds of the total membership of the Committee are to be selected from: industry; consumers; health economists; practising community pharmacists; general practitioners; clinical pharmacologists; and specialists. As at July 2024, PBAC had 20 members including the chair. PBAC has established two subcommittees: ESC and DUSC (see paragraph 2.21). |
Meets 6 times per year Usually holds standard meetings in March, July and November and intracycle meetings in May, September and December |
|
Australian Community Pharmacy Authority (ACPA) |
Considering applications for approval of PBS suppliers under section 90 of the NHA and providing recommendations to the secretary of Health regarding the approval of these applications |
ACPA has 6 members consisting of:
As at April 2024, ACPA had 6 members including the chair. |
Meets 7 to 10 times a year Also considers applications out of session |
|
Pharmaceutical Benefits Remuneration Tribunal (PBRT) |
Determining the fees paid by the Commonwealth to approved pharmacies for supplying PBS medicines |
PBRT consists of:
At least 1 additional member must have been, but is no longer, engaged directly or indirectly in community pharmacy (after consultation with the Pharmacy Guild). As at April 2024, the PBRT had 4 members including the chair. |
Meets once per year |
|
Pharmaceutical Services Federal Committee of Inquiry (PSFCI) |
Inquiring into and reporting to the minister or the secretary of Health on matters referred to it relating to the services or conduct of approved pharmacists in connection with the supply of PBS medicines |
PSFCI consists of the secretary (or their delegate) and 4 pharmacists appointed by the minister. As at April 2024, PSFCI had 4 members. |
Meets monthly Also holds out-of-session meetings |
Source: ANAO analysis of internal Health records.
2.22 Two PBS statutory bodies (ACPA and PSFCI) and the two PBAC subcommittees (ESC and DUSC) had governance documents to guide their operations. Governance documentation for these statutory bodies was in the form of a terms of reference document or member guidelines. In addition to including the legislative requirements for membership and functions, this documentation included information on appointment procedures and remuneration, procedures for conducting meetings and arrangements for managing confidentiality and conflicts of interest.
2.23 PBAC and PBRT did not have governance documents to guide their operations. Health advised the ANAO on 18 April 2024 that the Fair Work Commission, rather than Health, provides secretariat support to the PBRT.18 Meeting records show that the PBRT meets for around 15 minutes once a year and therefore requires minimal support to assist with its operations.
Opportunity for improvement
2.24 To support effective governance and administration, Health could develop governance documentation for PBAC (such as terms of reference or member guidelines) that outlines the legislative requirements for membership and functions. This could include information on appointment procedures and remuneration, procedures for conducting meetings and voting, and arrangements for managing confidentiality and conflicts of interest.
Has Health established an appropriate bilateral arrangement to oversee Services Australia’s delivery of PBS services and payments?
Health and Services Australia have established a Bilateral Management Arrangement, which includes bilateral agreements and bilateral governance arrangements that relate to the delivery of PBS services and payments.
- PBS-related program agreements were fit for purpose, with clear objectives and defined roles and responsibilities. All protocols supporting the bilateral arrangement were reviewed and updated between November 2023 and September 2024.
- While bilateral governance meetings have not occurred at the most senior levels, there has been regular engagement between the two entities at lower levels. Governance committees relevant to the PBS began considering risk, performance reporting, and updates to bilateral agreements in late 2023.
Bilateral agreements with Services Australia
2.25 Health and Services Australia have an ‘appropriated partnership’ bilateral arrangement. Services Australia is accountable for delivering PBS services and prioritising service delivery within its funding budget (appropriation), while Health retains policy responsibility for PBS services.19
2.26 To support bilateral engagement Health and Services Australia have established a Bilateral Management Arrangement (BMA) for the delivery of health and aged care programs, which comprises:
- a Statement of Intent between the Health secretary and Services Australia CEO (October 2022), which outlines strategic principles and governance arrangements for the BMA and covers all health and aged care programs delivered by Services Australia;
- six protocols covering communication and media (May 2024), compliance (September 2024), corporate services (June 2024), data exchange (August 2024), new and changed work (July 2024) and performance management (May 2024); and
- program agreements documenting specific bilateral activities that have been agreed between Health and Services Australia.
2.27 Health and Services Australia have two program agreements related to PBS services and payments:
- PBS Program Agreement (August 2023), which covers Services Australia’s assessment, processing and payment of PBS claims to PBS suppliers; and
- Approval of PBS Suppliers Program Agreement (February 2024), which relates to the systems access and data Services Australia provides to support Health’s assessment and approval of PBS suppliers.
2.28 Services Australia has a Bilateral Agreement Framework guidance document (updated in January 2024) that outlines 12 ‘critical elements’ to consider, address and document in all new bilateral agreements (including protocols and program agreements).20 Table 2.5 provides an assessment of the six BMA protocols and two PBS-related program agreements against these 12 elements, as well as an assessment of whether agreements were up to date in accordance with agreement terms and review provisions.
Table 2.5: Health and Services Australia bilateral agreement elements, as of June 2024
|
Elements |
Protocols |
Program agreements |
||||||
|
|
Communication and media |
Compliance |
Corporate services |
Data exchange |
New and changed work |
Performance management |
PBS |
Approval of PBS Suppliers |
|
Clear objective |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
|
Defined roles and responsibilities of each party |
◆ |
◆ |
▲ |
◆ |
◆ |
◆ |
◆ |
◆ |
|
Suitable governance arrangements |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
|
Performance measures |
■ |
■ |
■ |
■ |
■ |
■ |
◆ |
◆ |
|
Reporting and communication arrangements |
◆ |
◆ |
▲ |
◆ |
■ |
■ |
◆ |
◆ |
|
Statements regarding risk management |
■ |
◆ |
▲ |
◆ |
◆ |
■ |
◆ |
◆ |
|
Issues escalation and dispute resolution processes |
▲ |
◆ |
◆ |
◆ |
▲ |
▲ |
◆ |
◆ |
|
Funding arrangements |
■ |
◆ |
◆ |
▲ |
■ |
◆ |
◆ |
◆ |
|
Appropriate approval/sign-off |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
|
Term of agreement |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
◆ |
◆ |
|
Review points and provisions |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
◆ |
|
Expert advice and stakeholder contributions |
■ |
■ |
■ |
■ |
■ |
■ |
■ |
■ |
|
Up to date (in accordance with term and review provisions)? |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
Key: ◆ Met ▲ Partly met ■ Not met ✔ Yes ✘ No N/A Not applicable
Source: ANAO analysis.
2.29 The PBS Program Agreement and Approval of PBS Suppliers Program Agreement were up to date and met all critical elements in place at the time of their development.21 All six BMA protocols were reviewed and updated between November 2023 and September 2024. All protocols lacked documented consideration of one or more of the 12 critical elements of Services Australia’s Bilateral Agreement Framework.
Opportunity for improvement
2.30 When Health and Services Australia develop updated bilateral protocols and program agreements, there is an opportunity to document the rationale for not including ‘critical elements’ from Services Australia’s Bilateral Agreement Framework.
Bilateral governance arrangements
2.31 The 2022 Statement of Intent outlines a governance structure for the BMA involving bilateral meetings at the accountable authority and Senior Executive Service (SES) Band 3 levels, and bilateral governance committee meetings at lower levels (see Figure 2.1). The Statement of Intent states that:
- the accountable authority and SES Band 3 level meetings will occur ‘as required to monitor progress in relation to the delivery of bilateral arrangements’;
- Strategic Business Committee (SBC) is the ‘highest governance committee’ and is responsible for setting the strategic direction of jointly managed work, ensuring early and regular collaboration on new programs, providing oversight of bilateral health and aged care programs, and monitoring program and payment performance; and
- program manager meetings are ‘regular formal meetings … held to provide operational oversight of bilateral arrangements’.
Figure 2.1: BMA governance structure
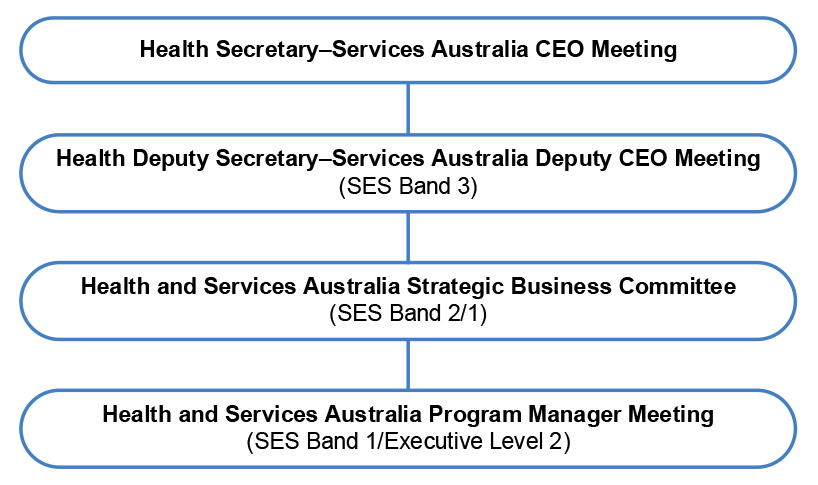
Source: Health & Services Australia, Statement of Intent, October 2022.
2.32 No secretary–CEO and deputy secretary–deputy CEO meetings were held in 2021–22, 2022–23 or 2023–24 under the formal governance structure of the BMA.
2.33 SBC met four to five times a year in 2021–22, 2022–23 and 2023–24. Analysis of meeting records indicates SBC meetings had an increased focus on program risks and performance reporting from late 2023 (after the commencement of this audit).
- In 2021–22 and 2022–23 SBC meetings functioned as a forum for Health and Services Australia to provide updates on priorities and initiatives. There was also discussion of the 2022 Statement of Intent review and preparation of the 2022–23 Annual Assurance Statement (discussed at paragraph 2.35).
- In 2023–24 SBC meetings became increasingly focused on reviewing bilateral agreements, performance reporting and discussion of key risks and issues. SBC developed a forward work program that indicated these matters would continue to be a focus.
2.34 In September 2023 Health and Services Australia established a PBS Committee at the SES Band 2 and Band 1 level, which meets quarterly to ‘provide governance and assurance mechanisms’ to support the delivery of the PBS.
Annual assurance statement
2.35 The 2022 Statement of Intent states that Services Australia will work collaboratively with Health to develop an annual assurance statement ‘providing assurance that health policies, developed by Health and delivered in partnership with Services Australia, meet the expectations of Government’. This process was first implemented for the 2021–22 financial year.
- The 2021–22, 2022–23 and 2023–24 annual assurance statements followed a consistent format which included a ‘high-level review’ of program agreements against a program assurance matrix (covering risk planning, performance measures, reporting and information exchange, and reviews). The statements included (as attachments) end-of-financial year assurance letters from Services Australia’s Chief Financial Officer to Health’s Chief Financial Officer, which stated that appropriate controls were in place and operating effectively, including for the PBS program.
- The 2023–24 Annual Assurance Statement included new sections on shared risk management, audit findings and recommendations, issues management and reporting against selected bilateral key performance measures (which included reporting for four key performance measures relating to the PBS).
Has an appropriate performance measurement framework been established?
Health has one external performance measure for the PBS, which is not outcome focused and does not provide meaningful performance information to the Parliament or the public. Health receives monthly reporting from Services Australia on bilateral performance measures. It has not used this data to oversee Services Australia’s service delivery. Health does not provide any regular performance reporting on the PBS to the minister or its executive committee.
Departmental performance reporting
External performance reporting
2.36 The PBS forms part of Program 2.3 (Pharmaceutical Benefits) in Health’s corporate plan, which has an objective to:
Provide all eligible Australians with reliable, timely, and affordable access to high-quality, clinically effective, cost-effective medicines, and pharmaceutical services by subsidising the cost of medicines through the [PBS] and the Life Saving Drugs Program (LSDP).22
2.37 In its 2023–24 corporate plan, Health identified two key activities under Program 2.3, one for the PBS and one the LSDP, each of which had an associated performance measure. The key activity, performance measure and planned performance results for the PBS were:
Key Activity: Provide all eligible Australians with reliable, timely, and affordable access to high-quality, clinically effective, cost-effective medicines recommended by [PBAC], by listing of new medicines on the [PBS].
Performance Measure: Percentage of new medicines recommended by [PBAC] that are listed on the [PBS] within 6 months of in principle agreement to listing arrangements.
Planned Performance Results (2023–24 to 2026–27): ≥80%23
2.38 Health has reported against this performance measure for the PBS in its annual performance statements since 2015–16. As shown in Table 2.6, Health reported that it exceeded its performance target for this measure every year since the measure was introduced.
Table 2.6: Percentage of new medicines recommended by PBAC listed on the PBS within 6 months of in principle agreement, 2015–16 to 2022–23
|
|
2015–16 |
2016–17 |
2017–18 |
2018–19 |
2019–20 |
2020–21 |
2021–22 |
2022–23 |
|
Target |
80% |
80% |
80% |
80% |
80% |
80% |
80% |
80% |
|
Result |
92% |
85% |
88% |
100% |
100% |
100% |
100% |
100% |
|
Target met? |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
Key: ✔ Yes ✘ No
Source: Department of Health, Department of Health Annual Report 2018–19, Commonwealth of Australia, Canberra, 2019, p. 85; and Department of Health and Aged Care, Department of Health and Aged Care Annual Report 2022–23, Commonwealth of Australia, Canberra, 2023, p. 72.
2.39 Health’s rationale for using this performance measure in 2022–23 was:
The measure reports the percentage of PBAC recommendations for which negotiations with product sponsors and activities for listing on the PBS are completed in a timely manner.
The 6-month timeframe provides sufficient time to discuss and agree complex pricing and budget impact issues, seek agreement to listing arrangements from other Government agencies (including all costs agreed with the Department of Finance), seek Government approval and finalise and distribute the amended PBS schedule. 6 months has been the metric for several years.
The Department uses this metric because sponsors must provide listing proposals that align with the PBAC’s recommendations on cost-effectiveness and financial implications among other matters before a listing can be finalised by Government.
2.40 In its 2023–24 Portfolio Budget Statements (published in May 2023), Health included eight key activities for the PBS under Program 2.3. When it published its 2023–24 corporate plan three months later (in August 2023), Health:
- retained one of the eight activities from the 2023–24 Portfolio Budget Statements as a key activity in its 2023–24 corporate plan (reproduced at paragraph 2.37 above);
- reclassified six of the eight activities as ‘additional activities [that] fall below [Health’s] materiality threshold for publishing and reporting against a program performance measure’24; and
- removed one activity relating to implementing PBAC’s recommendation to increase the maximum dispensing quantities of certain medicines to allow for 60-day dispensing.
In its 2024–25 Portfolio Budget Statements (published in May 2024), Health included one activity under Program 2.3 (the key activity reproduced at paragraph 2.37 above).
2.41 The ANAO’s audit of Health’s 2022–23 performance statements raised a significant finding relating to the completeness of Health’s performance measures.25 In particular, the audit found that a number of performance measures, including that for the PBS, did not appear to represent significant components of the related program reported on and that Health was unable to articulate the rationale for the selection of key activities, measures and targets. At the conclusion of the 2022–23 performance statements audit, Health accepted this finding and committed to improve its documentation of how performance measures and key activities are material representations of performance.
2.42 With respect to the PBS, Health’s single external performance measure does not address the three key components of the PBS objective. Health’s external performance measure for the PBS does not enable performance against the program objective to be accurately measured and assessed. It provides information only on the ‘timely’ component of the program objective, but not the ‘reliable’ and ‘affordable’ components. It does not adequately cover the range of PBS activities for which Health is responsible and does not provide the Parliament and the public with meaningful information on the performance of Health in administering this $19.5 billion program.
2.43 The ANAO’s 2015 performance audit of the Fifth Community Pharmacy Agreement (5CPA) found Health’s performance measures relating to the 5CPA, and the PBS more broadly, were not related to health outcomes, only addressed selected aspects of program objectives, and had been amended substantially. The audit recommended that Health review its performance reporting to improve alignment between the next Community Pharmacy Agreement and its performance measures and program objectives.26
2.44 In 2016 the ANAO conducted a follow-on audit to the 2015 5CPA audit. The follow-on audit assessed the recommendation relating to Health’s performance measures as implemented. The audit report noted that Health had conducted an internal staff workshop in September 2015 to develop an improved set of performance measures for the PBS that focused on: access to pharmacy and medicines; cost-effectiveness of PBS medicines and services; sustainability of PBS; and access to information for decision-making.27 The revised performance measures were first reported on in Health’s 2015–16 annual performance statements. Table 2.7 shows that most of these measures were removed from Health’s annual performance statements between 2017–18 and 2021–22.
Table 2.7: Quantitative performance measures for the PBS, 2015–16 to 2022–23
|
Performance measure descriptiona |
In annual performance statements? |
|||||||
|
|
2015–16 |
2016–17 |
2017–18 |
2018–19 |
2019–20 |
2020–21 |
2021–22 |
2022–23 |
|
Percentage of new medicines recommended by PBAC listed within 6 months of agreement of budget impact and price |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Percentage of new medicine listing submissions considered by PBAC within 17 weeks of lodgement |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
Percentage of Urban Centres in Australia (with ≥1,000 population) with approved PBS supplier |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✘ |
✘ |
|
Percentage of Urban Centres in Australia (with ≥1,000 population) with resident service provider or recipient of Medscheck, Home Medicines Review, Residential Medication Management Review or Clinical Intervention |
✔ |
✔ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
|
Percentage of subsidised PBS units delivered to community pharmacies within agreed timeliness requirements of the Community Service Obligation |
✔ |
✔ |
✔ |
✔ |
✘ |
✔ |
✘ |
✘ |
|
Average cost per subsidised script funded by the PBS |
✔ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
Average cost per subsidised script paid by consumers for subsidised medicines |
✔ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
Percentage of post-market reviews completed within scheduled timeframes |
✔ |
✔ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
|
Percentage of Government-accepted recommendations from post-market reviews implemented within 6 months |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
✘ |
✘ |
|
Estimated savings to Government from price disclosure |
✔ |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
|
Percentage of eligible medicines assessed in accordance with PBS price disclosure requirements |
✘ |
✘ |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
Key: ✔ Yes ✘ No
Note a: Minor changes were made to performance measure descriptions over the analysis period.
Source: ANAO analysis of Health’s annual reports 2015–16 to 2022–23.
2.45 After removing most of the measures in Table 2.7 from its annual performance statements, Health has not continued to monitor and report on performance against these measures in internal or external reporting. Health does not undertake any internal performance reporting for the PBS to inform its management of the program and drive business improvement. Aside from the single PBS-related performance measure that has continued to be included in Health’s annual performance statements, no additional performance reporting on the PBS has been provided to the Minister for Health and Aged Care, Health secretary or Health’s executive committee. This means that key decision-makers for the PBS have not had visibility of program performance and outcomes. It also increases the risk that program evaluation will not be based on reliable and verifiable data.
Recommendation no.2
2.46 The Department of Health and Aged Care establish and report against a performance management framework for the Pharmaceutical Benefits Scheme that:
- includes an appropriate mix of output, efficiency and effectiveness performance measures for key program activities, including those of third-party delivery partners; and
- enables the department’s performance in administering the Pharmaceutical Benefits Scheme purposes to be measured and assessed.
Department of Health and Aged Care response: Agreed.
2.47 The Department of Health and Aged Care is currently remediating findings in the ANAO 2022–23 annual performance statements audit. This includes developing an appropriate mix of output, efficiency and effectiveness performance measures per the Commonwealth Performance Framework.
2.48 The Department will work to develop appropriate performance measures for the Pharmaceutical Benefits Scheme (under Program 2.3) that are consistent with the Department’s Performance Reporting Materiality Policy and that will dearly articulate and present the linkages between the purpose of the program, key activities and performance measures, including those of third-party delivery partners.
Third party performance measurement
2.49 Health’s Performance Measurement and Reporting Framework (January 2021) states:
When multiple stakeholders contribute to the outcomes, objectives and performance of our programs, when designing performance information, program owners should also consider:
- how it holds third parties to account in delivering programs (e.g. through key performance indicators and milestones in funding agreements); and
- how it reports on program performance when it is not responsible for the delivery [of] program outputs, including specifying what assurance frameworks are in place.
Bilateral performance measures with Services Australia
2.50 The PBS Program Agreement (August 2023) between Health and Services Australia includes 10 bilateral performance measures (see Table 2.8). Services Australia has provided Health with a monthly dashboard report since 2022 outlining performance against nine of the 10 performance measures. The program agreement states that the PBS 10 performance measure (accuracy of PBS claim processing) is reported annually, but there has been no bilateral reporting against this performance measure.
Table 2.8: PBS Program Agreement performance measures
|
No. |
Name |
Description |
Frequency |
Target |
Resultsa |
|
PBS 1 |
PBS authorities telephony |
Average speed of answer for calls to PBS Authorities telephony line |
Monthly |
≤ 30 secs |
0/23 |
|
PBS 2 |
PBS online claims |
Percentage of online claims processed within 9 days |
Monthly |
≥ 98% |
23/23 |
|
PBS 3 |
PBS manual pharmacy claims |
Percentage of manual claims processed within 30 days |
Monthly |
≥ 82% |
17/23 |
|
PBS 4 |
PBS Safety Net claims |
Percentage of manual Safety Net card claims processed within 60 days |
Monthly |
≥ 82% |
14/23 |
|
PBS 5 |
Patient refunds |
Percentage of manual patient refunds processed within 60 days |
Monthly |
≥ 82% |
8/23 |
|
PBS 6 |
Authority approval requests |
Percentage of written requests processed within 5 working days (uploaded electronically)b or 10 working days (complex) |
Monthly |
≥ 82% |
10/23 |
|
PBS 7 |
Remote Area Aboriginal Health Services |
Percentage of claims processed within 30 days |
Monthly |
≥ 82% |
23/23 |
|
PBS 8 |
Paraplegic and Quadriplegic Program |
Percentage of claims processed within 7 daysc |
Monthly |
≥ 82% |
21/23 |
|
PBS 9 |
Stoma Appliance Schemed |
Percentage of claims processed within 7 daysc |
Monthly |
≥ 82% |
21/23 |
|
PBS 10 |
PBS claims payment quality standard |
Percentage of claims processed accurately |
Annually |
≥ 98% and no adverse findings from ANAOe |
Not reported |
Note a: Number of months target was met over the 23-month period from August 2022 to June 2024.
Note b: Prior to August 2023 the agreed timeframe for requests uploaded electronically was 3 working days.
Note c: Prior to August 2023 agreed timeframes ranged between 7 to 30 calendar days depending on the claim type.
Note d: The Stoma Appliance Scheme provides free stoma appliances and products to people who have a stoma (ostomates). It is not part of the PBS Schedule but is included as a performance measure in the PBS Program Agreement.
Note e: The ANAO undertakes an annual audit of Health’s financial statements, which includes testing of Health’s management of the PBS claims process. The effectiveness of systems and processes for managing PBS claims is discussed further at paragraphs 4.3 to 4.31 (Chapter 4).
Source: Health & Services Australia, PBS Program Agreement, August 2023, pp. 9–11; and Services Australia, monthly dashboard reports for PBS Program Agreement, August 2022–June 2024. ANAO analysis of Services Australia internal documents.
2.51 Services Australia’s reported performance against the nine monthly-reported performance measures under bilateral arrangements between August 2022 and June 2024 (see Table 2.8 and Appendix 4).
- Services Australia reported that it met its targets every month for two performance measures (PBS 2 and PBS 7). More than 99.9 per cent of PBS claims are online claims (covered by PBS 2), so almost all PBS claims were processed within benchmark timeframes.
- For six performance measures (PBS 3, PBS 4, PBS 5, PBS 6, PBS 8 and PBS 9) targets were reported as not met in some months. In its dashboard reporting to Health, Services Australia attributed its failure to meet performance measure targets for these measures to staff shortages, claim backlogs and needing to focus on other priorities.28
- For one performance measure (PBS 1), the target was reported as not met in all months. Services Australia attributed its failure to meet this performance measure target to staff resourcing and needing to focus on a broad range of payment and claim priorities.
2.52 Health has not used this monthly performance measure dashboard reporting to hold Services Australia to account for its PBS service delivery performance. In February 2024 the newly formed PBS Committee discussed performance measure performance, after being presented a quarterly dashboard report, with no decisions or actions recorded for this agenda item. The PBS Committee did not consider performance measure performance at its meeting in May 2024.
2.53 The Approval of PBS Suppliers Program Agreement (February 2024) between Health and Services Australia contains one performance measure: Services Australia to issue an RSA secure identification token29 within 10 business days of a request from Health. Services Australia has not provided reporting to Health on this measure.
Other third-party performance measures
2.54 Health has established performance reporting arrangements with other third parties (described in Box 1). Performance measures for these third-party reporting arrangements are largely output focused.
|
Box 1: Performance reporting for third-party performance measures |
|
Community Pharmacy Agreement progress report In 2022 Health worked with peak bodies to develop key performance measures for the Seventh Community Pharmacy Agreement (7CPA), with the aim of providing an evaluation framework for the 7CPA. Health’s Post-Implementation Review of the 7CPA noted that:
Health has published four progress reports against the 7CPA key performance measures since 2022. The measures and reporting are focused on presenting output and expenditure data to promote transparency and accountability, rather than on assessing the outcomes of the 7CPA. Health commenced a review of the 7CPA KPMs in 2023, but the review was not completed and thus did not inform the development of the Eighth Community Pharmacy Agreement. Strategic Agreement with Medicines Australia The Government’s 2021 Strategic Agreement with Medicines Australia included three key performance indicators:
The agreement also included a commitment to develop more key performance indicators during the term of the agreement (1 July 2022 to 30 June 2027). As of June 2024, Health was working with Medicines Australia to develop the additional key performance indicators and no performance reporting had occurred. Pharmaceutical Benefits Advisory Committee operations report Since 2009–10 Health’s annual report has included a PBAC report on the operations of the committee that has presented data on meeting outputs. The report does not include any performance targets or analysis. |
Note a: The NHA provides that listed medicines be assigned to formularies identified as F1 or F2. Generally, F1 is for single brand medicines and F2 for medicines with multiple brands or in a therapeutic group with other multiple brand medicines.
Have appropriate arrangements been established for managing risks?
Health has not undertaken appropriate risk assessments or developed appropriate risk management plans for the PBS at the divisional or program level. Its risk assessments and plans do not adequately cover key program activities for which Health is responsible. Health’s shared risk management plan with Services Australia covers risks relating to the services and payments Services Australia delivers for the PBS. From late 2023, bilateral governance bodies began discussing operational risks relevant to the PBS.
Departmental risk management arrangements
2.55 Health’s Risk Management Policy (April 2023) outlines its approach to risk management, enterprise risks, risk appetite and risk tolerance. Under the policy, departmental divisions are required to prepare business and risk plans annually and maintain risk registers for all programs.
Divisional risk management
2.56 As noted at paragraph 2.11, two divisions within the Health Resourcing Group have key responsibilities for PBS administration: Technology Assessment and Access Division (which manages PBS policy, listing, pricing and pharmacy approvals) and Benefits Integrity Division (which manages PBS provider compliance activities).
2.57 Technology Assessment and Access Division’s 2023–24 business and risk plan identified three risks relevant to the PBS:
- inability to attract and retain staff with health technology assessment skills and capabilities to support advisory committees (such as PBAC);
- balancing the delivering of ‘critical [business as usual] work’ (such as monthly PBS listings) with government priorities and timeframes; and
- the ANAO’s performance audit of PBS administration.
2.58 Benefits Integrity Division’s 2023–24 business and risk plan identified general risks to its health provider compliance program30, all of which are relevant to the PBS, but did not identify any risks specific to the PBS.
Program-level risk management
2.59 The 2022 internal audit of the PBS Program Agreement with Services Australia (see paragraph 2.12) identified that Health did not have a ‘program risk plan [for the PBS] that considers the Department’s program level risks’. The internal audit recommended that Health:
develop a risk assessment and plan that outlines the risks and mitigating controls for the holistic administration of the PBS. This risk assessment can then be used to confirm the completeness and appropriateness of the shared risk management plan used to manage the relationship with Services Australia.
2.60 In response to the recommendation, Health agreed to develop a PBS ‘program risk register’ by March 2023. Health developed a PBS Program Risk Assessment Plan in August 2023.
2.61 The plan replicated shared risks outlined in the bilateral risk management plan with Services Australia (discussed at paragraphs 2.66 and 2.67 below). It did not cover PBS program activities for which Health is responsible, such as developing new PBS policies, making legislative changes, assessing, listing and managing pricing for new and existing PBS medicines, approving PBS suppliers, or negotiating relevant agreements with peak industry bodies.
2.62 Case study 1 provides an example of stakeholder engagement risks that emerged in relation to a PBS policy change to allow 60-day prescriptions, introduced from September 2023 to reduce out-of-pocket costs for patients with stable ongoing health conditions.31
|
Case study 1. Stakeholder engagement risks from PBS 60-day prescription change |
|
In August 2018 PBAC endorsed a proposal from Health to allow certain medicines to be prescribed for 60-day supply. The government did not proceed with the change at that time. In December 2022 Health asked PBAC to reconsider the list of medications it originally found suitable for 60-day prescriptions. In April 2023 the Minister for Health and Aged Care announced it would be ‘allowing millions of Australians to buy two months’ worth of medicine for the price of a single prescription’ from 1 September 2023 with the aim of ‘easing cost of living pressures’. Health advised the government there would be strong opposition to the 60-day prescription measure from the Pharmacy Guild and pharmacy owners and recommended a well-resourced communications campaign to mitigate the risk. Shortly after the minister’s April 2023 announcement, the Pharmacy Guild commenced a campaign against the measure. In June 2023, in developing the legislative instrument for the change, Health identified that the disallowance period for the instrument would span until after the 1 September 2023 implementation date. In August 2023 a disallowance motion was introduced in the Senate which was defeated. While the disallowance risk was not realised, Health and Services Australia undertook contingency planning to prepare for the possibility that administrative changes scheduled to take effect from 1 September 2023 would need to be reversed. In September 2023 the Pharmacy Guild announced that it had ‘paused’ its campaign against the 60-day prescription measure after ‘securing an agreement from the Albanese Government to immediately enter negotiations for an 8th Community Pharmacy Agreement’.32 |
2.63 In November 2023 the Health Resourcing Group identified the integrity of payments as a ‘top five’ risk in reporting to Health’s Audit and Risk Committee, noting:
- Fraudulent and serious non-compliant Medicare Benefits Schedule (MBS) claims are known to be submitted and paid, thereby compromising the integrity of Medicare …
- While Services Australia manages the administration and claiming process, [Health] is accountable for the policy, business rules, Constitutional risk and legislative instruments for the MBS (and the PBS).
- These issues are likely to extend to the Pharmaceutical Benefits Scheme (PBS) – thus initiatives and activities arising from the MBS Integrity Taskforce should be considered for reforms across PBS.
In addition to the gaps outlined at paragraph 2.61, Health’s August 2023 PBS Program Risk Assessment Plan did not include PBS compliance risks.
Recommendation no.3
2.64 The Department of Health and Aged Care undertake a risk assessment for the Pharmaceutical Benefits Scheme program that covers activities for which the department is responsible.
Department of Health and Aged Care response: Agreed.
2.65 The Department will undertake a risk assessment specific to the Pharmaceutical Benefits Scheme to supplement the organisational risk assessments currently undertaken. This will be guided by the Department’s Risk Management Policy and Framework, including development of a targeted risk register and processes for monitoring and reviewing these on an ongoing basis.
Bilateral management of shared risks with Services Australia
2.66 Health and Services Australia have a bilateral PBS shared risk management plan (last updated October 2023) for the PBS Program Agreement, which identifies five shared risks:
1. Services Australia and Health cannot deliver the PBS effectively;
2. ICT systems do not support PBS delivery;
3. approved prescribers and suppliers do not prescribe and supply PBS items correctly;
4. PBS data is unavailable or insufficient; and
5. breaches occur (including data and financial).
2.67 The plan includes a detailed assessment of risk causes, consequences and related controls, including details of completed and planned controls testing. Controls were assessed in the plan as ‘fully effective’ for risks one, two and four, and ‘partially effective’ for risks three and five. All five risks had a risk tolerance level of ‘very high’, ‘high’ or ‘medium’, and the risk owners decided to accept the risks after control assessment resulted in the residual risks being rated as ‘low’ (see Appendix 5 for more detail on the bilateral shared risk assessment). For risk five (breaches occur), despite controls having been assessed as ‘partially effective’, the inherent risk rating of ‘very high’ was reduced through the application of controls to a residual risk rating of ‘low’.
2.68 Health and Services Australia committed in the Approval of PBS Suppliers Program Agreement to developing a shared risk management plan for the agreement within six months of signing the November 2022 agreement. A shared risk management plan for this agreement was endorsed in October 2023. The same commitment was included in the updated February 2024 Approval of PBS Suppliers Program Agreement, with a revised shared risk management plan yet to be developed.
2.69 As discussed at paragraph 2.33, bilateral governance meetings in 2021–22 and 2022–23 were focused on providing updates on priorities and initiatives. There was no agenda item for discussion of PBS risks, although issues related to the PBS were noted in some meetings. Since the establishment of the bilateral PBS Committee in September 2023 there has been evidence of bilateral engagement on shared PBS risks. For example, discussion has covered integrity risks relating to PBS Safety Net signature requirements and legislative barriers to efficient and effective program delivery.
Have appropriate arrangements been established for stakeholder engagement?
Health’s arrangements for stakeholder engagement for the PBS include the provision of information through websites, invitation of written submissions from stakeholders on specific PBS issues, agreement-making with industry bodies, and hosting regular stakeholder engagement forums. These arrangements have not been informed by a systematic analysis of stakeholder engagement needs or an overarching stakeholder engagement plan or strategy.
Stakeholder engagement planning
2.70 Health has published a Stakeholder Engagement Framework that provides key principles to guide stakeholder engagement activities so that they are purposeful, inclusive, timely, transparent and respectful. It also outlines a five-step process for engagement that includes steps for identifying stakeholders, analysing needs and setting objectives (see Table 2.9).
Table 2.9: Five-step stakeholder engagement process
|
Step |
Description |
|
Think |
We develop an overall consideration of strategic business objectives, how these relate to stakeholders and specific issues, and how you can undertake an initial prioritisation of stakeholders and issues for further analysis. |
|
Plan |
Introduce different levels of engagement, and guide the analysis of existing relationships, available resources and organisational constraints. It also helps you to learn more about specific stakeholder’s representatives, and to decide on what kind of relationship you want to develop with these stakeholders. |
|
Prepare |
Address questions of internal and external competencies and capacities to engage, and how you can ensure that all parties to an engagement are able to join and take part in it effectively. |
|
Engage |
Address and outline different engagement techniques, and — building on the previous steps — design an approach that suits the needs of your specific situation and help you to reach your objectives. |
|
Evaluate |
Follow-up on the outputs of engagement and ensure that your stakeholders feel assured regarding the quality of your efforts. |
Source: Department of Health, Stakeholder Engagement Framework, Commonwealth of Australia, Canberra, 2018, page 4, available from https://www.health.gov.au/resources/publications/stakeholder-engagement-framework [accessed 7 June 2024].
2.71 The Program Management Plan developed by Health in 2023 (see paragraph 2.14) includes a section on ‘Stakeholder and Communications Management’ (contained in a section on ‘Assurance and Reporting’) which states that the PBS ‘has well established communication and stakeholder engagement channels.’ The plan’s purpose includes defining ‘roles and responsibilities of key stakeholders’. The plan identifies some stakeholder groups in its program logic diagram, but it does not include health practitioners or patients as stakeholders.
2.72 Health has not developed an overall plan or strategy for stakeholder engagement for the delivery of the PBS. Health has prepared communication materials and employed strategies for engagement with stakeholders on specific PBS issues.
- For example, Health has developed an online ‘Office of Health Technology Assessment (OHTA) consultation hub’ with a dedicated section for consultations by PBAC.33
- Health has had targeted consultations with stakeholders, including meetings, written correspondence and webinars, on issues such as changes to the supply of medicines to treat opioid dependence, changes to maximum quantities for medicine dispensing and the 8CPA negotiations.
Stakeholder engagement arrangements
Website content
2.73 Health has developed a website to provide information to stakeholders on the PBS, such as:
- the process for listing medicines on the Schedule;
- the Schedule document;
- the role and procedures of PBAC and its subcommittees;
- the outcomes of PBAC considerations;
- guidance on the requirements for prescribing and supplying PBS medicines; and
- PBS statistics.34
The website content is aimed at health providers and members of the pharmaceutical industry.
2.74 The website attracted an average of 1,104 users and 6,994 page views each month from July 2021 to April 2024. The website provides users the option to subscribe to receive notifications of news and updates, with 6,927 people subscribed to the main email list as at 25 June 2024.
2.75 Services Australia has information on its website about PBS services and payments that it delivers, which is contained in webpages for specific audiences (for example, health professionals and patients).35 Services Australia also publishes reports on its website containing statistics on claims for PBS medicines paid by Services Australia.36
Stakeholder agreements
2.76 As noted in paragraph 1.18, the Commonwealth has entered into four written agreements with industry stakeholders. The agreements are not binding but include commitments that set medium-term policy reforms for the PBS that have a significant influence on the cost of the PBS, such as pharmacy remuneration, and reforms relating to the price of medicines (see Table 2.10).
Table 2.10: Agreements with PBS industry stakeholders
|
Agreement |
Term |
Key commitments |
|
Strategic Agreement with Medicines Australia |
6 September 2021 to 30 June 2027 |
|
|
Strategic Agreement with Generic and Biosimilar Medicines Association (GBMA) |
6 September 2021 to 30 June 2027 |
|
|
Strategic Agreement on Pharmacist Professional Practice with the PSA |
1 July 2024 to 30 June 2029 |
|
|
8CPA |
1 July 2024 to 30 June 2029 |
|
Source: ANAO analysis of stakeholder agreements.
Stakeholder forums
2.77 The stakeholder agreements referred to in Table 2.10 include commitments for Health to establish forums for oversight and monitoring the progress of commitments (see Table 2.11).
Table 2.11: Health stakeholder forums
|
Forum |
Membership |
Role |
Meeting frequency |
|
Joint Oversight Committee (Medicines Australia) |
Senior executives from Health and Medicines Australia. |
To monitor the implementation of the Medicines Australia Strategic Agreement. |
Once a year. |
|
Access to Medicines Working Group |
Senior executives from Health and Medicines Australia. |
To facilitate engagement between parties on issues relating to the Medicines Australia Strategic Agreement. |
Twice a year. |
|
Metrics and KPI Subgroup |
Senior executives from Health and Medicines Australia. |
To design and monitor metrics and KPIs for the successful implementation of the Medicines Australia Strategic Agreement. |
At least twice a year. First met in April 2024. |
|
GBMA Joint Oversight Committee |
Senior executives from Health and GBMA. |
To monitor the implementation of the GBMA Strategic Agreement. |
Twice a year. |
|
Biosimilar Working Group |
Senior executives from Health and GBMA. |
To facilitate engagement between parties on issues relating to the GBMA Strategic Agreement. |
The group has met 6 times since August 2023. |
|
Community Pharmacy Consultation Committee (CPCC) |
Senior executives from Health and the Pharmacy Guild. |
To monitor the implementation of the Seventh Community Pharmacy Agreement (7CPA). |
Twice a year. |
|
Pharmacy Stakeholder Consultation Committee (PSCC) |
Senior executives from Health, Pharmacy Guild, PSA, Consumers Health Forum and the National Aboriginal Community Controlled Health Organisation. |
To facilitate engagement between parties on issues relating to the 7CPA. |
Twice a year. |
Note: The CPCC and the PSCC were suspended in 2023 due to the commencement of negotiations for the 8CPA.
Source: ANAO analysis of Health internal documents.
2.78 Forums meet once or twice a year (with the exception of the Biosimilar Working Group which has met more frequently) and have discussions within the relevant scope of the forum.
2.79 Health has not established a forum to engage with state and territory government stakeholders on issues specifically related to the PBS. State and territory governments lead the management of public hospitals and other frontline health services that may procure, prescribe and supply PBS medicines. Some states also conduct health technology assessments for medicines to determine the use of these medicines in public hospitals.
Public contributions to the audit
2.80 In September 2023, the ANAO wrote to 32 organisations, including peak industry bodies, independent research bodies and relevant government entities, to invite written submissions regarding the subject of this audit. In addition, the ANAO accepted public contributions to the audit through the ANAO website between September 2023 to April 2024.
2.81 A total of 32 contributions to the audit were received from 21 organisations and six individuals (five contributors provided two contributions). Contributions to the audit raised concerns relating to stakeholder engagement by Health and Services Australia. All stakeholder groups, including business entities, individuals, peak bodies and state and territory governments, provided feedback that opportunities for engagement were limited. This was mainly attributed by the contributors to a lack of transparency of how decisions are made prior to implementation. Table 2.12 provides a summary of key themes raised in contributions.
Table 2.12: Key themes raised in public contributions to the audit
|
Stakeholder group |
Key themes raised |
|
Business entitiesa |
|
|
Individuals |
|
|
Peak bodies |
|
|
State and territory governments |
|
Note a: Business entities included pharmaceutical companies and retail pharmacies.
Source: ANAO analysis of public contributions to the audit.
Recommendation no.4
2.82 The Department of Health and Aged Care:
- develop a stakeholder plan for the Pharmaceutical Benefits Scheme that identifies all stakeholder groups, consultation objectives and methods of engagement; and
- publish a stakeholder strategy that informs stakeholders of Health’s planned approach to engaging with stakeholders on the Pharmaceutical Benefits Scheme, including where written agreements or partnerships may be used.
Department of Health and Aged Care response: Agreed.
2.83 As noted in the report, the Department of Health and Aged Care consults broadly with a range of stakeholders to help deliver a high quality efficient, and effective Pharmaceutical Benefits Scheme. The Department has, for instance a specific work program supporting and enhancing consumer engagement in the Pharmaceutical Benefits Scheme.
2.84 The Department will work to identify other stakeholder groups, goals of consultation and channels of engagement to formalise an overarching stakeholder plan for the Pharmaceutical Benefits Scheme.
2.85 The Department will also develop and publish a strategy that outlines the planned approach to engage with stakeholders in relation to the Pharmaceutical Benefits Scheme, and which highlights relevant written agreements or partnerships that support this engagement.
3. Managing the cost of the Pharmaceutical Benefits Scheme
Areas examined
This chapter examines the appropriateness of the Department of Health and Aged Care’s (Health) arrangements to manage the cost of the Pharmaceutical Benefits Scheme (PBS).
Conclusion
Health’s arrangements to manage the cost of the PBS are largely appropriate. Arrangements were in place to assess the cost-effectiveness of individual PBS medicines and manage the cost of listed medicines. Arrangements have been established to manage pharmacy remuneration through successive Community Pharmacy Agreements (CPAs), negotiated with the pharmacy industry, which Health supported through impact analysis for the eighth CPA signed in June 2024. Health has established processes for managing patient out-of-pocket costs and monitoring and forecasting the overall cost of the PBS. Health has not established arrangements to automate patient access to the Safety Net or engaged in horizon scanning analysis to anticipate potential future costs of new and novel medicines.
Areas for improvement
The ANAO identified an opportunity for improvement relating to conducting horizon scanning analysis to inform whole of PBS expenditure.
3.1 Australian Government funding for the PBS subsidises the cost of medicines for patients. The primary drivers of cost to the government for the PBS are the cost of medicines and pharmacy remuneration. Out-of-pocket costs to patients are limited through a co-payment and Safety Net. Around 31 per cent of prescriptions are below the maximum co-payment amount.37 If medicines exceed the patient co-payment amount the government incurs the cost of the difference. As at 30 June 2023, there were 928 different medicines with 5,261 brands listed on the Schedule of Pharmaceutical Benefits (the Schedule). The total government contribution as a proportion of overall cost has increased from 79.8 per cent in 1991–92 to 91.4 per cent in 2022–23.
3.2 Delivering great policy, available from the Australian Public Service Academy38, states that great policy advice is clear on intent, well informed, practical to implement and influential. It is emphasised that high-quality analysis and robust evaluation are critical to inform policy and achieve desired outcomes. Regulatory Impact Analyses, administered by the Office of Impact Analysis (OIA), ensure ‘that advice to government is accompanied by robust analysis, data and an accurate overview of the effects of proposed policies on [the] community.’39 Regulatory impact and the potential costs of implementing a proposed policy are justified through a cost-benefit analysis. Cost-benefit analyses ensure that the full monetary impact of policy, including effects on the community and economy, are considered when developing policy.
Have appropriate arrangements been established to manage the prices of PBS medicines?
Arrangements for assessing medicine cost-effectiveness outlined in the Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee have been followed. Health has complied with administrative procedures for listing medicines on the Schedule and agreeing medicine prices with sponsors. Health has negotiated deeds of agreement with medicine sponsors (covering special pricing arrangements and risk-sharing agreements) to minimise the cost of PBS medicines to government. Statutory price reductions are in place to decrease the cost of listed medicines. Medicines are delisted from the Schedule by medicine sponsors with no regular delisting process performed by Health.
Pharmaceutical Benefits Advisory Committee assessment of medicines
3.3 The Pharmaceutical Benefits Advisory Committee (PBAC) is an independent expert committee established under the National Health Act 1953 (NHA) to make recommendations to the Minister for Health and Aged Care (the minister) on which medicines should be subsidised under the PBS. PBAC is required by the NHA to consider the cost-effectiveness of medicines before it can make a recommendation.40 PBAC is required to make decisions by majority vote.
3.4 PBAC is supported in its consideration by two sub-committees:
- the Economics Sub-committee, which assesses clinical and economic evaluations of medicines submitted for listing; and
- the Drug Utilisation Sub-Committee (DUSC), which assesses estimates of projected usage and financial cost for medicines.
3.5 Health has developed the Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC guidelines)41 to support sponsors in applying to list medicines on the PBS. The PBAC guidelines outline the requirements for medicine sponsors to follow in developing their submissions for PBAC evaluation. The PBAC guidelines detail a standardised and systematic submission plan based on providing supporting evidence consistent with the aims of the NHA. The PBAC guidelines are also employed by PBAC to evaluate medicines for their listing on the Schedule.
3.6 The Procedural guidance for listing medicines on the Pharmaceutical Benefits Scheme42 (the procedural guidance) outlines the administrative steps that medicine sponsors must take to have their medicine listed on the Schedule, which are summarised in Figure 3.1.
Figure 3.1: Summary of the steps required to have a medicine listed on the PBS
Source: ANAO analysis, based on Department of Health and Aged Care, Listing process, Commonwealth of Australia, Canberra, 2024, available from https://www.pbs.gov.au/info/industry/listing/procedure-guidance/2-listing-process/listing-process [accessed 30 July 2024].
3.7 The Australian Government charges sponsors to recover the costs of PBAC’s evaluation. In 2024–25 the fees recovered from sponsors ranged from $12,785 to $264,595 depending on the type of submission.
3.8 The PBAC evaluation concludes with a decision by PBAC to recommend or not recommend a medicine for listing on the PBS. Following a positive recommendation, sponsors must initiate a second process with Health to finalise the price of the medicine, expected financial impacts, deeds of agreement (if required) and any restrictions for its prescription according to the recommendation made by PBAC.
Assessment of cost-effectiveness
3.9 When submitting a proposal to PBAC for evaluation, medicine sponsors must include either a cost-effectiveness or cost-minimisation analysis in a format prescribed by the PBAC guidelines, which state:
A full [cost-effectiveness analysis] is appropriate where the clinical evaluation has concluded that the proposed medicine is:
- therapeutically superior to the main comparator, but likely to result in additional costs to the health system; or
- therapeutically inferior to the main comparator, but likely to result in lower costs to the health system.
A cost-minimisation approach is appropriate where there is a therapeutic claim of noninferiority (or superiority), the safety profile is equivalent or superior (in both nature and magnitude), and use of the proposed medicine is anticipated to result in equivalent or lesser costs to the health system.43
3.10 Requirements for these two processes are outlined in Sections 3A and 3B of the PBAC guidelines. Table 3.1 summarises the required components of the two processes.
Table 3.1: Cost-effectiveness and cost minimisation analysis components
|
Cost-effectiveness |
Cost minimisation |
|
|
3A.1 Overview and rationale
3A.2 Methods and structure
3A.3 Population and setting
3A.4 Transition probabilities, variables and outcomes
|
3A.5 Health outcomes
3A.6 Resource use and costs
3A.7 Model validation
3A.8 Base-case results
3A.9 Uncertainty analysis
|
3B.1 Overview and rationale
3B.2 Equi-effective doses
3B.3 Additional costs and/or cost offsets
3B.4 Results
|
Source: Department of Health and Aged Care, Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee, Commonwealth of Australia, Canberra, 2016, available from https://pbac.pbs.gov.au [accessed 24 June 2024].
3.11 As part of PBAC’s evaluation of medicines it also recommends restrictions or specific requirements for obtaining authority for the medicine to be prescribed. Medicines can be listed under one of four categories: unrestricted, restricted, authority-required and authority-required (streamlined) (see Table 4.5). A restricted medicine can be prescribed only for specific therapeutic uses. Authority-required medicines, with the exception of authority-required (streamlined) medicines, require approval from Services Australia in order to be prescribed and dispensed for the subsidised PBS price. Medicines can have multiple item codes to address specific therapeutic needs and uses.
3.12 During the application process, sponsors can recommend a restriction or an authority requirement for listing the medicine on the Schedule. PBAC assesses the cost-effectiveness of medicines based on the sponsor-designated medical condition and population when determining whether a restriction or authority requirement should apply to the medicine listing.
3.13 PBAC considerations for applying authority requirements include:
- the potential for use in a population in which the proposed medicine is not cost-effective or where PBAC has not yet determined it to be cost-effective; and
- the potential for a high cost per patient or high total opportunity cost to the health system.
Other considerations by PBAC include the ‘quality use of the medicine’, medicine safety, and administrative burdens.
3.14 The ANAO assessed 13 medicines that were listed on the Schedule between July 2021 and April 2024 to determine whether the arrangements described in the PBAC guidelines for cost-effectiveness or cost-minimisation analysis and assessment have been operating in practice.44 The ANAO’s assessment found that:
- when PBAC recommended medicines for listing on the Schedule, cost-effectiveness was a primary focus for the recommendation;
- factors such as clinical efficacy and cost-effectiveness were considered in determining PBS restriction levels;
- PBAC did not recommend medicines for listing on the Schedule when they were not deemed cost-effective.45 The primary reasons for not recommending medicines were uncertainties relating to the clinical and cost-effectiveness not being sufficiently addressed in the information provided by the sponsor; and
- PBAC supported or recommended deeds of agreement (see paragraph 3.18) to reduce the cost of medicines and/or financial uncertainty related to the use of the medicine.
3.15 When PBAC made a positive recommendation, this was based on a cost-effective price of the medicine. If a medicine sponsor disagrees with the PBAC recommendation, they may decide not to proceed with listing on the Schedule, or may make a new submission to PBAC seeking reconsideration.46
Reporting to the minister
3.16 For the 13 medicines with new listings assessed by the ANAO (see footnote 44), after the PBAC meeting Health provided a brief to the minister that detailed whether PBAC had recommended medicines be listed on the Schedule and whether any of the proposed listings had the potential to attract media attention. Further detail was provided in the brief on decisions for medicines that Health considered may attract media attention, which sometimes included whether PBAC considered the listing to be cost-effective.
Pricing of medicines and listing on the Schedule
Pricing pathways
3.17 After PBAC has recommended a medicine for listing on the Schedule, medicine sponsors must then apply to Health to settle the price of the medicine. The process includes finalising the approved ex-manufacturer price (AEMP)47 and any prescription restrictions for the medicine. The AEMP is determined through a price agreement or price determination.
3.18 The procedural guidance details five different pricing pathways that can be followed once a positive recommendation has been made by PBAC (summarised in Table 3.2). The five pathways reflect whether there is a requirement to negotiate a deed of agreement with the medicine sponsor, as recommended by PBAC.48
Table 3.2: Pricing pathways for listing medicines on the Schedule
|
Pathway |
Summary |
|
A — Facilitated |
Available for medicines that meet the criteria of:
PBAC determines if the submission is eligible for this pathway. |
|
B — New deed |
Where no similar arrangements are in place a negotiation process occurs. Risk-sharing agreements, managed entry and special pricing arrangements may be considered. |
|
C — Existing deed |
Submissions which require notification of changes to a third party responsible for an existing deed of agreement and/or where an applicant has received a positive PBAC recommendation to list within the scope of existing arrangements. |
|
D — No deed |
No negotiation of a new or existing deed of agreement. |
|
Secretariat pricing |
Changes to listings of existing medicines that do not require a new price. |
Source: Department of Health and Aged Care, Procedural guidance for listing medicines on the Pharmaceutical Benefits Scheme, Commonwealth of Australia, Canberra, 2020, available from https://www.pbs.gov.au/industry/listing/procedure-guidance/files/Procedure-guidance-for-listing-medicines-on-the-Pharmaceutical-Benefits-Scheme-v2.5.pdf [accessed 24 June 2024].
3.19 Deeds of agreement allow the Australian Government to receive information on, and be reimbursed for, the costs of PBS medicines. Deeds of agreement have standard clauses which cannot be amended unless PBAC or the government identifies that medicines require different treatment. Deeds are reviewed at the end of their term or after a PBAC recommendation to review. There are two broad types of arrangements which are covered by a deed: special pricing arrangements and risk sharing agreements (see Box 2).
|
Box 2: Special pricing arrangements and risk sharing agreements |
|
Special pricing arrangements The Australian Government may enter into special pricing arrangements with medicine sponsors to enable access to PBS medicines at a lower price (recommended by PBAC to be cost-effective) without making the price publicly available and thereby impacting the medicine sponsors’ pricing in other countries. There are five criteria that must be met for special pricing arrangements to be negotiated:
Risk sharing agreements Risk sharing agreements are made between the Australian Government and medicine sponsors to mitigate risks related to uncertainties in cost estimations, cost-effectiveness, usage amounts and anticipated health outcomes of medicines. |
Approval of medicines for listing on the Schedule
3.20 The final step in the listing process is for the minister to make a determination listing a medicine at a specified price on the Schedule. Under subsection 85AD(1) of the NHA, the minister has delegated this authority to the secretary and deputy secretaries in Health, as well as first assistant secretaries, assistant secretaries and certain executive level 2 officers in the Health Resourcing Group.
3.21 Before a determination can be made, Australian Government policy requires Cabinet to approve the listing of medicines estimated to cost over $20 million per year through its usual processes.49 Medicines under $20 million may be approved by the minister after reviewing a ministerial submission that details the cash and fiscal impact of these medicines to the PBS over the forward estimates. Medicines with no financial impact may be approved by the First Assistant Secretary of the Technology Assessment and Access Division.
3.22 Once the listing has been approved, the relevant legislative instrument is made50 by the Assistant Secretary of the Technology Assessment and Access Division.
Assessment of compliance with pricing and listing requirements
3.23 The ANAO assessed the operation of listing processes and procedures for 13 medicines listed on the Schedule between July 2021 and April 2024 (see footnote 44).
3.24 Health followed appropriate processes for finalising the price of medicines for listing on the PBS for all assessed medicines. The processes aligned with the procedural guidance, and the medicine price aligned with the PBAC recommendation. The secretariat pricing pathway was used for medicines which did not require pricing changes.
3.25 Health followed its procedures to ensure deeds of agreement with the purpose of reducing the price of the sampled medicines were negotiated where appropriate. The deeds had consistent standard clauses, and were entered into on the advice of PBAC. Health had processes in place to invoice medicine sponsors for monies owed through the deeds of agreement.
3.26 For all assessed medicines, the processes followed for approval for the medicine price, listing of the medicine, and updating of the legislative instrument aligned with a valid exercise of statutory powers and government policy.
Post-listing changes to medicine prices
3.27 Medicines are listed on the Schedule in either F1 or F2 categories. The F1 category is for single brand medicines (including some single-branded combination medicines where the component medicines are not otherwise listed). The F2 category is for multiple brand (largely generic off-patent) medicines. When a medicine is in the F2 category it is subject to legislated price decreases through statutory price reductions and price disclosure. F1 medicines are subject to anniversary price reductions on the fifth, 10th and 15th anniversary of their listing on the Schedule. F2 medicines are subject to price disclosure reductions unless they are exempt items.51 These legislated mechanisms arise from the strategic agreements with Medicines Australia and the Generic and Biosimilar Medicines Association (discussed at paragraph 2.83 in Chapter 2).
3.28 The price of listed medicines can be changed through statutory price reductions triggered in circumstances listed in Division 3A or 3B of the NHA, or through sponsor-initiated requests for price increases or lower price offers.52 Figure 3.2 summarises the mechanisms for changing prices of F1 and F2 PBS medicines.
Figure 3.2: Summary of price change mechanisms for PBS medicines
Note: When a first new brand reduction is applied to a F1 medicine the medicine moves to the F2 Schedule.
Source: ANAO analysis.
Statutory price reductions — Division 3A
3.29 Division 3A of the NHA establishes a series of statutory price reductions that apply to PBS medicines.53 Statutory price reductions are capped to a maximum reduction of 60 per cent, which can bring the price of medicine to 40 per cent of the AEMP.54 As the statutory price reductions are tied to the strategic agreements with Medicines Australia and the Generic and Biosimilar Medicines Association they are currently limited to reductions up to 1 April 2027. Table 3.3 summarises the current statutory price reductions.
Table 3.3: Statutory price reductions under Division 3A
|
Reduction type |
Detail |
Reduction |
|
Anniversary price reduction |
Applied to F1 medicines when they have been listed for 5, 10 or 15 years |
5% at 5 and 10 years 26.1%, 30% and 1.48% at 15 years depending on the medicine and year it was listed |
|
First new brand reduction |
Applied when the first new biosimilar or bioequivalent brand with the same manner of administration is listed on the PBS |
25% |
|
Catch up reduction |
Applied on 1 April 2023 to medicines which have been listed on the PBS for 15 years or more without a price disclosure reduction |
Variable based on previous price reductions with maximum of 36.82% |
|
Combination flow on reduction |
Applied when 1 of the active ingredients experiences a statutory price reductiona |
In line with the active ingredient which experiences a statutory price reduction |
Note a: Combination medicines comprise two or more active ingredients, one of which is listed on the PBS.
Source: Department of Health and Aged Care, Ministerial discretion guidance material for statutory price reductions, Commonwealth of Australia, Canberra, available from https://www.pbs.gov.au/industry/pricing/ministerial-discretion/Ministerial-Discretion-Guidance-Material-for-Statutory-Price-Reductions.pdf [accessed 24 June 2024].
3.30 The ANAO assessed random samples of PBS medicines for 2021–22 and 2022–23 and determined that legislative requirements for statutory price reduction processes were reflected in the PBS for these years.
Ministerial waivers of statutory price reductions under Division 3A
3.31 The minister (or the minister’s delegate) may reduce or not apply a statutory price reduction under Division 3A.55 Sponsors of PBS-listed medicines may make a request to reduce or not apply the price reduction. The minister or delegate must consider the AEMP when reviewing these requests and may consider pricing history, clinical and viability aspects, and financial impacts.
3.32 As noted in Table 3.3, one reduction mechanism is the one-off catch-up. This applied on 1 April 2023 to medicines which had been listed on the PBS for 15 years or more and had not yet had a price disclosure reduction. Health received 421 requests for the application of ministerial discretion to waive the April 2023 catch up, of which 109 received no waiver, 262 were granted full waivers and 50 were granted partial waivers of varying percentages. The ANAO reviewed seven medicines56 for which a waiver was sought (see Table 3.4) to examine whether the guidelines governing the consideration of waivers were followed in practice.
Table 3.4: Medicines with a request to waive the April 2023 catch-up price reduction
|
Medicine |
Outcome of request |
Compliant with requirements |
|
Budesonide |
Full waiver approved for 1 form, partial waiver for 1 form |
✔ |
|
Ciclosporin |
Full waiver approved for 1 form |
✔ |
|
Deferiprone |
Full waiver approved for 3 strengths |
✔ |
|
Follitropin alfa |
1 strength granted a waiver while other strengths denied |
✔ |
|
Lithium carbonate |
Full waiver approved for 2 strengths |
✔ |
|
Methotrexate |
Full waiver approved for 2 strengths |
✔ |
|
Somatropin |
Partial waiver approved for 1 form |
✔ |
Key: ✔ Yes ✘ No
Source: ANAO analysis.
3.33 Medicines which PBAC advised would have an unmet clinical need without their listing on the Schedule were given full or partial waivers of the statutory price reductions. If PBAC advised there would be no unmet clinical need without the medicine listed on the PBS no waiver was granted. Somatropin was initially determined by PBAC to have no unmet clinical need if delisted. However, the medicine sponsor detailed the price decrease would be unviable and that the product is preservative free, unlike other somatropin products available, resulting in the item being granted a partial waiver.
3.34 Advice provided to the delegate to approve the waivers included the pricing history, clinical and viability aspects, and financial impacts.
Reductions as a result of price disclosure — Division 3B
3.35 Certain medicines with multiple brands listed on the Schedule are subject to price disclosure requirements to ensure that the average prices of the medicines remain aligned with market prices.57 Medicine sponsors must disclose pricing information to the Australian Government every six months, which enables the minister (or the minister’s delegate) to determine a weighted average disclosed price for the brand. The listed price of a medicine on the Schedule may be reduced if the current price exceeds the weighted average disclosed price by a specified margin. Price reductions under Division 3B may be made on 1 April and 1 October each year.
3.36 The ANAO assessed a random sample of PBS medicines for 2022–23 and determined that legislative requirements for price disclosure processes were met in 2022–23.
Price disclosure dispute resolution
3.37 Medicine sponsors may dispute the determination of a price disclosure reduction and request a waiver. There is no legislated mechanism for this dispute resolution process. The price disclosure reduction may be disputed and waived for one or more of the following reasons:
- weighted average disclosed price calculation errors;
- incorrect price disclosure threshold applied;
- market behaviour (such as unusual or aggressive discounting); or
- clinical issues (such as a price reduction resulting in a medicine shortage).
3.38 Between July 2021 and April 2024 Health received 60 requests for waivers of the price disclosure reduction, of which 16 were granted a waiver. Figure 3.3 summarises the reasons why disputes have been raised by the sponsor and granted by Health.
Figure 3.3: Summary of reasons for raising and granting a price disclosure dispute
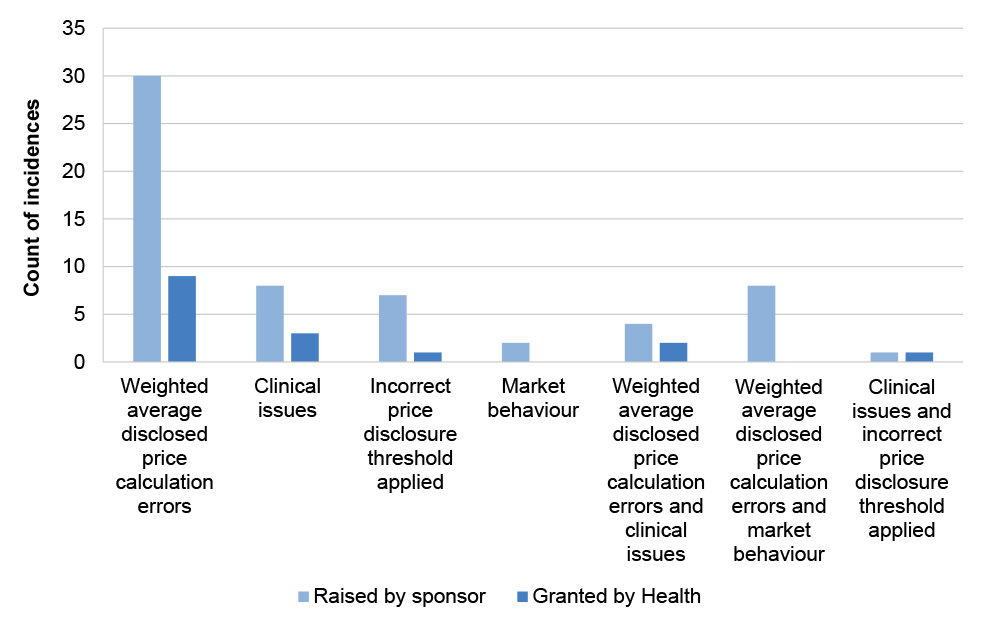
Source: ANAO analysis.
Requests for price increases
3.39 Medicine sponsors may request that the minister agree to a new maximum price. Price increases may only be agreed when they are necessary to ensure continued medicine supply and align with PBAC advice. Health’s procedures restrict medicine sponsors to one such request for each form of a medicine per year. If a price increase is agreed, it can take effect on either 1 April or 1 October. The authority to approve price increases is delegated to the First Assistant Secretary of the Technology Assessment and Access Division.
3.40 An application for a price increase must meet a number of requirements to be approved, including:
- there is a high risk the sponsor will withdraw the medicine from the PBS without a price increase;
- there would be unmet clinical need or increased costs to Government if the medicine was withdrawn; and
- the requested price increase is not above the cost-effective price recommended by PBAC.
3.41 Approval for increased financial expenditure as a result of a price increase is not required if the price increase request meets the criteria.
3.42 Between 1 July 2021 and 1 June 2024, 62 medicines were granted a full price increase, 55 medicines were granted a partial increase, 78 medicines were denied an increase and the decisions for 21 medicines were deferred. The ANAO assessed four medicines which had price increase requests (see Table 3.5) to determine if Health’s framework was operating.58
Table 3.5: Medicines with a price increase request
|
Medicine |
Outcome of request |
Compliant with framework |
|
Budesonide |
2 items granted a full increase 3 items denied an increase |
✔ |
|
Lithium carbonate |
2 items granted a full increase |
✔ |
|
Methotrexate |
2 items denied an increase |
✔ |
|
Pimecrolimus |
1 item granted a full increase |
✔ |
Key: ✔ Yes ✘ No
Source: ANAO analysis.
3.43 The assessment found that the criteria were considered, with clear advice provided to the delegate for the approval or denial of the price increase request.
Litigation seeking compensation for unrealised savings
3.44 Since 2011–12, Health has sought compensation from pharmaceutical companies, claiming that savings in PBS expenditure were denied as a result of interlocutory injunctions wrongfully granted in unsuccessful patent infringement litigation. The basis of the Commonwealth’s claims in these cases is that such injunctions delay the listing of generic medicines on the Schedule and any savings that would follow from that listing, such as consequential price reductions for existing medicines on the PBS. As of June 2024, the Commonwealth had brought four separate claims, in each case seeking compensation in excess of $100 million.
Post-listing reviews of PBS medicines
Reviews of major listings after 24 months
3.45 PBAC’s DUSC conducts routine monitoring of major new listings and changes to existing listings 24 months after listing. Reviews of predicted versus actual usage may be triggered or declined for the reasons listed in Table 3.6.
Table 3.6: Reasons for DUSC review at 24 months
|
Reasons not to review |
Reasons to review |
|
|
Source: DUSC agenda papers.
3.46 The DUSC analysis allows for the identification of inefficient medicine utilisation and off-script prescribing.59 DUSC reviews can also lead to broader analyses of the market. For example, DUSC’s post-listing consideration of bictegravir + emtricitabne + tenofovir alfenamide led to a wider review of Human Immunodeficiency Virus antivirals.60
Post-market reviews
3.47 Post-market reviews of medicines were established in the 2011–12 Federal Budget and began in 2015. In 2022 the post-market review framework was reassessed in the context of the Strategic Agreement with Medicines Australia. The reassessment sought to reduce the time to complete a post-market review to within 12 months, without limiting PBAC independence. In January 2024 a revised framework for post-market reviews was approved by the minister. Post-market reviews may be initiated due to concerns with medicine cost-effectiveness, clinical effectiveness, quality use of the medicine, high utilisation or identified international differences in medicine utilisation. Post-market reviews can be initiated by PBAC or DUSC and require ministerial approval.
3.48 Two post-market reviews have been completed since July 2021.
- Post-market review of medicines used for smoking cessation (June 2022) — which resulted in PBAC recommending extending restrictions for nicotine replacement therapy.
- Post-market review of opiate dependence treatment program medicines (March 2023) — which resulted in PBAC recommending amendments to the listings for these medicines and changes to authority approvals.
Delisting of medicines
3.49 On 1 January 2016, 17 low-cost medicines that were also available without prescription (termed ‘over-the-counter’), such as iron supplements and paracetamol, were removed from general availability through the PBS. While PBAC recommended retaining the medicines for specific groups (such as Aboriginal and Torres Strait Islander people and paraplegic and quadriplegic people), it recommended removing the medicines from general availability as they: were available over-the-counter, had a low PBS price, did not require specialists to prescribe, and clinical evidence did not support the subsidy arrangements. Since the removal of these medicines in 2016, no further medicines have been removed from the PBS through post-listing review processes.
3.50 Delisting of medicines from the Schedule has occurred at the request of medicine sponsors. Sponsors delist a medicine because the medicine will no longer be manufactured, the medicine is being replaced by a different form of the medicine, or it is not financially viable to keep the medicine on the PBS.61 Sponsors submit delisting requests for review at PBAC meetings. PBAC identifies whether there will be an unmet clinical need if a medicine is delisted and can determine whether a ‘supply only’ period62 should be applied to a medicine being delisted.
3.51 If PBAC identifies an unmet clinical need when a medicine is delisted, it may ask Health to seek to retain the medicine on the PBS.
Internal review of PBAC arrangements
3.52 The policies and methods followed by PBAC to assess new medicines have been subject to a recent review supported and resourced by Health in accordance with the Strategic Agreement with Medicines Australia (see Table 2.10). The review committee was formed and had adopted its terms of reference by March 2023.63 The review considered PBAC policies and methods such as:
- economic evaluations and the use of medicine comparators;
- new technology which may or may not provide substantial improvements in health outcomes;
- how to improve clinical, economic, and financial certainties throughout the lifecycle of technology; and
- how technology for conditions with a high unmet clinical need, particularly those with clinical and economic uncertainty, are assessed.64
3.53 The review was due to be completed in June 2023. It was extended twice, first to December 2023 then to May 2024. The final report was published in September 2024.65 The report recommended several improvements to the PBAC cost-effectiveness assessment process, among other recommendations, including:
- triaging applications to determine appropriate evaluation and assessment processes;
- streamlined assessment for applications with cost-minimisation analysis;
- improving the early/facilitation resolution pathways to be more flexible for applications with a high therapeutic value in clinical areas with unmet need;
- introducing a framework to support high-cost/high-impact medicines as an alternative to the current price per unit approach;
- improving post-listing review mechanisms to support the regular examination of medicine performance, utilisation, displacement and therapeutic utility; and
- undertaking research on when certain medicines should be accepted at higher prices.
Have appropriate arrangements been established to determine pharmacy remuneration for dispensing PBS medicines?
The Australian Government has negotiated Community Pharmacy Agreements (CPAs) with the pharmacy sector to determine pharmacy remuneration for dispensing PBS medicines since 1990. CPAs offer flexibility to include terms such as the remuneration adjustment mechanism to mitigate unexpected expenditure for the Australian Government. The choice to negotiate a CPA rather than allowing remuneration to be set by an independent tribunal was not supported by adequate impact analysis for the seventh CPA. Health prepared an Impact Analysis for the eighth CPA, signed in June 2024, which supported continuation of pharmacy remuneration setting through a CPA.
3.54 The Pharmacy Benefits Remuneration Tribunal (PBRT) was established in 1981 to independently determine the Commonwealth price66 for pharmacy dispensing of PBS medicines. In 1989 the Pharmacy Guild of Australia (Pharmacy Guild) disagreed with a change to pharmacy remuneration made by the PBRT and subsequently negotiated directly with the minister to develop the first CPA. As part of this, a legislative change was introduced requiring the PBRT, under subsection 98BAA(1) of the NHA, to give effect to pricing terms agreed in a CPA. If a CPA cannot be agreed between the minister and Pharmacy Guild, pharmacy remuneration can be determined by the PBRT.
3.55 Since 1990, pharmacy remuneration (money paid to pharmacies to supply PBS medicines) in Australia has been determined through negotiations of successive CPAs. Each CPA has operated for a term of roughly five years. The eighth CPA (8CPA) commenced on 1 July 2024 following the early termination of the seventh CPA (7CPA). The 8CPA is an agreement between the Minister for Health and Aged Care (on behalf of the Commonwealth of Australia) and the Pharmacy Guild.67
Pharmacy remuneration under the 7CPA
3.56 The 7CPA commenced on 1 July 2020 and was due to end on 30 June 2025. The 7CPA set fee parameters for pharmacy administration, dispensing, and wholesale costs along with specifying the amount pharmacists could charge for recording items for the Safety Net and issuing Safety Net cards. The 7CPA allowed for adjustment of these fees against the consumer price index (CPI).
3.57 Clause four of the 7CPA allowed for additional charges to patients for medicines below the co-payment amount. Pharmacists could choose to charge the patient the Commonwealth price, a Safety Net recording fee (if applicable), and an additional patient charge. The sum of these components could not exceed the maximum co-payment charge. The additional patient charge component did not accumulate towards a patient’s Safety Net balance.
3.58 Pharmacies are reimbursed the difference between the Commonwealth price and the patient co-payment. If medicines are sold below the patient co-payment amount, pharmacies will receive no payment from the Commonwealth. Pharmacies can discount medicines below the co-payment amount, forgoing any profits when they do so. Health noted when briefing the minister in June 2022 that discount pharmacy chains often choose to discount medicines to be price competitive.
3.59 The 7CPA included, for the first time, a remuneration adjustment mechanism (RAM), that shared risks between the government and the Pharmacy Guild relating to higher or lower than estimated prescription volumes. This was intended to provide greater certainty for government and predictability for community pharmacies. The RAM resulted in a proposed decrease of the Commonwealth price due to be paid by government starting from 1 July 2024 (see Appendix 7), but this was superseded by the commencement of the 8CPA on 1 July 2024.
3.60 From 1 January 2016 pharmacists could choose to discount medicines at or above the Commonwealth price by one dollar. If pharmacists offer this discount the patient will pay $30.60.
Expenditure on pharmacy remuneration under the 7CPA
3.61 For the first three financial years of the 7CPA expenditure on pharmacy remuneration averaged 6.5 per cent higher than forecasted, totalling an additional $532 million in expenditure. During these financial years the expenditure was impacted by higher than expected CPI increases and a decrease in the general patient co-payment amount on 1 January 2023 (paragraph 3.76). Anticipated impacts for 2023–24 included further CPI increases and the introduction of 60-day dispensing for selected medicines.68
Post-implementation review of the 7CPA
3.62 The Office of Impact Assessment (OIA) requires impact analysis to be performed for:
Any policy proposal or action of government, with an expectation of compliance, that would result in a more than minor change in behaviour or impact for people, businesses or community organisations.69
3.63 The 7CPA was signed on 11 June 2020 before a Regulation Impact Statement (RIS) was finalised and assessed by the OIA.70 The draft RIS was published on the OIA website for transparency but was not formally assessed.
3.64 Health conducted a post-implementation review (PIR) of the 7CPA71, as a RIS had not been provided for assessment to OIA before the 7CPA was signed. The PIR was limited to 7CPA activities undertaken prior to 1 July 2022. Health completed the PIR in November 2022 and concluded that:
the 7CPA is an appropriate mechanism for supporting arrangements for pharmacy remuneration, community pharmacy programs, and CSO funding pool arrangements and related services, consistent with previous CPAs. 72
3.65 In developing the 7CPA PIR, Health sought feedback from various organisations on the 7CPA process. Key themes emerging from the feedback were: issues with the stakeholder engagement processes; questions about the necessity of a CPA; a lack of transparency in medicine costs; and a lack of reviews and evaluations of community pharmacy programs.
3.66 OIA found the PIR inadequate as it did not provide ‘robust evidence demonstrating how the 7CPA delivers a net benefit to community’.73 OIA also found data gaps in relation to health outcomes and accessibility of medicines, with no plan to address the gaps. OIA suggested further analysis of the performance of pharmacies in order to determine whether community pharmacies were reliant on the 7CPA to be viable and to ensure the intervention was appropriate and targeted.
3.67 The Prime Minister wrote to the minister on 31 January 2023 in response to the OIA assessment. In the letter, the Prime Minister noted that the PIR had not supported ‘robust evidence-based policymaking’. The Prime Minister asked that Health prepare for the end of the 7CPA by completing an impact analysis at the required standard to inform the next CPA, including analysing whether negotiating another CPA would be necessary or appropriate.
Pharmacy remuneration under the 8CPA
3.68 In August 2023 the minister announced that negotiations for the 8CPA would commence early and conclude by 30 June 2024. An impact analysis to inform the new CPA, as requested by the Prime Minister, was not completed before the negotiations for the 8CPA commenced. No other reviews of the 7CPA pharmacy remuneration mechanism were completed before the negotiations for the 8CPA commenced.
3.69 Table 3.7 outlines the main negotiation points for pharmacy remuneration between the Pharmacy Guild and the government and the outcomes of negotiations.
Table 3.7: Outcomes and cost impact of 8CPA
|
Negotiation point |
Outcome |
Cost |
|
Dispensing remuneration |
$22.5 billion over 5 years ($15.8 billion for dispensing PBS subsidised medicines and $6.7 billion in remuneration for unsubsidised scripts) |
$3 billion cost to Government |
|
Introduction of administration, handling and infrastructure fee for medicines with 60-day dispensing allowance |
Introduction of an additional community supply support payment which includes an administration, handling and infrastructure fee for 60-day scripts above the co-payment |
$2.1 billion over 5 years |
|
Remuneration adjustment mechanism |
Six-month assessment periods with remuneration adjustments based on prescription numbers |
No forecasted cost impact as it is dependent on prescription numbers exceeding thresholds |
|
Changes to co-payment and 1 dollar discount |
General and concessional co-payment reduced by 1 dollar and 1 dollar discount removed |
$486 million cost to Government |
Source: ANAO analysis of Health internal documentation.
3.70 On 13 March 2024 a heads-of-agreement was signed between Health and the Pharmacy Guild. The agreement detailed an additional investment of up to $3 billion in community pharmacy and in cheaper medicines above existing projections, an administration, handling and infrastructure fee for 60-day prescriptions, and a revised remuneration adjustment mechanism. The agreement also detailed a freeze to the indexation of co-payments, which is to be compensated for by reducing the one dollar discount yearly by indexation until it reaches zero. The effect of this decision is that indexation will be frozen for the general co-payment for one year, and for the concessional co-payment for five years.
Impact analysis
3.71 When approving the commencement of the negotiations for the 8CPA in August 2023, the Prime Minister informed the minister that a high-quality impact analysis would need to be completed. Health finalised an Impact Analysis for the negotiation of the 8CPA in June 2024.74 The Impact Analysis considered three policy options:
- Option 1 — continuation of the 7CPA until 30 June 2025 and no new CPA upon its expiry;
- Option 2 — an 8CPA that includes existing pharmacy programs; and
- Option 3 — an 8CPA for pharmacy remuneration and pharmacy programs delivered directly through community pharmacies.
3.72 The Impact Analysis contained a net benefit analysis of each option against a range of qualitative criteria: person-centred dispensing and programs; equity, sustainability and access for consumers and businesses; accountability and transparency; and innovation and continuous improvement. Option 3 scored highly against all criteria, with potential improvement through developing an improved understanding of medicine costs with stakeholders. Option 1, which was considered to lack pharmacy remuneration certainty, scored lower on a range of criteria. Option 2, which lacked the inclusion of community pharmacy programs, scored lower on person-centred programs, and innovation and continuous improvement.
3.73 The OIA rated the Impact Analysis ‘good practice’. In order to receive an ‘exemplary’ rating the OIA advised that the Impact Analysis would require:
- Further in-depth analysis on the flow-on and distributional costs and benefits of the policy options.
- A detailed discussion of the implementation risks of the policy, including their likelihood, consequences and how they will be managed.
3.74 The government agreed to implement option 3 based on the Impact Analysis. The 8CPA was signed on 3 June 2024 and began on 1 July 2024.
Have appropriate arrangements been established to monitor and advise government on out-of-pocket costs to patients for PBS medicines?
Health has used monitoring data to model the impact of proposed changes to patient co-payment amounts and Safety Net thresholds on patient out-of-pocket costs. Based on this modelling, Health has provided advice to government on proposals to help patients achieve greater cost-savings through these mechanisms. Health has not established arrangements to automatically determine eligibility for the Safety Net. Health has estimated that 640,000 patients become eligible for the Safety Net each year but do not apply, foregoing $100 million in medicine subsidies.
Patient out-of-pocket costs for PBS medicines
3.75 Out-of-pocket costs for patients are largely influenced by two features of the PBS: patient co-payments and the Safety Net.
- The co-payment represents the maximum amount patients will pay for PBS medicines (not including discretionary pharmacy dispensing fees), above which any remaining cost is subsidised by the Australian Government. The co-payment is currently $31.60 for general patients or $7.70 for concession card holders.
- The Safety Net limits the amount patients will expend on medicines over the calendar year. When a patient’s co-payment expenditure on PBS medicines in a calendar year reaches the relevant Safety Net threshold ($1,647.90 for general patients or $277.20 for concession card holders), the patient’s co-payment is reduced for the remainder of the year (currently $7.70 for general patients or free for concession card holders).75
3.76 The co-payment and Safety Net increase in line with the CPI annually on 1 January and may also change in accordance with Australian Government policy. Table 3.8 outlines the changes to the co-payment and Safety Net amounts since 1 January 2021. Appendix 8 shows the changes to the co-payment amount and Safety Net threshold since 1960 and 1990 respectively.
Table 3.8: Changes to co-payment and Safety Net amounts since 1 January 2021
|
Date of change |
Co-payment |
Safety net |
||
|
|
General ($) |
Concessional ($) |
General ($) |
Concessional ($) |
|
1 January 2021 |
41.30 |
6.60 |
1497.20 |
316.80 |
|
1 January 2022 |
42.50 |
6.80 |
1542.10 |
326.40 |
|
1 July 2022 |
No change |
No change |
1457.10 |
244.80 |
|
1 January 2023 |
30.00 |
7.30 |
1563.50 |
262.80 |
|
1 January 2024 |
31.60 |
7.70 |
1647.90 |
277.20 |
Source: Department of Health and Aged Care, Fees, Patient Contributions and Safety Net Thresholds, Canberra, 2024, available from https://www.pbs.gov.au/info/healthpro/explanatory-notes/front/fee#_1 [accessed 24 June 2024].
3.77 Out-of-pocket costs for patients are also influenced by pricing decisions made by pharmacists. Pharmacists may choose to offer a discount of up to one dollar (for medicines priced above the co-payment threshold) or any amount (for medicines priced below the co-payment threshold). Pharmacies can impose certain fees on top of the co-payment (see Table 3.9).
Table 3.9: Fees additional to the co-payment
|
Fee |
Description |
|
Brand premium |
Certain brands of a medicine attract extra costs due to manufacturers requesting an additional charge. Patients can avoid this fee by choosing a brand without a premium. |
|
Therapeutic group premium |
The difference between the lowest cost medicine in a group and another medicine in a therapeutic group with comparable safety and health outcomes. Patients can avoid this fee through choosing medicines in the therapeutic group without the premium. If they are unable to take the lowest cost medicine for medical reasons, their doctor may request an exemption. |
|
Special patient contributions |
When the supplier and the government cannot agree on a price, a listed medicine may have a higher price. If a patient’s doctor believes there is no therapeutic alternative available, for example due to adverse reactions, medicine interactions, or anticipated poor medicine compliance, the government may pay this fee. |
Source: Department of Health and Aged Care, About the PBS, Commonwealth of Australia, Canberra, 2024, available from https://www.pbs.gov.au/info/about-the-pbs#What_you_pay_for_PBS_medicines [accessed 24 June 2024].
3.78 If a pharmacy has discounted the price of a medicine, only the cost the patient has paid counts towards the Safety Net. Pharmacies may charge an additional patient charge for medicines below the co-payment. This fee does not count towards the Safety Net. Section 4.3 of the 8CPA states:
The Pharmacy Guild must use its best endeavours during the Term to ensure that, prior to dispensing a Pharmaceutical Benefit, Approved Pharmacists make consumers aware of any Safety Net Recording Fee and Additional Patient Charge to be charged, the fact that the Additional Patient Charge is not Commonwealth initiated, and that fees and charges may differ between pharmacies.76
3.79 Patients can claim a refund if they spend over the Safety Net threshold before obtaining the Safety Net card.77 If patients change between being a concessional and general patient during the calendar year, they need to apply for a Safety Net card for their current circumstances.
Advice on patient co-payments
2023 reduction to general co-payment
3.80 In the October 2022–23 Federal Budget, the Australian Government decreased the general co-payment amount from $42.50 to $30 from 1 January 2023. This was a commitment made by the Australian Labor Party in the May 2022 election. This policy change was estimated to cost $696.1 million over the following four years. Health developed a two-page RIS78 for the co-payment change. Health did not develop an evaluation plan or performance metrics to assess the outcomes of the change.
3.81 Prior to the March 2022–23 Federal Budget, the Pharmacy Guild had called for the general co-payment to be reduced to $19. Modelling by Health indicated that decreasing the co-payment to $19 would have increased costs for a larger number of general patients than if the co-payment was reduced to $30. Health advised the minister that this is because pharmacies can choose to discount medicines below the co-payment or apply a $1 discount for medicines at or above the co-payment amount.
3.82 The Australian Government committed to no patient being worse-off from the 1 January 2024 reduction in the co-payment. Health identified through modelling that, due to pharmacy discounting rules, some patients would pay more for medicines than before the 1 January 2023 co-payment decrease. Health advised the Australian Government that legislation could be introduced to enable pharmacies to offer an increased discount to prescriptions between $30 and $42.5079, which would ensure no patient would be worse-off.80 This approach was adopted by the Australian Government.
3.83 Health conducted an advertising campaign with a $5.9 million budget for the co-payment reduction that aimed to reduce the number of people delaying purchases of PBS medicines due to cost. The campaign ran from December 2022 to May 2023, and included media advertising, letters to community pharmacies, factsheets, social media posts, posters, videos, and a newsletter article and table explaining the changes for pharmacists. Health completed an evaluation of the campaign in September 2023. The evaluation found the campaign raised awareness of the co-payment changes, but it did not change the number of patients delaying purchases of PBS medicines due to cost.
3.84 The Productivity Commission’s Report on Government Services 2024 stated that 7.6 per cent of respondents delayed or did not get prescription medicines when needed in 2022–23 due to cost, an increase from 5.6 per cent in 2021–22.
Consideration of other co-payment options
3.85 In April 2023 Health conducted policy analysis that examined international and Australian evidence on co-payments. The paper considered opportunities to modify the current co-payment system to minimise financial hardship for patients while ensuring the financial stability of the PBS. Policy options considered included:
- removing the co-payment or decreasing the Safety Net threshold for certain patient populations;
- introducing additional co-payment tiers;
- incentivising medication adherence and developing tools to identify non-adherence;
- increasing prescription quantities;
- combining the PBS and Medicare Safety Nets;
- automating Safety Net threshold calculations; and
- capping indexation increases to the co-payment and Safety Net.
3.86 Health advised the ANAO in April 2024 that:
There are a number of items already being progressed by the Department at this time around further changes to PBS affordability, this includes items currently being prepared for Government consideration and some still in development.
This includes potential further changes to PBS co-payments, however further consultation and Government consideration is still required to finalise these potential changes.
3.87 As part of the 2024–25 Federal Budget, co-payment indexation increases were announced to be temporarily frozen from 1 January 2025. For general patients, indexation will resume on 1 January 2026. For concessional patients, indexation will resume on 1 January 2030. These changes to the co-payment stem from the 8CPA (see paragraph 3.68).81
Reporting on under co-payment prescriptions
3.88 Since 2012–13 Health has publicly reported data on the number of PBS prescriptions under the co-payment amount. Since 2018–19 this reporting has also included the net cost to patients. Reporting is provided against the generic name of the medicine and by the formulation and concentration.
Advice on the PBS Safety Nets
Assessment of Safety Net thresholds
3.89 There is no formal mechanism to evaluate Safety Net thresholds. On 1 July 2022 the Safety Net thresholds were decreased to $1,457.10 for general patients and to $244.80 for concession card holders. This change was announced as part of the March 2022–23 Federal Budget. The Treasury Laws Amendment (Cost of Living Support and Other Measures) Bill 2022 was introduced to amend the legislation and decrease the Safety Net. The changes were estimated to increase costs to government by $525.3 million over four years and provide more than 2.4 million patients earlier access to the Safety Net.
Patient tracking of their Safety Net balance
3.90 As patients purchase medicines through the calendar year they are required, with assistance from their pharmacists, to record their purchases on a paper form. If a patient uses multiple pharmacies the onus is on the patient to track their own expenditure. Once the Safety Net threshold is reached the pharmacist submits the form to Services Australia and provides the patient a Safety Net card. Patients cannot apply directly to Services Australia for the Safety Net card.
3.91 Section 84B of the NHA defines the family relationships for the PBS Safety Net. Under the PBS Safety Net a family includes the initial patient, their partner, dependent children under 16 and full-time dependent students under the age of 25. The PBS Safety Net legislation does not limit the number of families a patient may belong to nor limit the ability of prescriptions to be recorded against more than one family group.
3.92 Eligibility for the Medicare Safety Net has been automatically determined since legislative changes were introduced in 2015. The Medicare Safety Net can be checked by patients through the MyGov app, using the Express Plus Medicare app or by calling Services Australia. Patients can also confirm their family members through the aforementioned methods or through a confirmation form. The Medicare Benefits Schedule allows children to be part of more than one family but limits recording of expenses incurred by a child to the adult that incurred the expense.
External reviews of the Safety Net
3.93 The ANAO’s 2010 performance audit of the administration of the PBS recommended that:
Medicare Australia and [Health] examine how the PBS system and data capture arrangements could be enhanced to enable patients to be advised when [they] have reached the PBS Safety Net Threshold, and advise government on options.82
Health disagreed with the Recommendation as ‘the matter of an automated safety net is a policy issue with significant program design and cost implications and is a matter for Government to consider.’83
3.94 A 2017 Review of Pharmacy Remuneration and Regulation, conducted for the sixth CPA, recommended that the Australian Government:
a. require the PBS Safety Net to be managed electronically for consumers. This functionality should be automatic from the consumer’s perspective;
b. investigate whether the PBS Safety Net scheme can be adjusted to spread consumer costs over a twelve-month period;
c. provide sufficient transparency in the way a patient’s progress towards the PBS Safety Net is collated, including information on any gaps in how it is calculated; and
d. investigate and implement an appropriate system which allows payments for opiate dependence treatments to count towards the PBS Safety Net.84
The government accepted this Recommendation but noted ‘that it poses a number of policy and implementation issues that would need to be considered further.’85
Automation of the Safety Net
3.95 Between 2021 to 2024 Health advised the Australian Government on how the Safety Net application process could be automated. In 2021 Health estimated that 640,000 patients become eligible for the Safety Net each year but do not apply, foregoing $100 million in medicine subsidies. Services Australia is currently progressing a project which will seek to digitise the Safety Net recording process for pharmacists (see paragraph 4.68), but this does not extend to automatically applying the Safety Net to eligible patients.
Have appropriate arrangements been established to monitor and advise government on the overall cost of the PBS?
Health has established arrangements for modelling the overall cost of the PBS and the impact of new medicine listings, and it provides advice to the government and Parliament through the annual Budget processes.
- Health has established a system to model PBS expenditure based on the current legislative requirements, which it uses to model the impact of new and amended medicine listings.
- Reporting on PBS expenditure is available through an annual report and reporting on Services Australia’s website.
- Health has not performed horizon scanning analysis to forecast PBS expenditure and identify potential policy changes.
Monitoring of PBS expenditure
3.96 Health uses a system called Pharmacy Remuneration and Negotiation Consolidated Information System (PhRANCIS) to model PBS expenditure for budgetary purposes. PhRANCIS uses prescription volume forecasts and pricing assumptions, based on currently legislated price changes, to estimate PBS expenditure. PhRANCIS can produce reports for individual medicines and can be used for pricing and budget estimates for medicines. Health also uses PhRANCIS to model CPA scenarios and expenditure and has used it to inform negotiations for the 8CPA. PhRANCIS does not capture PBS expenditure for community pharmacy programs, community service obligations under the CPA, or the Life Saving Drug Program.
3.97 Services Australia provides Health three different monthly reports on PBS expenditure:
- end of month balances for unpresented cheques or EFTPOS;
- expenditure through the online authority system; and
- monthly PBS expenditure broken down by constituent elements of the PBS (such as ordinary claims by general and concessional patients, claims made under the Safety Net by general and concessional patients, and claims made for highly specialised medicines).
3.98 Services Australia also provides Health annual reports on the accrual of outstanding claims. The annual reports contain a summary of the way claim payments for PBS medicines are made; for example, through PBS suppliers, online or manually. A final accrual figure for outstanding claims is provided.
Reporting of PBS expenditure
3.99 Health has published an annual PBS Expenditure and Prescriptions Report since 2002–03.86 These reports detail the overall expenditure of the PBS and break down expenditure into categories such as general or highly specialised medicines, high-cost medicines, high prescription medicines, expenditure by general or concessional Safety Net or non-Safety Net, and expenditure under the CPA.
3.100 Services Australia has websites reporting on PBS expenditure and prescription numbers.87 These reports can provide either prescription numbers or expenditure for each PBS item number, medicinal therapeutic group, or patient group. Reports can be generated by state or territory, month, financial year, or calendar year.
3.101 Health provides briefings on whole-of-PBS expenditure to the government during Portfolio Budget Statements processes.
PBS budget
3.102 PBS budget expenditure continues to grow in nominal terms. In 2022–23 expenditure on the PBS was reported to be $17 billion, up 15.6 per cent from $14.7 billion in 2021–22, which was up 6.7 per cent from $13.8 billion in 2020–21.
3.103 The PBS budget has also been increased in recent Mid-Year Economic and Fiscal Outlook (MYEFO) processes.88 For new and amended PBS listings the budget was increased by:
- $682.6 million over four years in 2020–21;
- $1.1 billion over four years in 2021–22; and
- $3.5 billion over four years in 2023–24.
The 2021–22 MYEFO also had a $638.3 million increase for medicine price increases and increases in concession card holders due to COVID-19 unemployment measures.
3.104 Through the deeds of agreement for individual medicines (see Box 2), Health has recovered money owed from sponsors for PBS medicines.
3.105 Total PBS expenditure and money recovered by Health since 2018 is shown in Figure 3.4.
Figure 3.4: Revenue from deeds of agreement
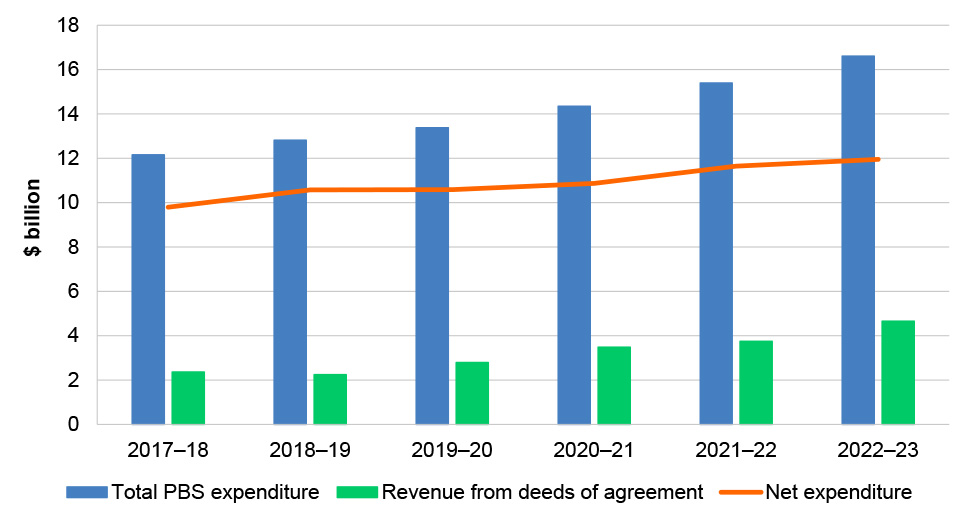
Source: ANAO analysis of Health annual reports.
Forecasting of PBS expenditure
3.106 As of 30 June 2023, there were 928 different medicines with 5,261 brands listed on the PBS Schedule. In 2022–23 the top five PBS medicines for government expenditure were priced from $1,089 to $21,377. These five medicines cost government over $2 billion.
3.107 The Australian population is steadily ageing, with 17 per cent of the population aged 65 or older in 2023. Data from the Australian Bureau of Statistics indicates that as the Australian population ages there will be an increase in expenditure on medicines. Figure 3.5 shows the supply of PBS medicines by age and sex in 2020–21.
Figure 3.5: Percentage consumption of PBS medicines by age and sex in 2020–21
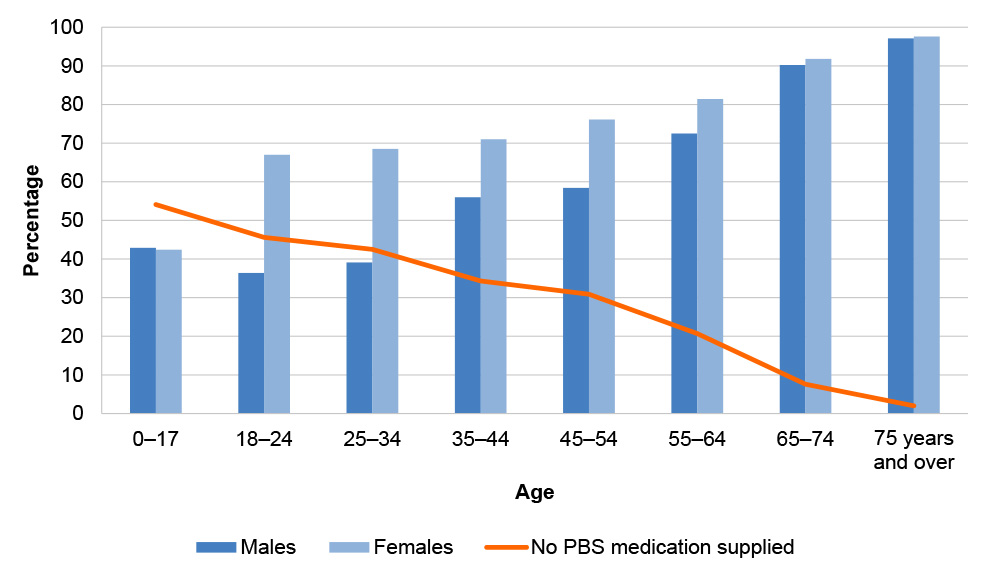
Source: Australian Bureau of Statistics, Pharmaceutical Benefits Scheme Supplied Medications, ABS, Canberra, 2022, available from https://www.abs.gov.au/articles/pharmaceutical-benefits-scheme-supplied-medications [accessed 24 June 2024].
3.108 Age also increases the number of medications a person consumes. Persons aged 65–74 who require medication consume an average of 5.7 medication types, rising to 7.7 for those aged 75 and over. In comparison, persons aged between 25–44 who require medication consume an average of 2.8 medication types.89
3.109 Modelling of projected PBS growth was performed by the Parliamentary Budget Office in 2020,90 which determined that from 2018–19 to 2030–31 the PBS would remain at 0.7 per cent of gross domestic product and by 2030–31 be 2.5 per cent of total government expenditure. Estimated expenditure on pharmaceutical benefits for the 2024–25 financial year was 2.7 per cent of all government expenditure.
3.110 Public contributions to this audit received from pharmaceutical companies indicated that current medicine pricing policies effectively stem increases in the cost of the PBS. Public contributions to the audit suggested that further price savings could be found through incentivising the uptake of biosimilars.
3.111 Advice provided by Health in the 2022 incoming government brief stated that:
The governance arrangements for the PBS and pricing reforms have supported the listing of significant numbers of new medicines for patients, while containing spending growth at a modest rate, particularly in comparison to the [Medicare Benefits Schedule]. PBS costs have been very effectively contained through the institution of price disclosure and other statutory price reductions for listed medicines.
3.112 Horizon scanning analysis91 is performed by countries, including Canada, the United States of America and the United Kingdom, to assist in preparing health systems for new and impactful technologies. Health has not performed any horizon scanning analysis for the government to identify emerging technology that may impact the PBS budget. A recent review of PBAC processes (discussed at paragraph 3.52) considered horizon scanning in its advice to government.
Opportunity for improvement
3.113 Health could undertake horizon scanning analysis of whole of program expenditure to inform whether future policy changes are required to manage the cost of the PBS.
4. Delivery of services and payments
Areas examined
This chapter examines the effectiveness of the Department of Health and Aged Care’s (Health) and Services Australia’s arrangements to manage the delivery of Pharmaceutical Benefits Scheme (PBS) services and payments.
Conclusion
Health and Services Australia’s arrangements to manage the delivery of PBS services and payments are partly effective. Processes and systems for PBS claims processing are not fully effective at ensuring that legislative requirements for PBS claims are met, as Services Australia is not ensuring that PBS suppliers certify claims in accordance with legislative timeframes. While payment integrity is reviewed, it is not subject to performance monitoring or reporting. Payment timeliness is monitored, and targets are regularly met. The results are not included in Services Australia’s Annual Performance Statement. The provision of authority approvals is based on an automated system. There were differences in approval rates between authority applications made online and by phone, and Services Australia’s performance target for reporting on answering authority calls in its Annual Performance Statements does not align with the performance target agreed with Health in bilateral agreements. PBS Safety Net card claims and patient refunds are reliant on manual processes and timeliness performance measures have not been consistently met.
Areas for improvement
The ANAO made three recommendations aimed at ensuring the backlog of uncertified PBS claims is addressed, and ensuring that Services Australia reports against the integrity and timeliness of PBS claims processing and authority approvals consistent with performance measures agreed with Health.
The ANAO also identified an opportunity for improvement to investigate the discrepancy in authority approvals processed online and over the phone.
4.1 Under an appropriated partnership arrangement, a service delivery entity is funded by direct Budget appropriation to deliver services of a program which is the responsibility of a policy entity. For these arrangements, the policy entity retains responsibility for the outcomes of its programs and the service delivery entity is directly accountable to the Australian Government and Parliament for delivering services to the Australian community and for prioritising that service delivery within its budget funding.
4.2 Health and Services Australia have an ‘appropriated partnership’ bilateral arrangement (refer to paragraphs 2.25 to 2.26). Services Australia is funded by direct Budget appropriation to deliver certain services and payments for the PBS, among other programs. Under the Bilateral Management Arrangement which governs this relationship, Services Australia (the service delivery entity) is responsible for implementing effective systems and processes to deliver PBS services and payments and for monitoring and reporting on its performance to Health (the policy entity). Services Australia also reports to Parliament against its performance for service delivery through its annual performance statement contained in its annual report.
Have effective processes and systems been established to ensure the integrity and timeliness of PBS claims processing?
Almost all claims (99.9 per cent) made by PBS suppliers are submitted through Services Australia’s Online Claiming for PBS system, which automatically assesses claims against legislative rules before processing advance payments. Due to an absence of controls to ensure advance payments to PBS suppliers are certified within statutory timeframes, over one-third of approved PBS suppliers have uncertified claims totalling $1.514 billion (as at 30 June 2024). Payment integrity is reviewed but is not subject to performance monitoring or reporting. Payment timeliness is monitored, and targets are regularly reported as met, but it is not included in public reporting.
4.3 PBS suppliers are entitled to be paid for supplying PBS medicines to eligible patients, in line with requirements set out in the National Health Act 1953 (NHA). Payments to PBS suppliers provide reimbursement for the cost of medicines and remuneration for the pharmaceutical services required to dispense medicines. The National Health (Supply of Pharmaceutical Benefits–Under Co-payment Data and Claims for Payment) Rules 2022 set out the rules for processing, determining and making any payments of the claims made by PBS suppliers.92
4.4 Funding for the PBS (as well for the Medicare Benefits Scheme) is managed through accounts established under the Medicare Guarantee Act 2017 and administered by the Treasurer and the secretary of Health.
4.5 In 2022–23, Services Australia processed 219 million claims for payment and paid a total of $17.2 billion to approved PBS suppliers.93 The majority (95 per cent) of PBS suppliers are retail pharmacies.94 As at 30 June 2024, there were a total of 6,323 PBS suppliers (see Table 4.1).
Table 4.1: Number of approved suppliers, as at 30 June 2024
|
Type of PBS supplier |
Number of PBS suppliers |
Percentage of totala |
|
Pharmacist (retail pharmacies) |
5,977 |
94.5 |
|
Private hospitals |
166 |
2.6 |
|
Public hospitals |
173 |
2.7 |
|
Dispensing doctors |
7 |
0.1 |
|
Total |
6,323 |
100.0 |
Note a: Percentages do not add to 100 due to rounding.
Source: Department of Health and Aged Care.
4.6 The processing of PBS claims relies on the successful coordination of functions undertaken by both Health and Services Australia, such as the timely provision of changes to the Schedule of Pharmaceutical Benefits (the Schedule), the approval of PBS suppliers, and the financial reconciliation of PBS payments.
PBS claims processing system
4.7 Health publishes an updated Schedule on the first day of each month. The Schedule compiles all the legislative changes resulting from recommendations from the Pharmaceutical Benefits Advisory Committee (PBAC), statutory changes to PBS medicine prices, and the indexation of relevant fees.
4.8 Almost all claims (99.9 per cent) made by PBS suppliers are submitted through Services Australia’s Online Claiming for PBS system (CPS). The incorporation of the updated Schedule into the CPS supports correct and accurate processing of PBS claims. Manual claims can also be made by providing paper prescriptions and claim paperwork to Services Australia, but such claims are rare and incur a fee to be paid to Services Australia.95
4.9 The CPS automatically assesses claims against 18 sets of rules, underpinned by the requirements set out in the National Health (Supply of Pharmaceutical Benefits–Under Co-payment Data and Claims for Payment) Rules 2022. Claims that do not satisfy all rules are assigned reason codes that signify the reasons for the assessment outcome. Reason codes are categorised as information, warning, time-based warning, or rejection codes according to the impact on the assessment outcome (see Table 4.2). A rejection-type reason code signals that the claim is not payable.
Table 4.2: Overview of reason codes for the assessment of PBS claims
|
Reason code type |
Description |
Assessment outcome |
Number of unique codes |
|
R |
Rejection — Claim has been rejected for payment due to an issue with the prescription. PBS supplier can correct the error and resubmit the claim. |
Claim not payable |
364 |
|
W |
Warning — Issue has been identified with the prescription. PBS supplier must address any warning displayed and be satisfied that claim is correct. |
Claim payable |
51 |
|
I |
Information — PBS supplier should take note of additional information in relation to the prescription claimed. |
Claim payable |
30 |
|
X |
Time-based warning — Warning for a set time that varies depending on the scenario. |
Claim payable, until such time as a rejection code is returneda |
17 |
|
Total |
462 |
||
Note a: Time-based warnings do not change to a rejection if the issue is not addressed. However, if the issue is not addressed and a new claim is submitted with the same details, after the set time period, a rejection reason code will return.
Source: ANAO analysis of internal documents from Services Australia; Services Australia, Manage PBS reason and rejection codes, available from https://www.servicesaustralia.gov.au/manage-pbs-reason-and-rejection-codes?context=20 [accessed 20 June 2024].
4.10 To make a claim for payment, PBS suppliers provide prescription and patient details to Services Australia and receive an assessment result advising of whether the prescription is approved for payment before supplying the PBS medicine to a patient. Once the payment is approved by Services Australia, PBS suppliers supply the PBS medicine to the patient.
4.11 If a claim is assessed as payable, the CPS initiates a request for payment in a separate system, which provides the PBS supplier’s banking details and details of the payment request to the Reserve Bank of Australia (RBA). The RBA makes the payment directly into the PBS supplier’s bank account, generally on the same day the request for payment is sent to the RBA. The process for approving payments to PBS suppliers is outlined in Figure 4.1.96
Figure 4.1: Process for approving payments to PBS suppliers
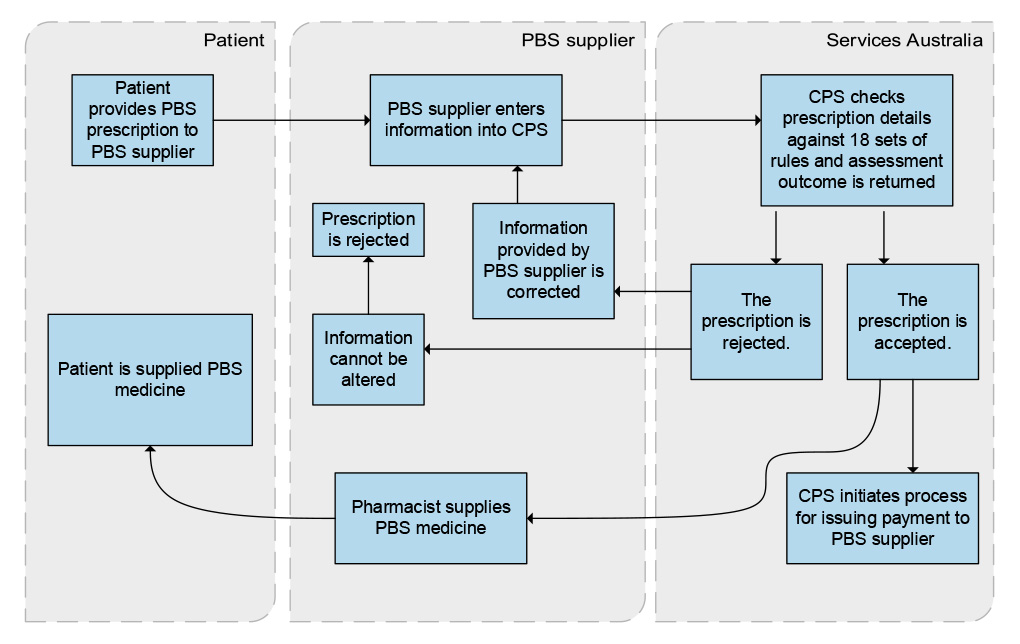
Source: ANAO analysis of Services Australia internal documents.
4.12 To finalise a claim, a PBS supplier must complete a statement to certify that the supply of PBS medication has been made in accordance with legal requirements. This certification is usually done by PBS suppliers after the PBS medicine has been dispensed.
4.13 Payments issued to PBS suppliers in advance of finalising a claim are allowable under section 99AB of the NHA. PBS suppliers can amend or cancel their claim after the payment is made. Services Australia undertakes a reconciliation of payments to PBS suppliers and amends payment amounts according to any changes made by the PBS supplier as part of certifying the claim.
Uncertified claims
4.14 The ANAO’s 2010 performance audit of the administration of the PBS found that in August 2008 there were 47 PBS suppliers with over $6 million in ‘outstanding claims’ (defined in the audit report as claims not certified within 35 days).97
4.15 Services Australia advised the ANAO in July 2024 that PBS suppliers are given 90 days to make changes to claims for which they have already received an advance payment. There is no legislative basis for providing PBS suppliers 90 days to amend their claims.
4.16 Health provided the ANAO with internal advice from August 2024 that states that section 6 of the National Health (Supply of Pharmaceutical Benefits–Under Co-payment Data and Claims for Payment) Rules 2022 provides for PBS suppliers to certify claims within a period not exceeding 65 days.98 Subsequent advice obtained in October 2024 stated that uncertified claims could potentially be recovered as debts to the Commonwealth if it was found that suppliers were not entitled to amounts that had been paid under s99AB of the NHA.
4.17 Services Australia advised the ANAO in July 2024 that as of 30 June 2024 there were 3,813 PBS suppliers with $1.514 billion in claims not certified within 65 days (see Table 4.3). Until these claims have been processed and determined by Services Australia on behalf of Health, the potential debts arising from uncertified claims, and their value, are unknown.
Table 4.3: Summary of PBS claims not certified within 65 days, as at 30 June 2024a
|
Description |
Number/value |
|
Number of claims not certified within 65 days |
15,023 |
|
Total number of prescriptions in claims not certified within 65 days |
34.0 million |
|
Total value of claims not certified within 65 days |
$1.514 billion |
|
Number of PBS suppliers with claims not certified within 65 days |
3,587 |
|
Number of PBS suppliers with claim not certified for over 2 years |
2,414 |
|
Highest value of claims not certified within 65 days from a single PBS supplier |
$130 million |
Note a: Data for all PBS suppliers are included, including those whose approval has been revoked.
Source: Services Australia.
4.18 The secretary of Health is responsible for the recovery of debts from PBS suppliers, as well as for broader health provider compliance. Uncertified claims are not addressed in risk documentation for the PBS including in Health and Services Australia’s Joint PBS Risk Management Plan 2023-2024 (see Appendix 5). Services Australia provides Health with a monthly report on PBS ‘outstanding’ claims in accordance with the reporting requirements set out under the PBS Program Agreement.
Recommendation no.5
4.19 The Department of Health and Aged Care and Services Australia document and implement a strategy for addressing the backlog of uncertified Pharmaceutical Benefits Scheme claims.
Department of Health and Aged Care response: Agreed.
4.20 The Department of Health and Aged Care is working with Services Australia to develop, document and implement a strategy and workplan for addressing the backlog of uncertified Pharmaceutical Benefits Scheme claims at the earliest opportunity.
Services Australia response: Agreed.
4.21 Services Australia will work together with Department of Health and Aged Care to document and implement a strategy for addressing the backlog of uncertified Pharmaceutical Benefits Scheme claims.
Payment integrity
4.22 Services Australia undertakes post-payment assurance tests across 16 categories of PBS claims as part of its Administered Assurance Framework, developed to provide the Chief Financial Officer with assurance that payments on behalf of partner agencies are accurate for inclusion in the financial statements. Each post-payment assurance test involves validating the details for a random sample of 50 claims and re-performing calculations for manual payments. Details validated include the payment amount, relevant dates and approvals, and patient eligibility. A description of each payment category and procedures for testing is at Appendix 9.
4.23 Services Australia advised the ANAO in August 2023 that testing can occur quarterly, monthly or annually depending on the payment category. Post-payment assurance testing for 2021–22 to 2023–24 is summarised at Table 4.4.
Table 4.4: Post-payment assurance testing for PBS payments
|
Payment category |
2021–22 |
2022–23 |
2023–24 |
|
Community pharmacy — PBS general Schedule items |
✔ |
✔ |
✘ |
|
Remote Area Aboriginal Health Services |
✔ |
✔ |
✘ |
|
Stoma, ostomya and paraplegic and quadriplegic supplies |
✔ |
✔ |
✘ |
|
Dental PBS items |
✔ |
✘ |
✘ |
|
Payments during system outages |
N/Ab |
✔ |
N/Ab |
|
Chemotherapy |
✔ |
✘ |
✘ |
|
Hospital authorities |
✔ |
✔ |
✘ |
|
Prescriber bag supplies |
✔ |
✔ |
✘ |
|
Highly specialised drugs, botulinum toxin, in-vitro fertilisation, human growth hormone |
✔ |
✘ |
✘ |
|
Optometrists |
✔ |
✔ |
✘ |
|
Nusinersen |
✔ |
✔ |
✔ |
|
Onasemnogene abeparvovec (Zolgensma) |
✔ |
✔ |
✔ |
|
Extemporaneously prepared medicines |
✔ |
✘ |
✘ |
|
Standard formula preparations |
✔ |
✘ |
✘ |
|
Safety Net card claim |
✔ |
✘ |
✘ |
|
Patient refunds |
✔ |
✘ |
✘ |
|
Total number of categories tested |
15 |
9 |
2 |
Key: ✔ Testing performed ✘ No testing performed N/A Not applicable
Note a: Claims for stoma and ostomy items are not within the scope of the PBS Schedule.
Note b: There were no system outages that met the threshold for post-payment assurance testing.
Source: ANAO analysis of Services Australia internal documents.
4.24 Each PBS payment category has been tested at least once over the past three years. The total number of categories with post-payment assurance tests has declined since 2021–22 (see Table 4.4).
4.25 In its 2022–23 Annual Performance Statements Services Australia reported against ‘Strategic Performance Measure 3 — Administrative correctness of payments’. The target for this measure was the achievement of a correctness rate of greater than 98 per cent. The administrative correctness of PBS claims is not measured and does not contribute to performance reported for Strategic Performance Measure 3.99
4.26 The PBS Program Agreement between Health and Services Australia includes a performance measure for PBS claims payment integrity, to be reported annually: ‘The percentage of PBS claims that have been processed accurately’ (see PBS 10 in Table 2.8 and Appendix 4). The target for this measure is the achievement of a correctness rate of greater than 98 per cent. Services Australia has not reported to Health on the performance measure and does not track it internally. Health has not sought reporting on this measure from Services Australia (see paragraph 2.52).
Payment timeliness
4.27 The PBS Program Agreement between Health and Services Australia contains five performance measures relating to the timeliness of manual and automatic payments for PBS claims (see PBS 2, PBS 3, PBS 7, PBS 8 and PBS 9 in Table 2.8 and Appendix 4). Services Australia’s reporting to Health on payment timeliness indicated that targets were regularly met.
4.28 Services Australia’s reporting against ‘Strategic Performance Measure 5 — Work processed within timeliness standards’ does not include claims for the PBS.100
Recommendation no.6
4.29 Services Australia report to the Department of Health and Aged Care on payment accuracy for the Pharmaceutical Benefits Scheme (PBS) in accordance with the PBS Program Agreement, and separately report on the integrity and timeliness of PBS payments in its Annual Performance Statements.
Services Australia response: Agreed.
4.30 Services Australia will work with Department of Health and Aged Care to report on payment accuracy for the Pharmaceutical Benefit Scheme (PBS) in accordance with the PBS Program Agreement. Services Australia is also undertaking a review and expansion of work types to include PBS integrity and timeliness in its Annual Performance Statements for 2024–25.
Have effective systems and processes been established to manage authority-required approvals?
A system to manage authority-required approvals has been established that is consistent with Health and Services Australia’s respective responsibilities under the PBS bilateral agreement. There are differences in approval rates depending on the method used by an applicant to apply for an authority. Reported results for the timeliness of authority approvals against performance measures set out in bilateral arrangements have largely not met targets. Services Australia reports in its Annual Performance Statement on the achievement of a performance measure target of answering authority calls within 15 minutes. This does not align with the target of answering authority calls, on average, in less than 30 seconds.
PBS authority-required medicines
Process overview
4.31 Of the 81 per cent of medicines listed on the Schedule with prescribing restrictions, 32 per cent require prescribers to apply for and obtain authority from Services Australia before a PBS prescription can be issued (see Table 4.5). As noted at paragraphs 3.11 to 3.13, the requirement to obtain authority is designed to manage the safety and cost-effectiveness of PBS medicines by controlling the instances in which medicines may be used according to PBAC recommendations.
Table 4.5: Restriction levels for PBS medicines
|
PBS restriction level |
Description |
Percentage of PBS Schedule as at June 2024a (%) |
|
Unrestricted |
Can be prescribed for any purpose without prior approval and be eligible for PBS subsidies. |
18.8 |
|
Restricted |
Can be prescribed only for specific purposes to be eligible for PBS subsidies, though no check or control is applied at the time of prescription or dispensing. |
19.9 |
|
Authority-required (streamlined) |
Can be prescribed only for specific reasons defined in the Schedule. An authority code recorded against the item in the Schedule must be included on the prescription for checking at the time of dispensing. |
31.9 |
|
Authority-required |
Can be prescribed only with an approval from Services Australia. Applications for approval can be made by phone, in writing, or online. |
29.4 |
Note a: Calculated by ANAO using Services Australia data.
Source: ANAO analysis of Department of Health and Aged Care publications and Services Australia PBS processing data.
Authority-required arrangements
4.32 When a prescriber wishes to prescribe an authority-required PBS medicine, they must apply to Services Australia. The application process requires prescribers to confirm that the patient meets the eligibility conditions for the PBS medicine as required under the Schedule (an example is provided in Case study 2). If approved, Services Australia provides prescribers an authority code that relates to the prescribed medicine and clinical condition to include on the prescription.
|
Case study 2. Onasemnogene abeparvovec prescribing requirements |
|
The Schedule requires that the medication onasemnogene abeparvovec is prescribed — among other conditions — only to a patient who has not previously been treated with the medications nusinersen or risdiplam for the same indication (spinal muscular atrophy). This condition is presented as a question to be answered either in the affirmative or negative by the prescriber, and where the prescriber answers ‘no’, the authority will not be provided for them to prescribe the item. |
Authority-required (streamlined) arrangements
4.33 For certain PBS medicines, the requirement to obtain authority is subject to a streamlined process. This process requires prescribers to look up the authority code that relates to the prescribed medicine and clinical condition in the Schedule and include the code on the prescription. This streamlined process was developed in conjunction with the Australian Medical Association with the aim of reducing the administrative burden for prescribers to provide them with more time to devote to patient care without compromising the integrity of the authority system. The process for PBAC’s consideration of restrictions on the prescription of medicines is outlined at paragraphs 3.11 to 3.13.
System
4.34 Services Australia’s system for processing PBS claims checks that a valid authority approval code is included on a prescription; if the authority code aligns with the requirements of the Schedule, the PBS claim is approved and the medicine is dispensed (see Figure 4.2).
Figure 4.2: PBS prescribing flowchart
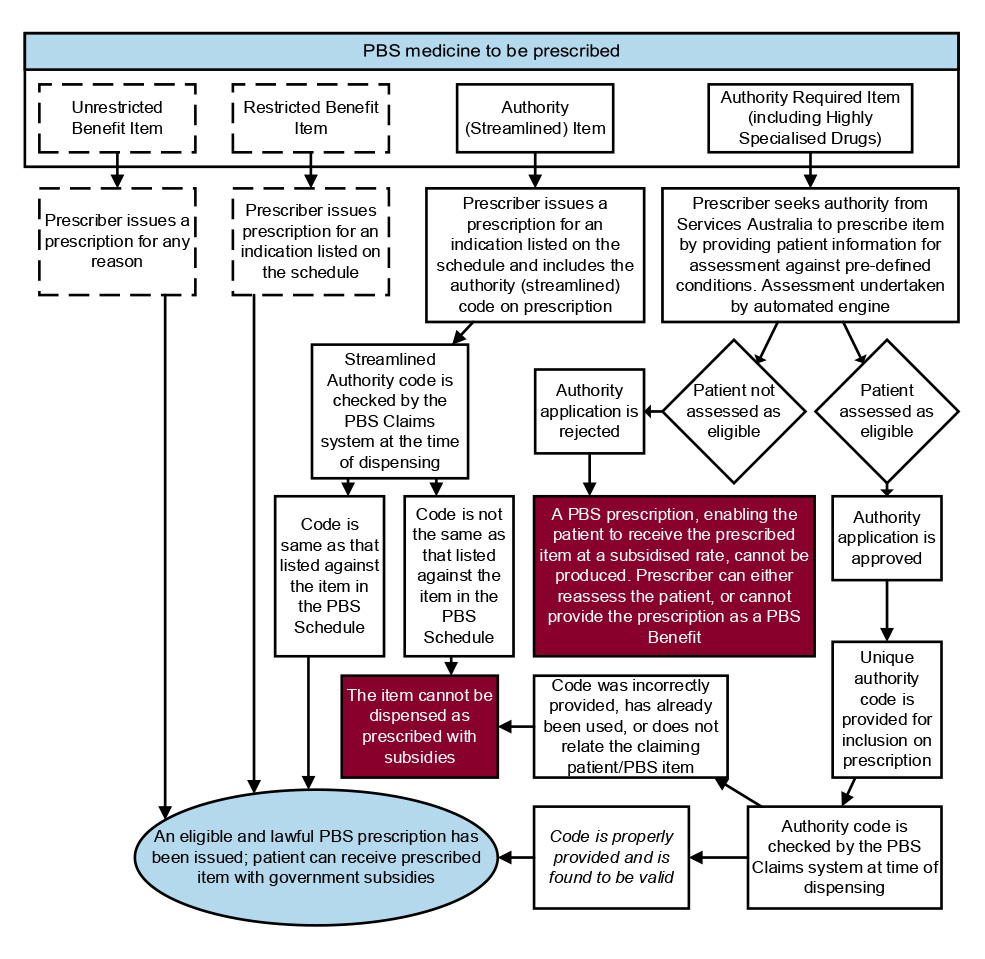
Source: ANAO analysis of Services Australia internal documents.
4.35 Services Australia uses the Online PBS Authorities (OPA) system to process authority applications made by PBS prescribers. This system automatically assesses requests for authority codes by checking the information provided by prescribers, in the form of answers to medicine-specific questions, against the requirements in the Schedule. Applications for an authority code can be made via an internet portal, phone, a paper-based form, or third-party clinical software that is configured to communicate with OPA (see Table 4.6).
Table 4.6: PBS authorities application channels
|
Channel |
Channel description |
Average number of applications per month in 2023–24 |
Average proportion of applications per month in 2023–24 (%) |
|
Services Australia-administered Health Professional Online Services portal (HPOS) |
PBS prescriber uses HPOS to access OPA and apply for an authority approval. |
204,976 |
34.7 |
|
Third-party clinical software |
PBS prescriber uses third-party software to access OPA, allowing them to apply for an authority approval. |
560 |
0.1 |
|
PBS Authorities telephony line |
PBS prescriber talks to a Services Australia officer, who uses OPA to assess the authority approval request. |
355,017 |
60.1 |
|
Written applications |
PBS prescriber writes to Services Australia requesting authority approval. Services Australia officer uses OPA to assess the authority approval request. |
30,092 |
5.1 |
Source: ANAO analysis of Services Australia records and data.
4.36 ANAO testing of OPA found that Schedule requirements were properly reflected in the system.
4.37 Analysis of data from July 2022 to December 2023 shows the majority of applications were made via phone (see Table 4.7). Over that period, the proportion of applications made via HPOS increased from 22.0 per cent in quarter 1 2022–23 to 40.5 per cent in quarter 4 2023–24, and the proportion of applications made via third-party prescriber software remained below 0.5 per cent. During 2023–24, there were increasing wait times for the authorities telephony line (see Appendix 4, Figure A.1).
Table 4.7: Authority applications by channela, by quarter, 2022–23 and 2023–24
|
|
Phone |
Written |
Prescriber software |
HPOS |
Total |
|
Q1 2022–23; total and proportion |
1,231,601 70.4% |
127,993 7.3% |
5,976 0.3% |
384,200 22.0% |
1,749,770 100.0% |
|
Q2 2022–23; total and proportion |
1,228,469 71.9% |
127,130 7.4% |
4,419 0.3% |
348,953 20.4% |
1,708,971 100.0% |
|
Q3 2022–23; total and proportion |
1,203,362 68.4% |
104,196 5.9% |
1,630 0.1% |
450,607 25.6% |
1,759,795 100.0% |
|
Q4 2022–23; total and proportion |
1,177,420 67.6% |
98,186 5.6% |
1,727 0.1% |
465,814 26.7% |
1,743,147 100.0% |
|
Q1 2023–24; total and proportion |
1,159,482 65.7% |
97,181 5.5% |
1,750 0.1% |
506,378 28.7% |
1,764,791 100.0% |
|
Q2 2023–24; total and proportion |
1,107,750 62.5% |
90,324 5.1% |
1,580 0.1% |
573,243 32.3% |
1,772,897 100.0% |
|
Q3 2023–24; total and proportion |
1,021,667 57.8% |
86,474 4.9% |
1,652 0.1% |
658,515 37.2% |
1,768,308 100.0% |
|
Q4 2023–24; total and proportion |
971,309 54.5% |
87,123 4.9% |
1,737 0.1% |
721,570 40.5% |
1,781,739 100.0% |
Note a: The analysis excludes applications that had not been processed to completion.
Source: ANAO analysis of Services Australia data.
4.38 The authority code received by a prescriber, and included on a prescription, is checked at the time of item dispensing by Services Australia’s claims processing engine (CPS, discussed at paragraph 4.7), which ensures that:
- the date of supply is equal to or after the lodgement date of the authority application;
- the dosage and repeats listed on the prescription are valid;
- the authority code is legitimate and was issued by Services Australia; and
- the code was generated and issued in line with Schedule requirements for the medicine.
4.39 Public contributions to the audit from individuals and organisations included commentary about the PBS authorities system, including that:
- there is little consultation with PBS prescribers and suppliers on policy changes regarding PBS authorities, nor with new or altered Schedule listings;
- multiple authority codes for single medicines creates confusion and administrative burdens for prescribers, and the authorities system and policy, generally, creates an administrative burden for practitioners by reducing contact time with patients and creating needs for specialist software; and
- the HPOS system is not user friendly, especially when access is needed frequently, and there is poor integration of OPA with otherwise necessary prescribing software.
Quality assurance
4.40 Services Australia undertakes internal monitoring of authority applications for reporting to executive management and to inform the targeting of support and training for Services Australia staff. This is done through a process of quality checking, which is reported through a monthly dashboard. The quality checking process involves checking approvals for online and written authority prescriptions against Services Australia’s Enterprise Quality Framework (a principles-based framework for setting a consistent approach to quality across Services Australia). The sampling plan for checking authority approvals requires that 0.5 per cent of the monthly total of authority approvals are randomly selected for quality checking. From this work, individual staff members can be identified for targeted training and treatment of errors.
4.41 In addition to the quality checking process, authority approvals are checked as part of post-payment assurance testing of the alignment of PBS claims against Schedule requirements (refer to paragraph 4.22 and Appendix 9). All authority applications, regardless of whether they are submitted via HPOS, a phone call, or in writing, are based on prescribers’ answers to the same set of questions reflecting the requirements in the Schedule.
4.42 Authority approvals data shows that the rejection rate for HPOS-based applications is more than double the rate for phone-based applications (see Table 4.8).
Table 4.8: Rejection rate of authority applications by phone and HPOS, 2019–20 to 2023–24
|
Application channel |
Applications — total |
Applications — rejected |
Rejection rate (%) |
|
Phone |
12,118,255 |
278,844 |
2.30 |
|
HPOS |
4,807,859 |
269,223 |
5.60 |
Source: ANAO analysis of Services Australia data.
4.43 The same processing engine is used to assess authority applications regardless of the medium by which an application is made. Box 3 illustrates how a factor could be unique to phone-based applications and therefore create the observed differences in rejection rates.
|
Box 3: An example of a factor unique to phone-based applications |
|
Services Australia’s internal guidance documentation states that PBS authority phone line operators can, ‘due to the sensitive nature of some diseases or conditions’, read a medicine-specific restriction criteria to the approved prescriber to elicit a response confirming or denying their patient’s applicability against a Schedule-based requirement. This guidance does not define which PBS medicines fall into this permissible category nor what sensitive aspects of conditions may warrant such action. |
4.44 Services Australia advised the ANAO in July 2024:
Services Australia acknowledges that there is a variance in rejections rates dependent on the channel. Nonetheless, rejection rates for both telephone and online channels are considered low when compared to total volumes (2.3 percent and 5.6% rejection rates for each channel).
Feedback from PBS prescribers and Service Australia operators indicates the following:
- when a PBS prescriber uses the PBS Authorities telephone line to request an authority approval, a service officer requests information from the prescriber to answer eligibility questions. The service officer may pick up where errors will result in a rejection before the point of submitting the request. The prescriber has the opportunity to reconsider prescription details during that call to meet requirements. This will not be reflected as a rejection for this prescribing instance.
- Services Australia staff have access to additional resources to assist the prescriber to select the correct PBS item code. Many medicines have a number of different listings under different item codes. It can be challenging for prescribers to identify the correct PBS item code to select when self-serving via HPOS.
- when a prescriber uses the self service option, the prescriber may enter details that results in the patient not meeting eligibility for PBS subsidy and receive a rejected assessment result.
Services Australia has recently released new functionality to the Online PBS Authorities (OPA) System. One of the new features is a dynamic questions and answer system (DQA) that will improve the way the eligibility questions are presented to the PBS prescriber. As this DQA system is rolled out for new and amended PBS listings, data may reflect a reduction in rejections using the OPA system over time.
Opportunity for improvement
4.45 There is an opportunity for Services Australia to undertake further investigation of why rejection rates for authority applications differ depending on whether an applicant applies via the Health Professional Online Services portal or over the phone.
Timeliness
4.46 Services Australia provides monthly reporting to Health on nine bilateral performance measures in its PBS Program Agreement with Health (see Table 2.8). Two measures relate to the timeliness of PBS authorities, specifically those processed via the phone (PBS 1) and in writing (PBS 6).101 Reported results for these measures from August 2022 to June 2024 (see Appendix 4) show that bilateral targets were not met for all months for PBS 1 (23 of 23 months) and most months for PBS 6 (13 or 23 months).
4.47 When counting the number of phone calls received and their duration, Services Australia’s telephone management system recognises a call as completed when it has been transferred between internal menus. This means that a single phone call from the perspective of a caller may be counted as two or more separate phone calls for performance monitoring purposes, each with a separate answer time. The PBS 1 measure does not account for this.
4.48 Services Australia’s reporting in its Annual Performance Statement against ‘Strategic Performance Measure 5 — Work processed within timeliness standards’ does not include authority approvals for the PBS.102
4.49 Services Australia’s external reporting against ‘Strategic Performance Measure 4 — Customers served within 15 minutes’ incorporates PBS authority approvals provided by phone. The target of 15 minutes was reported as met for authority approvals in 2021–21 and 2022–23. The target for this measure does not align with the target agreed in bilateral arrangements for PBS 1 of answering authority calls, on average, in less than 30 seconds.
4.50 The timeliness of authority approvals impacts doctors and other PBS prescribers who need to seek authority approvals, often during a consultation with a patient. Submissions to the audit stated there have been delays and extended wait times for obtaining authority approval from Services Australia.
Recommendation no.7
4.51 Services Australia align its reporting on the timeliness of issuing authority approvals in its Annual Performance Statement with performance measures and targets agreed in bilateral arrangements.
Services Australia response: Not agreed.
4.52 Services Australia has a range of tiered performance measures.
4.53 The 7 Strategic Performance Measures form Tier 1 of these performance measures. Bilateral partner agency reporting commitments form Tier 2 and Tier 3 measures.
4.54 Strategic Performance Measure 4 is customers served within 15 minutes and includes telephony and face to face services queue data. The telephony queue data includes answering authority calls. Aligning this measure with targets agreed in bilateral arrangements is not possible as the measure includes customers served across all Services Australia’s programs.
Have effective systems and processes been established to manage the PBS Safety Net and patient refunds?
Services Australia has established processes and systems to manage PBS Safety Net and patient refunds. Both systems are reliant on paper-based application forms which are submitted by post and manually processed by Services Australia. The reliance on manual processing means that performance is sensitive to staffing numbers, which has meant timeliness performance measures have not been consistently met. Services Australia’s quality checking process for Safety Net claims does not provide accurate data on the reasons for rejecting Safety Net card applications to inform education or compliance activities.
PBS Safety Net
Process overview
4.55 The PBS Safety Net (discussed at paragraphs 3.75 to 3.79 and 3.89 to 3.95) is managed using a system which places responsibility on patients to record evidence of eligibility and apply for Safety Net cards. Patients and PBS suppliers (usually pharmacists) use a hardcopy Services Australia prescription record form (PRF) or pharmacy dispensing software PRF to record the dispensing of PBS medicines throughout the calendar year for the patient and their family (if applicable) (see Figure 4.3).
Figure 4.3: Extract of the prescription record form (PB240)
Note: The prescription record form is part of the application form for a Safety Net card – PB240.
Source: Services Australia, available from https://www.servicesaustralia.gov.au/pb 240 [accessed 30 August 2024]
4.56 PBS suppliers are required to record the dispensing of a PBS medicine on a PRF when it is provided to them; they are not required, though are permitted, to notify patients when their PRF demonstrates expenditure above the Safety Net threshold.
4.57 When the Safety Net threshold has been met for an individual or their family, a PBS supplier can issue a Safety Net card to the patient so they can access higher subsidies on future medicine purchases. Each transaction for a PBS medicine that has counted towards the Safety Net must be recorded on a PRF and, when the card is issued to a family unit, the PBS supplier needs to check that the family is eligible.
4.58 After issuing one or more Safety Net cards, a PBS supplier submits a claim to Services Australia for dispensing these cards using the ‘PBS Safety Net claim for payment form (PB241)’ for up to 12 cards. The claim form needs to be accompanied by the corresponding PRF and application forms as evidence of patient eligibility. As at 1 January 2024, PBS suppliers are entitled to a card issuance payment of $12.04 from Services Australia for each Safety Net card they have issued.
System
4.59 PBS suppliers need to submit claim forms and supporting evidence via post to Services Australia for assessment. Services Australia assesses claims using a dedicated Safety Net Claims System. This assessment validates patient eligibility for the Safety Net card, as issued by PBS suppliers, and triggers payments to suppliers for validly issuing cards. Figure 4.4 depicts a process map of the card issuance and claims process undertaken by patients, PBS suppliers, and Services Australia. Services Australia advised the Australian Government in April 2023 that processing 150,000 Safety Net card claims requires the manual scanning and processing of 3.8 million pieces of paper.
4.60 The system is more difficult to administer for patients and PBS suppliers when a patient purchases medicines from multiple PBS suppliers, creating complexity in tracking eligible spending. When patients consistently purchase medicines from one PBS supplier, it is easier to track their spending. As discussed in paragraph 3.95, Health estimated that in 2021 640,000 patients were eligible for the Safety Net but did not apply, forgoing a potential reduction of $100 million in patient out-of-pocket costs.
Figure 4.4: PBS Safety Net card issuance and claims process
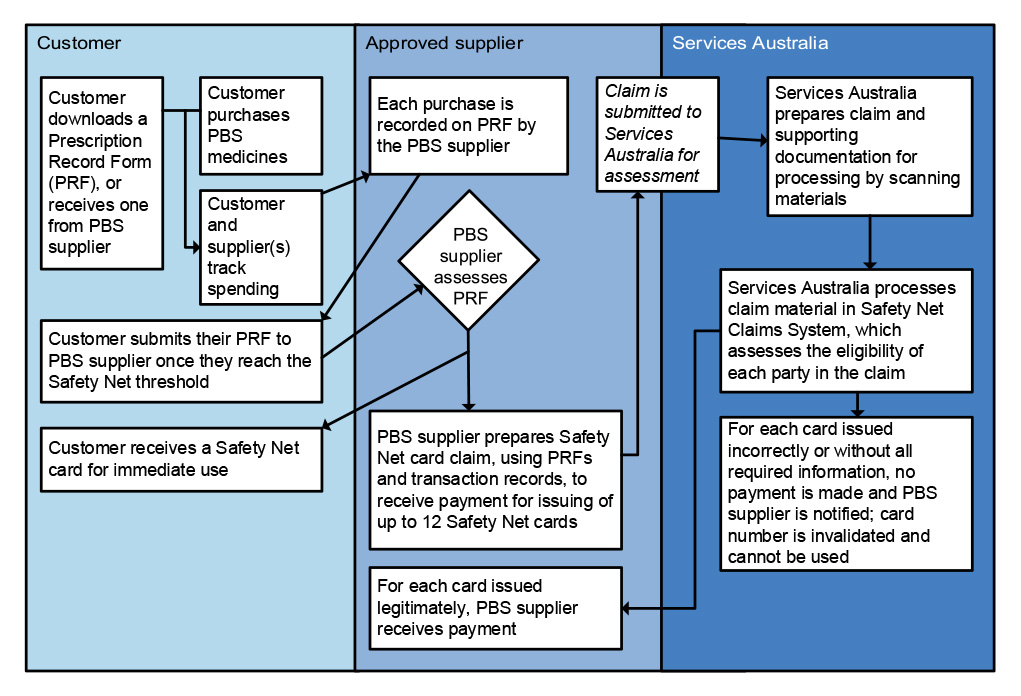
Source: ANAO analysis.
4.61 Services Australia does not conduct eligibility checks prior to a Safety Net card being issued, as cards are issued by a PBS supplier who has determined the patient, based on the PRF, is eligible. Services Australia validates the PBS suppliers’ assessment of eligibility post-issuance. Services Australia officers undertake a series of checks for each card which is part of a claim provided by a PBS supplier, including checks to:
- confirm the family structure of the card holder meets the legislative definitions of a family unit relevant to the PBS Safety Net program (if applicable);
- calculate spending against the Safety Net threshold for the individual or family; and
- determine when the Safety Net threshold was met by the individual or family.
These checks were previously done by manual interrogation of Services Australia systems; they are now undertaken through the Safety Net Claims System.
4.62 A PBS supplier will not receive payment for a card which is found to be invalid or when the claim lacks supporting evidence, and this card will also be invalidated and unusable by the patient. As part of processing a Safety Net card claim, Services Australia officers record reasons for not paying claims made by PBS suppliers. As shown in Figure 4.5, two reason codes for not paying a claim (‘patient did not sign or date form’ and ‘card already claimed’) have comprised the majority of reasons codes recorded since May 2023.
Figure 4.5: ‘Do Not Pay’ reasons for PBS Safety Net card claims, July 2022 to June 2024
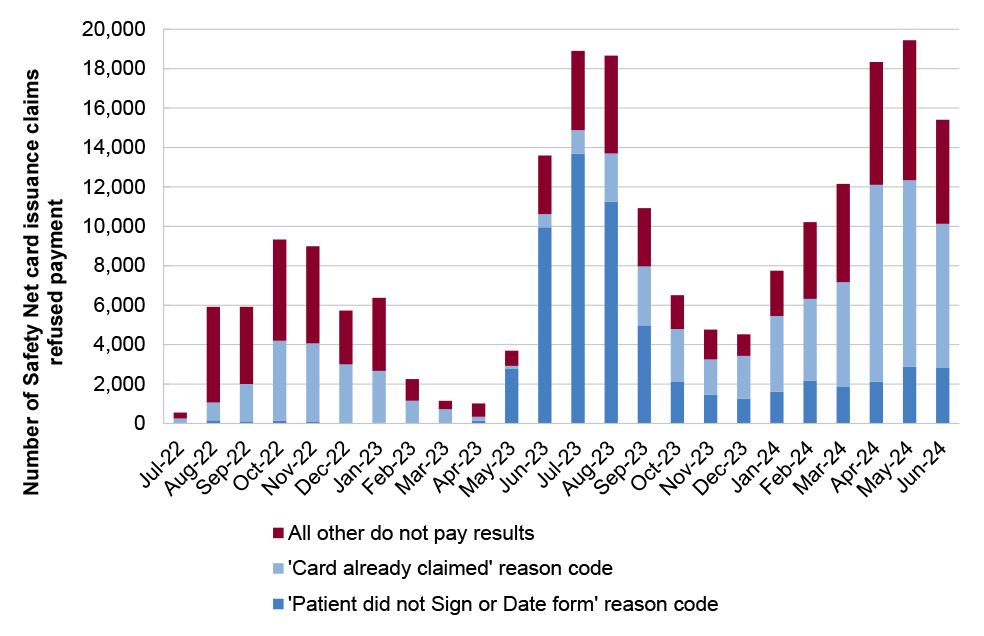
Source: ANAO analysis of Services Australia data.
4.63 Between April and July 2023, there was an increase in the number of Safety Net card claims that were rejected on the basis of the ‘patient did not sign or date form’ reason code. Services Australia advised the ANAO in July 2024 that this error code is used for when either a patient or PBS supplier incorrectly signed the application form for a Safety Net card. This means that it is not possible to determine from the data whether the increased prevalence of rejected applications during this period was due to issues with patient signatures in Safety Net application forms or issues with signatures from PBS suppliers.
4.64 Special exemptions were established during the COVID-19 pandemic which temporarily changed signature requirements for Safety Net application forms. Between 8 April 2020 and 31 December 2020, the requirement for patients, or agents acting on their behalf, to sign the Safety Net application form was removed. Between 25 September 2020 and 31 March 2023, the requirement for PBS suppliers to sign the PRF form was also removed.
4.65 Services Australia does not keep sufficient data on the reasons for rejecting Safety Net card applications to inform education or compliance activities related to applications for PBS Safety Net cards. Services Australia advised the ANAO in July 2024 that it was undertaking a review of the ‘patient did not sign or date form’ reason code.
Quality assurance
4.66 Services Australia reviews the quality of Safety Net claims as part of a quality checking process undertaken under its Enterprise Quality Framework. Services Australia’s checking process involves selecting a random sample of 0.5 per cent of the monthly total of Safety Net claims and 2 per cent of the monthly total of Safety Net card applications made by PBS suppliers. Claims are reviewed to identify errors in information processed or keyed that does not match the claim documentation. Services Australia uses these checks for reporting to executive management and to identify staff training and error treatments.
4.67 As shown in Figure 4.6, between July 2020 and June 2024 an average of 19 per cent of Safety Net activities checked through quality assurance processes had processing errors.
Figure 4.6: Services Australia quality checks on Safety Net activities
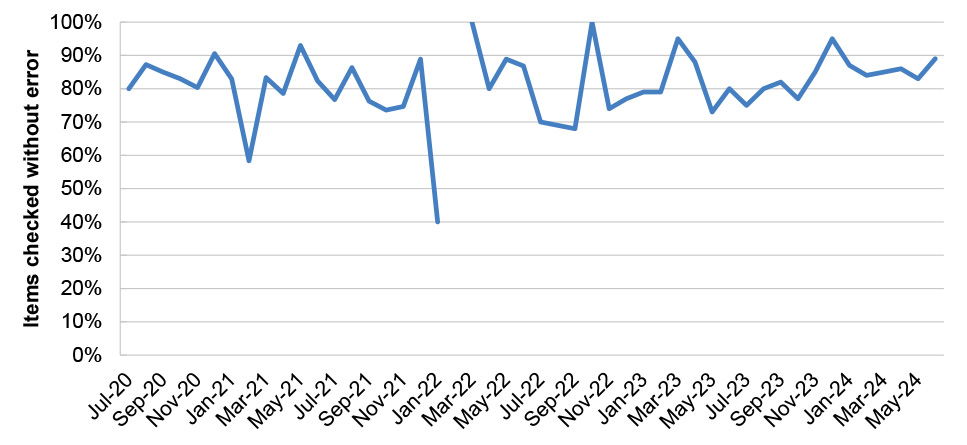
Note: No checks were conducted in February 2022.
Source: ANAO analysis of Services Australia internal quality assurance dashboards.
4.68 Services Australia is progressing a project to digitise the Safety Net claim for pharmacists.103 The proposed system upgrades would allow pharmacists to submit claims for Safety Net cards through HPOS and receive an immediate claim assessment result. The PBS Safety Net system would conduct eligibility assessment, calculate if the PBS Safety Net threshold has been reached, record the PBS Safety Net card number against the patient, and pay the card issuing fee to the pharmacist. This project is expected to be implemented by 1 January 2025.
4.69 The Safety Net digitisation project does not extend to automatically applying the Safety Net to eligible patients. As noted at paragraph 3.93, a 2010 ANAO audit of Medicare Australia’s administration of the PBS recommended that ‘Medicare Australia and DoHA [Health] examine how the PBS system and data capture arrangements could be enhanced to enable patients to be advised when [they] have reached the PBS Safety Net Threshold, and advise government on options.’
Timeliness
4.70 Services Australia provides monthly reporting to Health on the proportion of Safety Net claims processed and released for payment within 60 calendar days of submission (for the PBS 4 bilateral measure in its PBS Program Agreement with Health). The target for this measure is 82 per cent or higher. Services Australia reported to Health that this target was met from August 2022 to August 2023 and not met from September 2023 to May 2024 (see Appendix 4, Figure A.4). Services Australia reported to Health that its failure to meet targets between September 2023 and May 2024 was due to the redeployment of staff to other priority tasks within the agency. Services Australia recruited additional staff to assist with reducing various backlogs across the agency (refer to paragraph 2.51). The PBS 4 target was met in June 2024.
PBS patient refunds
Process overview
4.71 Patients may be eligible to claim a refund from Services Australia in circumstances where the patient has not received the full PBS benefits to which they are entitled (see Figure 4.7). To receive a patient refund, patients must demonstrate that they were eligible for a level of subsidy which they did not receive at the time of dispensing, either by failing to produce their Medicare or concession card at the time of item dispensing, not providing their Safety Net card, or receiving their Safety Net card after they were eligible for it.
- When claiming a refund on the basis that the patient had failed to produce their Medicare or concession card at the time of dispensing, the patient provides their Medicare number and Safety Net, Department of Veterans’ Affairs or concession card number in a standardised form, as well as pharmacist-verified purchase record documents which show how much was spent by the patient. The guidance, published on the PBS website, states that ‘responsibility for claiming entitlements rests with the patient.’
- When claiming a refund on the basis that a patient was entitled to but did not receive Safety Net subsidies, the patient and any relevant pharmacists manually record spending that was previously undertaken on purchases of PBS medicines on the PRF (see paragraph 4.55). This form is completed and submitted to Services Australia at the time or after a pharmacist issues the Safety Net card to the patient. The verification which Services Australia undertakes is retrospective, as the Safety Net card is active as soon as it is issued to the patient.
4.72 Once Medicare and/or concession status, and/or Safety Net status, has been validated by Services Australia, then the patient can request a refund for any overpayment of previously purchased PBS medicines. For both types of refunds, Services Australia’s key controls are manual verification that a patient claiming a refund was eligible for some form of PBS-related subsidy at the time of dispensing, and that they paid for a medicine at a level above their concession status.
Figure 4.7: PBS patient refund process map
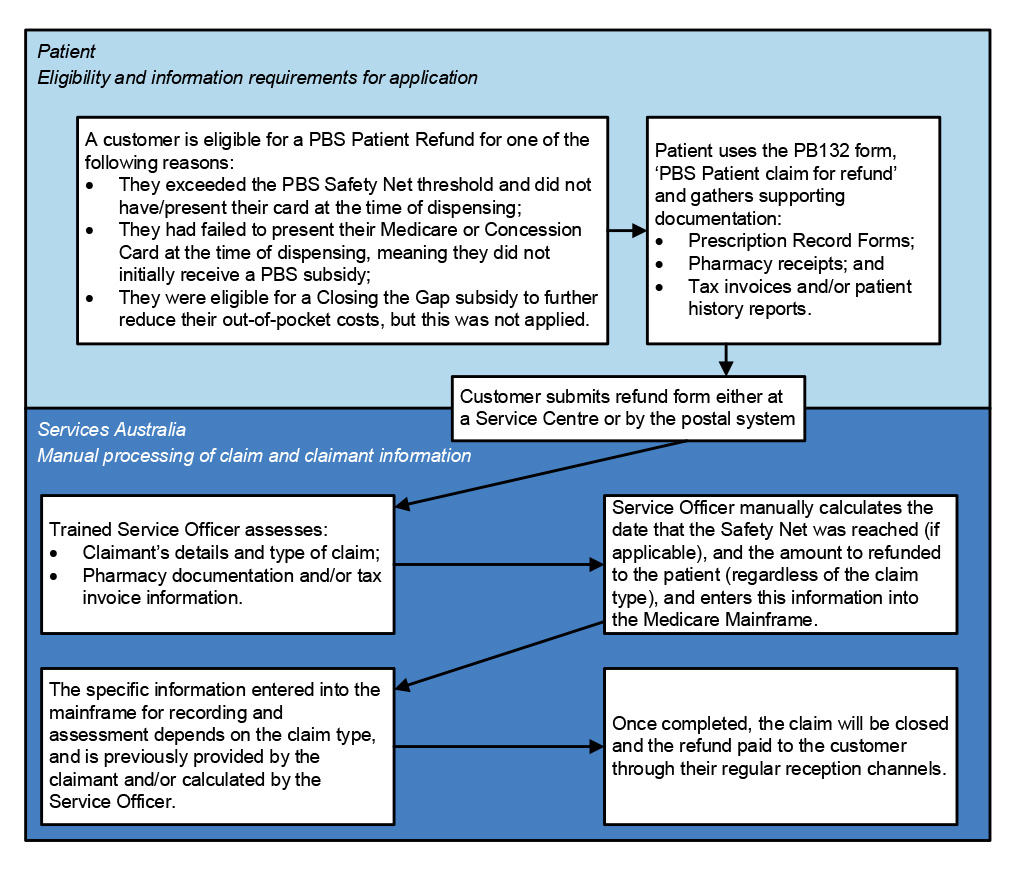
Source: ANAO analysis of Services Australia records.
System
4.73 Eligible patients apply for a refund using a Services Australia form (‘Patient claim for refund form — PB132) and by providing required supporting documentation. Between June 2019104 and June 2024:
- the average value of claims processed and paid out was $131;
- on average, 2,379 claims were processed each month for refunds totalling $18,779,222; and
- 10 claims were rejected from a total of 145,093 claims.105
4.74 Processing of patient refunds is done manually by Services Australia officers using a tool located in the ‘Medicare Mainframe’ system. Medicine and supply information relevant to a claim must be manually entered by the officer from a range of different data sources (see Figure 4.8).
Figure 4.8: Services Australia processing of patient refund claims
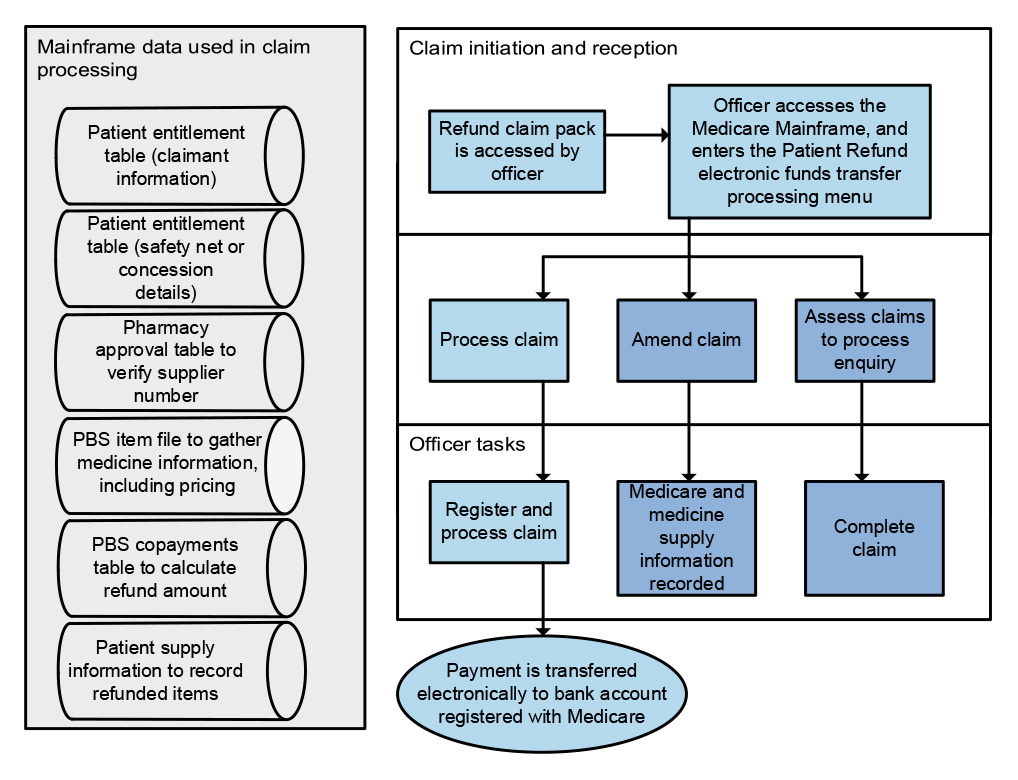
Source: ANAO analysis of Services Australia records.
4.75 If applicable, the officer will calculate the date on which the Safety Net threshold was reached to compare it to the date of the purchase being claimed in the refund. The information is processed using a tool in the Medicare Mainframe to determine the total amount claimable and the amount to be paid to the claimant.
4.76 Services Australia processing officers also access information stored in other systems, including the Workload Management System (when assessing outside of a Services Centre), the Medicare System (for bank account and personal information), and the PBS Safety Net Checker system (for claims against a Safety Net threshold). The overall process requires the manual collation of information held across different databases (see the ‘Mainframe data used in claim processing’ box in Figure 4.8) before the calculation can be performed in the central Medicare Mainframe tool.
Timeliness
4.77 Services Australia has a performance measure (PBS 5) in its PBS Program Agreement with Health which measures the monthly proportion of patient refund claims processed within 60 calendar days of submission. The target for this measure is 82 per cent.
4.78 As shown in Figure A.5 in Appendix 4, Services Australia reported that it did not meet the target between July 2023 and June 2024. In reporting to Health, Services Australia noted that ‘resources are being balanced between PBS [Patient] Refunds supporting the PBS Safety Net peak and other processing priorities.’106 Services Australia advised the ANAO in July 2024 that, as PBS patient refunds are dependent on PBS Safety Net entitlement, the resourcing for these work types can be balanced against each other and increased workloads in one area can affect the other.
Appendices
Appendix 1 Entity responses
Department of Health and Aged Care

Services Australia

Appendix 2 Improvements observed by the ANAO
1. The existence of independent external audit, and the accompanying potential for scrutiny improves performance. Improvements in administrative and management practices usually occur: in anticipation of ANAO audit activity; during an audit engagement; as interim findings are made; and/or after the audit has been completed and formal findings are communicated.
2. The Joint Committee of Public Accounts and Audit (JCPAA) has encouraged the ANAO to consider ways in which the ANAO could capture and describe some of these impacts. The ANAO’s Corporate Plan states that the ANAO’ s annual performance statements will provide a narrative that will consider, amongst other matters, analysis of key improvements made by entities during a performance audit process based on information included in tabled performance audit reports.
3. Performance audits involve close engagement between the ANAO and the audited entity as well as other stakeholders involved in the program or activity being audited. Throughout the audit engagement, the ANAO outlines to the entity the preliminary audit findings, conclusions and potential audit recommendations. This ensures that final recommendations are appropriately targeted and encourages entities to take early remedial action on any identified matters during the course of an audit. Remedial actions entities may take during the audit include:
- strengthening governance arrangements;
- introducing or revising policies, strategies, guidelines or administrative processes; and
- initiating reviews or investigations.
4. In this context, the below actions were observed by the ANAO during the course of the audit. It is not clear whether these actions and/or the timing of these actions were planned in response to proposed or actual audit activity. The ANAO has not sought to obtain assurance over the source of these actions or whether they have been appropriately implemented.
- A PBS Program Management Plan was finalised by the First Assistant Secretary of Technology Assessment and Access Division on 17 August 2023 (see paragraph 2.15).
- Health and Services Australia updated the following program agreements and protocols under their Bilateral Management Agreement (see paragraphs 2.26 and 2.27):
- PBS Program Agreement (updated in August 2023);
- Approval of PBS Suppliers Program Agreement (updated in February 2024);
- Communication and Media Protocol (updated in May 2024);
- Performance Management Protocol (updated in May 2024);
- Corporate Services Protocol (updated in June 2024);
- New and Changed Work Protocol (updated in July 2024); Health Protocol for External Data Release (updated in August 2024); and
- Compliance Protocol (updated September 2024).
- In September 2023 Health and Services Australia established a PBS Committee at the Senior Executive Service Band 2 and Band 1 level, which meets quarterly to ‘provide governance and assurance mechanisms’ to support the delivery of the PBS (see paragraph 2.34).
- A PBS Program Risk Assessment Plan was developed by Health in August 2023 (see paragraph 2.60).
- Health developed an Impact Analysis for the negotiation of the eighth Community Pharmacy Agreement in June 2024. The Office of Impact Assessment rated the Impact Analysis as ‘good practice’ (see paragraph 3.73).
Appendix 3 ANAO analysis of delegations made under the National Health Act 1953 and subordinate instruments
1. As noted at paragraph 2.5, the Minister for Health and Aged Care (the minister), the Secretary of the Department of Health and Aged Care (Health) and the Chief Executive Officer (CEO) of Services Australia have delegated specific powers under the National Health Act 1953 (NHA) and subordinate legal instruments to specified officers in Health and Services Australia through written delegation instruments. The ANAO found irregularities and anomalies in the instruments of delegation from the minister, the secretary of Health and the CEO of Services Australia. Examples of these irregularities and anomalies are outlined in Table A.1.
Table A.1: Examples of irregularities and anomalies identified
|
Irregularity or anomaly |
Example |
|
Sub-delegation has occurred without referencing in the instrument the power that allows for the sub-delegation |
The secretary of Health has delegated section 24 of the National Health (Pharmaceutical Benefits) Regulations 2017 to the CEO of Services Australia, which is then sub-delegated by the CEO of Services Australia. The CEO of Services Australia (as the Chief Executive Medicare) is authorised under subsection 8AC(3) of the Human Services (Medicare) Act 1973 to sub-delegate functions delegated to the CEO under another Act. However, the CEO of Services Australia has not referenced in the delegation instrument the power that was relied upon to sub-delegate. |
|
Sub-delegation has occurred where the power was not delegated |
The powers in section 91 of the NHA are given to the secretary of Health. The secretary did not delegate any of those powers to the CEO of Services Australia. The CEO of Services Australia, who does not hold any powers under section 91, either directly or through a delegation, then purported to sub-delegate section 91 of the NHA. |
|
Delegation has occurred where the section in the NHA vests powers in more than one person, and the delegation has been made of the whole section, which is in excess of the powers vested in any one person under that section of the NHA |
Section 133 of the NHA vests powers in both the secretary of Health and the minister. The secretary has delegated all of section 133 to specified officers in Health and to the CEO of Services Australia. The secretary of Health only has powers under subsection 133(1) of the NHA. The minister has powers under subsections 133(2) and 133(4). The secretary has delegated powers which belong to the minister and are in excess of the secretary’s powers. |
|
Delegation has occurred where there are express provisions against the delegation of a specific power of function |
Paragraph 6(1)(aa) of the NHA states that the minister’s power under subsection 90(10) cannot be delegated. The minister did not delegate this section. The secretary of Health (who did not have the power) has delegated all of section 90 of the NHA to specified officers in the department, which includes subsection 90(10). |
|
Delegation has occurred where powers or functions have been repealed |
Section 99ACE of the NHA was repealed effective 1 July 2022. The minister has delegated this section after the section was repealed. |
Source: ANAO analysis of delegation instruments.
Appendix 4 Bilateral Pharmaceutical Benefits Scheme performance measures between Health and Services Australia
Figure A.1: PBS 1 — PBS authorities telephony, August 2022–June 2024
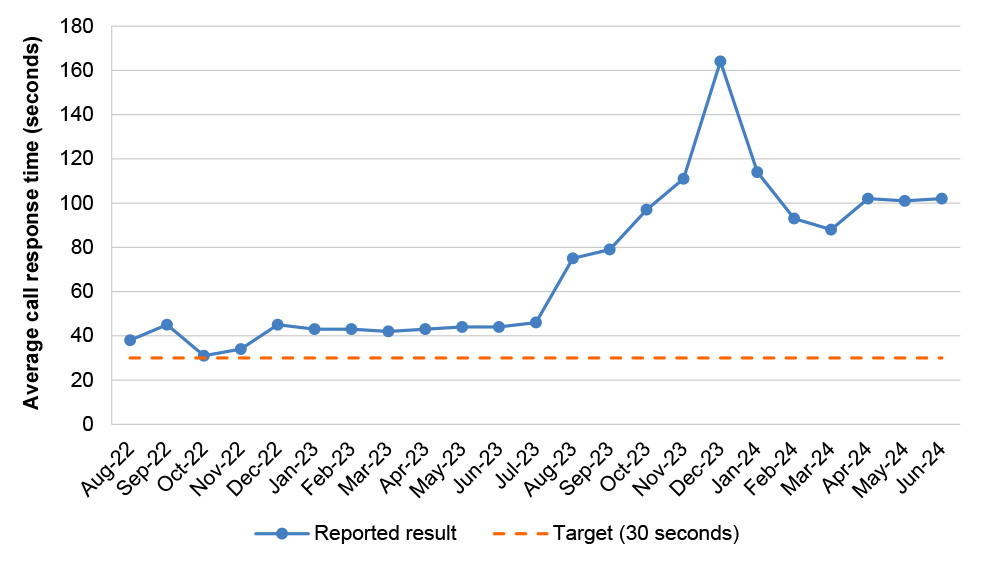
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.2: PBS 2 — PBS online claims, August 2022–June 2024
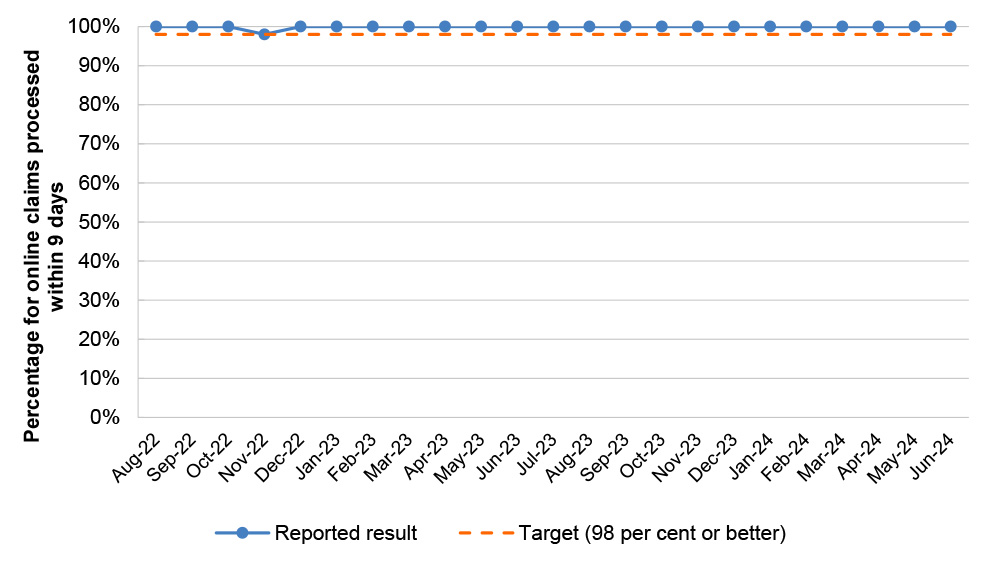
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.3: PBS 3 — PBS manual pharmacy claims, August 2022–June 2024
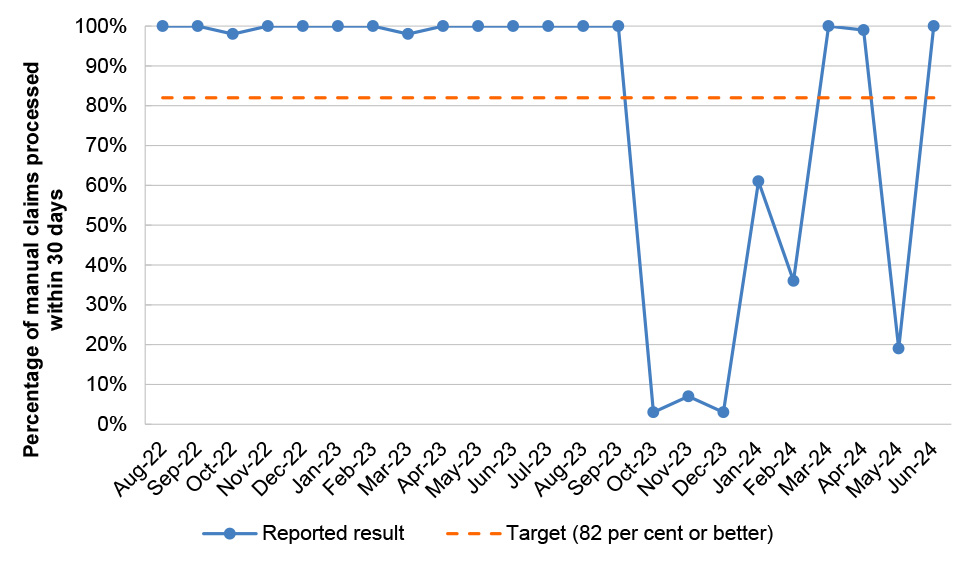
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.4: PBS 4 — PBS Safety Net claims, August 2022–June 2024
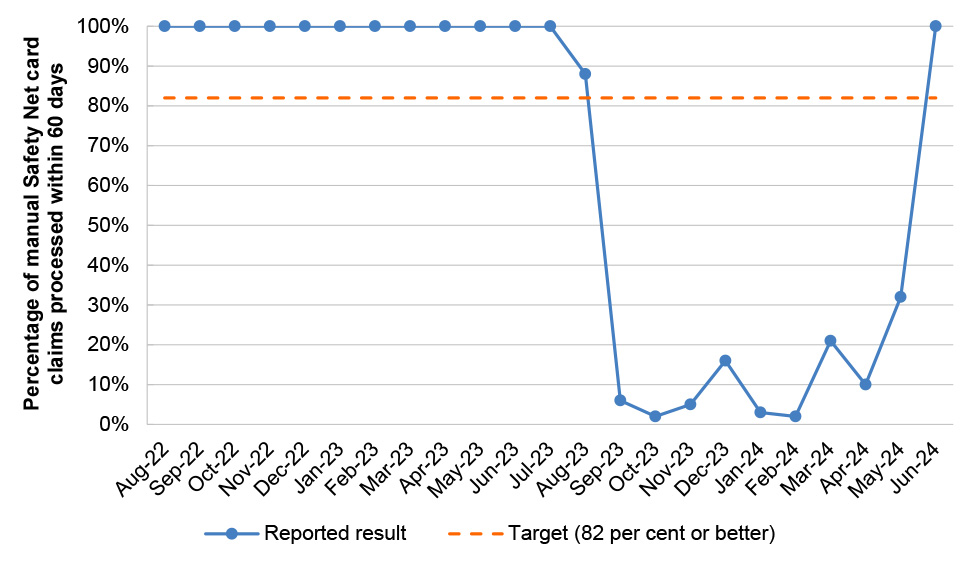
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.5: PBS 5 — Patient refunds, August 2022–June 2024
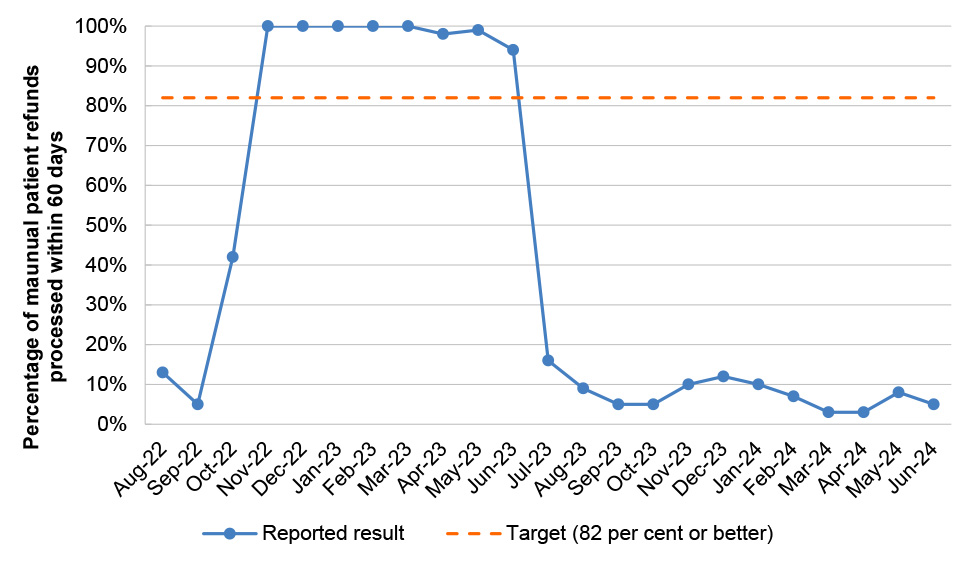
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.6: PBS 6 — Authority approval requests, August 2022–June 2024
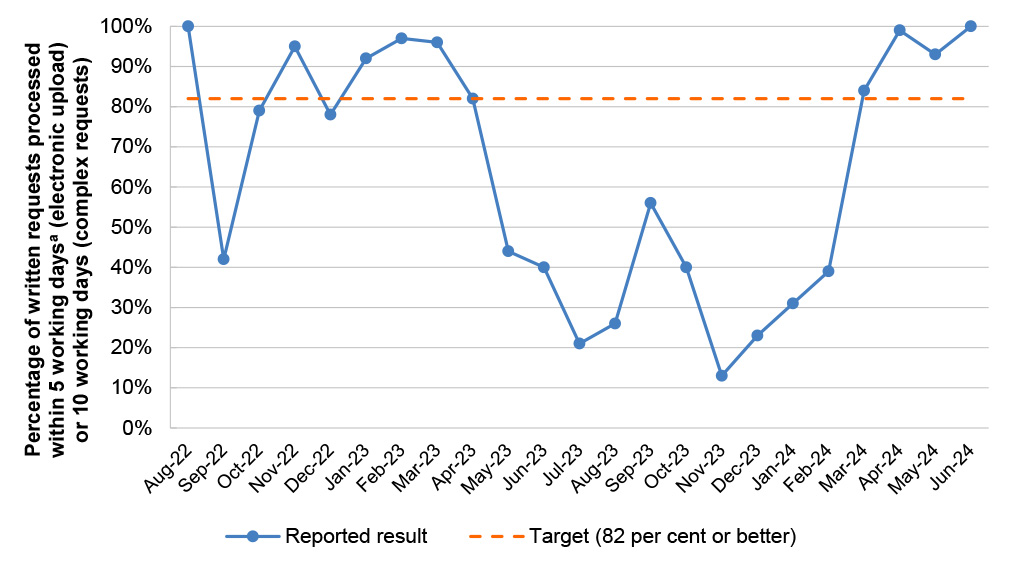
Note a: Prior to August 2023 the agreed timeframe for requests uploaded electronically was 3 working days.
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.7: PBS 7 — Remote Area Aboriginal Health Services, August 2022–June 2024
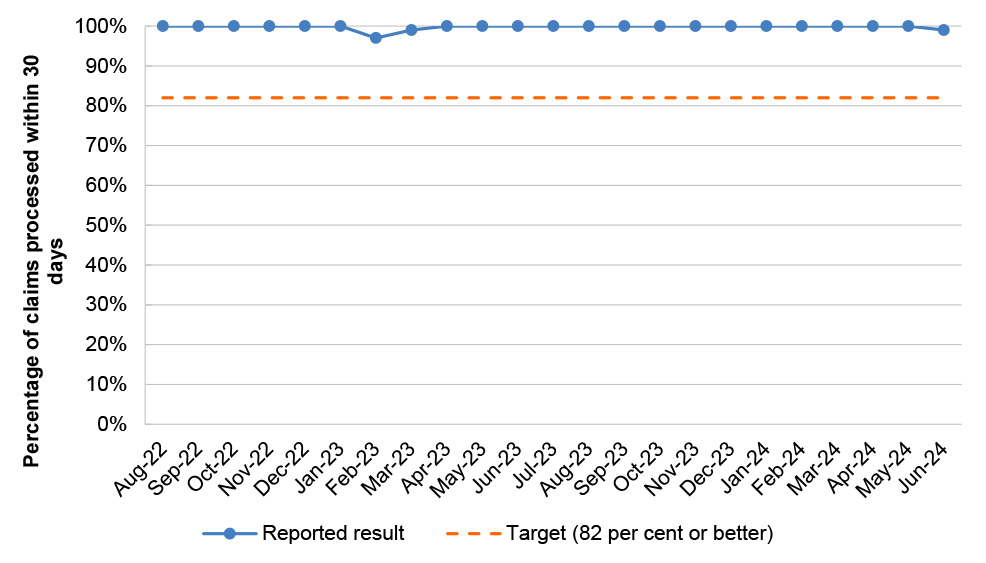
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.8: PBS 8 — Paraplegic and Quadriplegic Program, August 2022–June2024
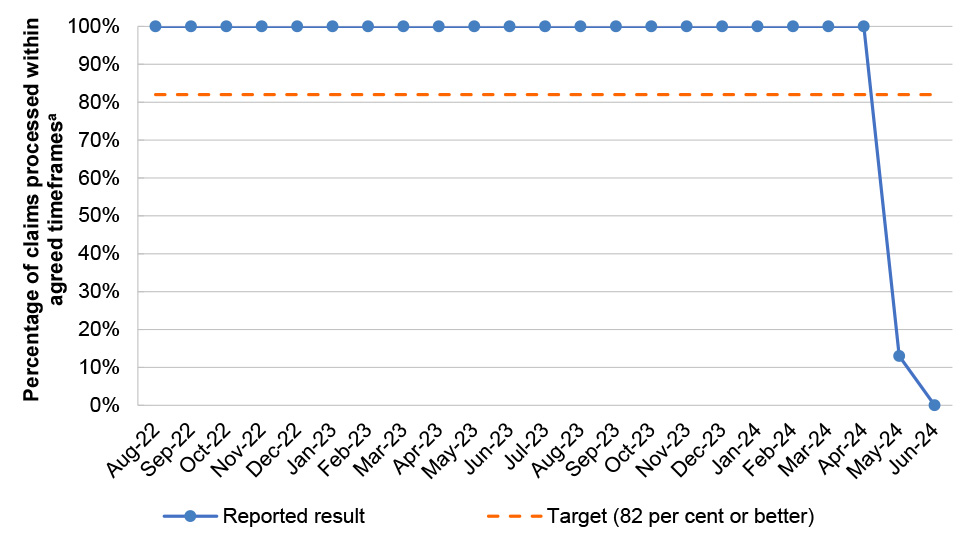
Note a: From August 2023 the agreed timeframe for processing claims was 7 calendar days. Prior to August 2023 agreed timeframes ranged between 7 to 30 calendar days depending on the claim type.
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Figure A.9: PBS 9 — Stoma Appliance Scheme, August 2022–June 2024
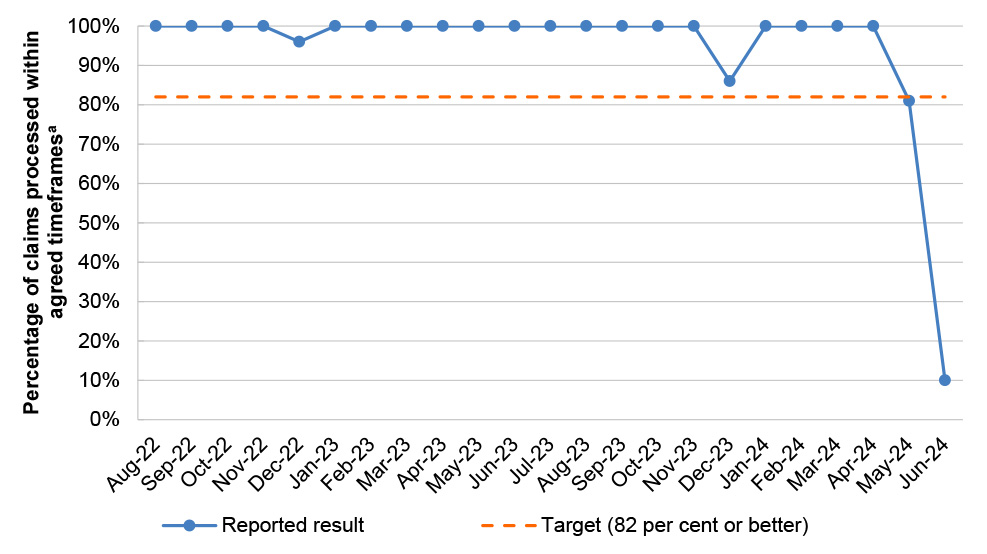
Note a: From August 2023 the agreed timeframe for processing claims was 7 calendar days. Prior to August 2023 agreed timeframes ranged between 7 to 30 calendar days depending on the claim type.
Source: Services Australia, monthly dashboard reports to Health under the PBS Program Agreement.
Appendix 5 Health and Services Australia shared Pharmaceutical Benefits Scheme risks
|
Risk |
Causes |
Consequences |
Inherent risk rating |
Controls |
Control rating |
Residual risk rating |
|
Services Australia and Health cannot deliver the PBS effectively |
|
|
High |
|
Fully effective |
Low |
|
ICT systems do not support PBS delivery |
|
|
Medium |
|
Fully effective |
Low |
|
Approved prescribers and suppliers do not prescribe and supply PBS items correctly |
|
|
Medium |
|
Partially effective |
Low |
|
PBS data is unavailable or insufficient |
|
|
Medium |
|
Fully effective |
Low |
|
Breaches occur (including data and financial) |
|
|
Very high |
|
Partially effective |
Low |
Source: Department of Health and Aged Care and Services Australia, Joint PBS Risk Management Plan 2023-2024, internal document, approved 5 October 2023.
Appendix 6 Medicines assessed as part of the audit
|
Medicine |
Year listed and indication |
Number of prescriptions 2023–24 |
Cost to Government 2023–24 ($) |
s85/s100 |
Authority restrictions |
|
Avatrombopag |
2023 — severe thrombocytopenia |
1,483 |
4,521,240 |
s100 HSD public and private |
Authority required |
|
Bictegravir + Emtricitabine + Tenofovir alafenamide |
2019 — HIV infection |
67,579 |
114,876,211 |
s100 HSD community access |
Authority required (streamlined) |
|
Budesonide |
1991 — chronic asthma 2002 — intractable rhinitis 2014 — Rectal foam 2022 — Crohn disease 2022 and 2023 – Eosinophilic oesophagitis |
246,706 |
12,453,571 |
s85 |
Authority required and Authority required (streamlined) |
|
Ciclosporin |
1992 — Transplant rejection 2021 — chronic severe dry eye disease with keratitis Also listed for the below indications:
|
75,990 |
10,351,005 |
s85 and s100 HSD public and private |
Authority required and Authority required (streamlined) |
|
Dapagliflozin |
2013 — diabetes mellitus type 2 2022 — chronic heart failure 2022 — chronic kidney disease |
1,524,707 |
69,288,179 |
s85 |
Authority required (streamlined) |
|
Deferiprone |
2004 — Iron overload |
696 |
1,054,027 |
s100 HSD public and private |
Authority required (streamlined) |
|
Elexacaftor + Tezacaftor + Ivacaftor & Ivacaftor |
2022 — cystic fibrosis |
26,590 |
568,332,758 |
s100 HSD public and private |
Authority required |
|
Eptinezumab |
2023 — chronic migraine |
3,980 |
5,579,914 |
s85 |
Authority required (streamlined) |
|
Follitropin Alfa |
2004 — with treatment for items 13200 or 13203 of the Medicare Benefits Scheme 2016 — assisted reproduction 2021 — assisted reproductive technology; anovulatory infertility; infertility |
53,725 |
65,415,303 |
s100 IVF and s85 |
Authority required (streamlined) and restricted benefit |
|
Lithium carbonate |
1971 — mood stabiliser 1999 — mood stabiliser, bipolar disorder |
179,478 |
6,740,698 |
s85 |
Unrestricted |
|
Methotrexate |
1964, 1981,1982, 1987 and 2011 — chemotherapy 2008 and 2018 — severe psoriasis & rheumatoid arthritis |
510,913 |
17,983,625 |
s85, Chemotherapy |
Authority required (streamlined), restricted benefit |
|
Molnupiravir |
2022 — SARS-CoV-2 infection |
361,710 |
416,758,196 |
s85 and prescriber bag |
Authority required (streamlined) |
|
Nivolumab |
2016 — melanoma 2017 — Renal Cell Carcinoma (RCC) & Non-Small Cell Lung Cancer (NSCLC) 2018 — squamous cell carcinoma of head and neck 2019 — stage 4 clear cell variant RCC 2020 — melanoma 2021 — unresectable malignant mesothelioma 2022 — gastro-oesophageal cancers; second-line squamous cell oesphageal carcinoma; first line treatment of advanced or metastatic gastro-oesophageal cancers 2023 — oesophageal or gastro-oesophageal cancers (adjuvant) 2023 — gastro-oesophageal cancers (restriction amendments) |
60,520 |
437,293,850 |
Chemotherapy |
Authority required (streamlined) |
|
Onasemnogene abeparvovec |
2022 and 2023 — spinal muscular atrophy |
9 |
22,749,711 |
s100 HSD public |
Authority required |
|
Patiromer |
2023 — chronic hyperkalaemia |
4,012 |
1,470,291 |
s85 |
Authority required and Authority required (streamlined) |
|
Pimecrolimus |
2005 — atopic dermatitis |
150,928 |
1,151,672 |
s85 |
Authority required (streamlined) |
|
Somatropin |
1996, 2002, 2006, 2007, 2008, 2010, 2011, 2011, 2012, 2015, 2016, 2018, 2019, 2020, 2023 — growth hormone deficiency |
16,522 |
26,698,478 |
s100 growth hormone |
Authority required |
|
Vericiguat |
2022 — chronic heart failure |
8,346 |
1,123,351 |
s85 |
Authority required and Authority required (streamlined) |
Source: Department of Health and Aged Care.
Appendix 7 Remuneration adjustment mechanism
1. The 7CPA introduced a remuneration adjustment mechanism (RAM) to minimise unexpected expenditure for the Government and allow a stable income for pharmacies. Increases or reductions to the Commonwealth price can be made if the predicted prescription volumes differ significantly from the actual prescription volumes, as determined through calculations outlined in Appendix B of the 7CPA. There are four assessment periods for the RAM:
- 1 July 2020 – 31 December 2020;
- 1 January 2021 – 31 December 2021;
- 1 January 2022 – 31 December 2022; and
- 1 January 2023 – 31 December 2023.
The RAM utilises the estimated and actual number of subsidised and unsubsidised prescriptions during the assessment periods to calculate whether adjustments to the RAM are required.
2. The first calculation of the RAM identifies the difference between the number of actual and estimated subsidised prescriptions. Table A.2 details prescription data and the calculation outcome.
Table A.2: First RAM calculation
|
Assessment period |
Estimated subsidised prescription number |
Actual subsidised prescription number |
Calculation outcome (% increase in actual from estimate) |
|
1 July 2020 – 31 December 2020 |
109,521,070 |
113,238,530 |
3.39 |
|
1 January 2021 – 31 December 2021 |
207,523,772 |
215,268,750 |
3.73 |
|
1 January 2022 – 31 December 2022 |
208,099,570 |
219,261,643 |
5.36 |
|
1 January 2023 – 31 December 2023 |
210,456,812 |
226,178,715 |
7.47 |
Source: ANAO analysis.
3. As the 2020 and 2021 assessment periods were below 5 per cent no further calculation or change to pharmacy remuneration was required. As the 2022 and 2023 assessment periods had a greater than 5 per cent increase in the actual number of prescriptions, this triggered a secondary calculation.
4. The secondary calculation incorporates the number of unsubsidised prescriptions. Table A.3 shows the secondary calculation for the 2022 and 2023 assessment periods.
Table A.3: Second RAM calculation
|
Assessment period |
Actual subsidised prescription number (A) |
Actual unsubsidised prescription number (B) |
Estimated unsubsidised prescription number (C) |
Estimated subsidised prescription number (D) |
Calculation outcome (A+(B-C))/D (%) |
|
1 January 2022 – 31 December 2022 |
219,261,643 |
103,440,474 |
104,377,953 |
208,099,570 |
4.91 |
|
1 January 2023 – 31 December 2023 |
226,178,715 |
103,721,362 |
107,648,589 |
210,456,812 |
5.6 |
Source: ANAO analysis.
5. As the second calculation did not exceed 5 per cent for the 2022 assessment period no change to pharmacy remuneration was required. The 2023 assessment period exceeded 5 per cent which triggered a decrease to the Commonwealth price. The calculation applied to determine the reduction in the Commonwealth price is shown below.
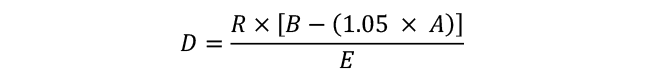
Where:
R is the sum of the first level of the administration and handling free plus the dispensing fee for the medicine at the end of the assessment period
A is the estimated subsidised prescriptions for the assessment period
B is the actual subsidised prescription of the assessment period
E is the total estimated prescriptions for the next financial year
D is the reduction, in dollars, to the first level of the administration and handling fee after indexation for the next financial year
6. Calculation of the reduction for 2023 resulted in decreasing the Commonwealth price by $0.21. This increase was due to start on 1 July 2024 but has been overridden by the introduction of the 8CPA.
7. While the 1 January 2023 co-payment decrease (discussed at paragraph 3.76) was modelled to decrease the number of unsubsidised scripts, the RAM was designed so that this would not trigger an adjustment.
8. Clause 3.5 of the 7CPA required Health and the Pharmacy Guild to each assess the RAM adjustment process in 2022–23. Health’s review of the RAM determined that the intent of the RAM was being achieved with spending falling within the expected ranges.
Appendix 8 Changes to patient co-payment amounts and Safety Net thresholds
Figure A.10: Changes to patient co-payment amounts since 1960
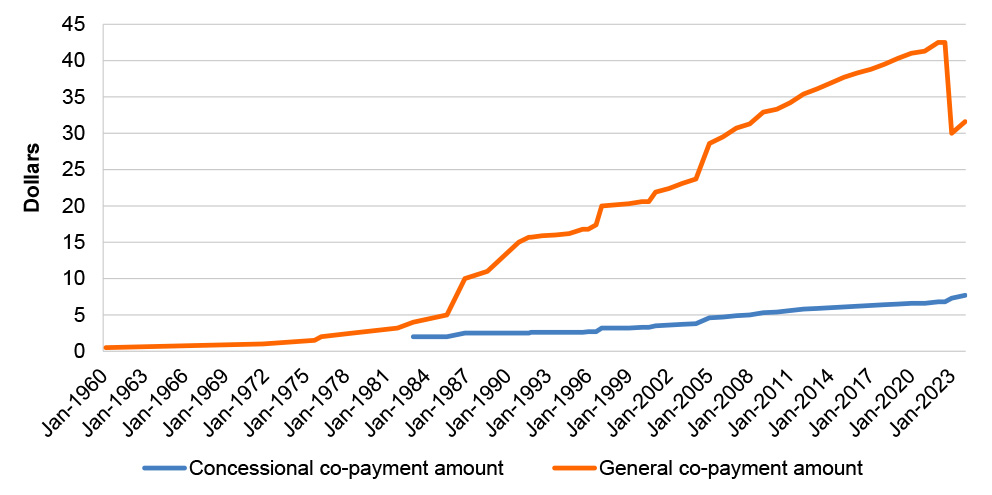
Source: Department of Health and Aged Care, Fees, Patient Contributions and Safety Net Thresholds, Canberra, 2024, available from https://www.pbs.gov.au/info/healthpro/explanatory-notes/front/fee#_1 [accessed 15 May 2024].
Figure A.11: Changes to patient Safety Net amounts since 1990
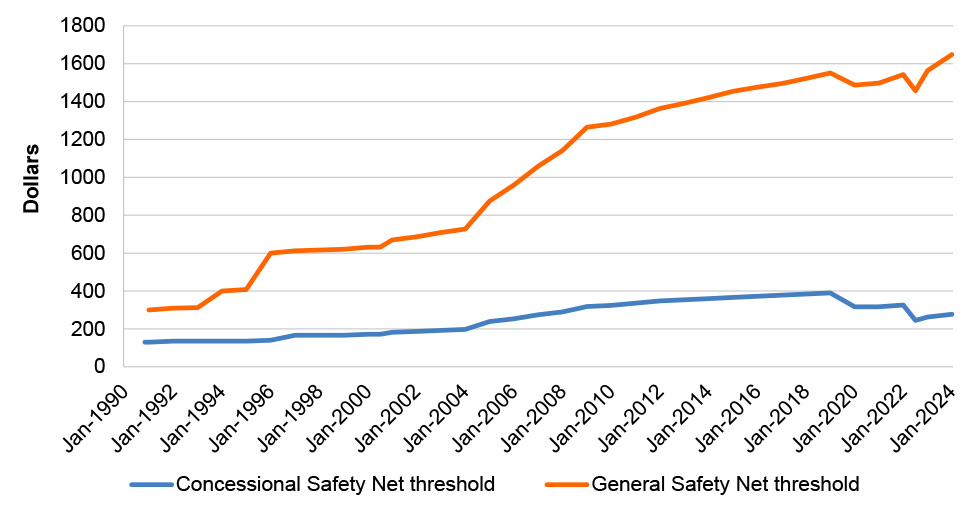
Source: Department of Health and Aged Care, Fees, Patient Contributions and Safety Net Thresholds, Canberra, 2024, available from https://www.pbs.gov.au/info/healthpro/explanatory-notes/front/fee#_1 [accessed 15 May 2024].
Appendix 9 PBS payment categories for post-payment assurance testing
|
Payment category |
Description |
|
Community pharmacy — PBS general Schedule items |
General schedule items are PBS medicines that are listed under section 85 of the NHA. Payment testing includes checking dates of prescription and supply, health provider approvals, patient eligibility, payment accuracy and alignment against PBS schedule requirements. |
|
Remote Area Aboriginal Health Services |
Aboriginal health services (AHS) in remote areas can provide free PBS medicines to their patients. Payment testing includes checking dates of prescription and supply, AHS approval, payment accuracy and alignment against PBS schedule requirements. |
|
Stoma, ostomy and paraplegic and quadriplegic supplies |
The Stoma Appliance Scheme provides free stoma appliances to people who have a stoma (ostomates), through 21 stoma associations. Similarly, PBS benefits for paraplegic- or quadriplegic-related products can be supplied through authorised paraplegic or quadriplegic associations. Payment testing includes validity of stoma association, alignment with PBS Schedule and Stoma Appliance Schedule requirements. |
|
Dental PBS items |
Dentists have a separate Dental formulary from which they can prescribe PBS medicines. Payment testing includes checking dates of prescription and supply, health provider approvals, patient eligibility, payment accuracy and alignment against PBS schedule requirements. |
|
Payments during system outages |
Payment testing of items dispensed during a PBS online system outage includes checking dates of prescription and supply, health provider approvals, patient eligibility, payment accuracy and alignment against PBS schedule requirements. |
|
Chemotherapy |
Special arrangements, under section 100 of the NHA, for supplying chemotherapy medicines that require reconstitution or preparation for individual patients. Payment testing includes checking dates of prescription and supply, health provider approvals, payment accuracy and alignment against PBS schedule requirements. |
|
Hospital authorities |
Public and private hospital authorities that are approved to prescribe PBS medicines under the NHA may prescribe and dispense up to 1 month’s supply of PBS medicines to outpatients, patients on discharge and chemotherapy medicines. Payment testing includes checking dates of prescription and supply, health provider approvals, payment accuracy and alignment against PBS schedule requirements. |
|
Prescriber bag Supplies |
Prescriber bag supplies refer to certain PBS medicines that are provided without charge to prescribers who in turn can supply them free to patients for emergency use. Payment testing includes checking dates of prescription and supply, health provider approvals, payment accuracy and alignment against PBS schedule requirements. |
|
Highly specialised drugs, botulinum toxin, in-vitro fertilisation, human growth hormone |
Highly specialised drugs, botulinum toxin, in-vitro fertilisation, human growth hormone refers to PBS medicines supplied under special arrangements under section 100 of the NHA. Payment testing includes checking dates of prescription and supply, health provider approvals, patient eligibility and alignment against PBS schedule requirements. |
|
Optometrists |
Optometrists can only prescribe certain PBS medications. Payment testing includes checking dates of prescription and supply, health provider approvals, and alignment against PBS schedule requirements. |
|
Nusinersen |
Nusinersen is a high-cost PBS medicine approved for the treatment of spinal muscular atrophy. Payment testing includes checking dates of prescription and supply, health provider approvals, patient eligibility, payment accuracy and alignment against PBS schedule requirements. |
|
Onasemnogene abeparvovec (Zolgensma) |
Zolgensma is a high-cost PBS medicine approved for the treatment of spinal muscular atrophy. Payment testing includes checking dates of prescription and supply, health provider approvals, patient eligibility, payment accuracy and alignment against PBS schedule requirements. |
|
Extemporaneously prepared medicine |
An extemporaneous preparation (compound) is a medicine or mixture of medicines prepared or compounded in a pharmacy according to the order of a prescriber. Payment testing includes checking dates of prescription and supply, health provider approvals and payment accuracy and alignment against PBS schedule requirements. |
|
Standard formula preparation |
Standard formula preparations are extemporaneous preparations specifically listed in the PBS Schedule. Payment testing includes checking dates of prescription and supply, health provider approvals and payment accuracy. |
|
Safety Net card claim |
PBS suppliers can claim payment for issuing PBS Safety Net cards to eligible patients. Payment testing includes checking PBS supplier approval, patient details and payment accuracy and alignment against PBS schedule requirements. |
|
Patient refunds |
Patients are entitled to a refund when they fail to show their Medicare card or concession card when purchasing a PBS medicine or spend over their yearly PBS Safety Net threshold. Payment testing includes checking PBS supplier approval, patient details and accuracy of refund payment and alignment against PBS schedule requirements. |
Source: ANAO analysis of Services Australia internal documents.
Footnotes
1 Department of Health and Aged Care, Schedule of Pharmaceutical Benefits, available from www.pbs.gov.au [accessed 18 May 2024].
2 Department of Health and Aged Care, PBS Expenditure and Prescriptions, Canberra, 2023, available from https://www.pbs.gov.au/info/statistics/expenditure-prescriptions/pbs-expenditure-and-prescriptions [accessed 7 November 2024]. Medicines can be prescribed outside of the PBS, including through in-hospital or private prescribing.
3 The Department of Health and Aged Care advised the ANAO in June 2024 that as at 20 June 2024 there were 144 legal instruments governing the PBS. The ANAO found that, of the 144 legal instruments identified by Health, some were no longer in force or had been repealed before 20 June 2024, and some instruments were in effect that had not been identified.
4 Department of Health and Aged Care, Minister for Health and Aged Care, available from https://www.pbs.gov.au/info/industry/listing/participants/minister [accessed 30 May 2024].
5 Department of Health and Aged Care, PBAC Public Summary Documents, available from https://www.pbs.gov.au/info/industry/listing/elements/pbac [accessed 30 May 2024].
6 Department of Health and Aged Care, Medicine Status Website, available from https://www.pbs.gov.au/medicinestatus/home.html [accessed 30 May 2024].
7 Persons authorised to prescribe pharmaceutical benefits are listed under section 88 of the NHA. There are separate arrangements for PBS prescriptions in certain public hospitals. To gain access to pharmaceutical benefits under this arrangement a patient must attend a participating public hospital and be a discharge patient or non-admitted patient. Only a medical practitioner providing medical treatment or a midwife providing midwifery treatment or a nurse practitioner providing nurse practitioner treatment within a participating public hospital may prescribe PBS subsidised medication from a hospital. The states of Victoria, Queensland, South Australia, Western Australia and Tasmania, and the Northern Territory have agreed to implement these arrangements.
8 Authority-required medicines, with the exception of authority-required (streamlined) medicines, require approval from Services Australia in order to be prescribed and dispensed for the subsided PBS price. Services Australia assesses applications for approval against the requirements listed in the Schedule and, if approved, provides a code to the prescriber to be included on the prescription.
9 The approval of PBS suppliers is outlined in sections 90, 92 and 94 of the NHA respectively. Detailed arrangements for the supply of PBS medicines depend on the type of medicine and are outlined in subordinate legal instruments.
10 The Commonwealth Price (Pharmaceutical Benefits Supplied by Approved Pharmacists) Determination 2020 describes the components of the Commonwealth price, which allow the Commonwealth price to be calculated.
11 Department of Health and Aged Care, Eighth Community Pharmacy Agreement, available from https://www.health.gov.au/topics/primary-care/what-we-do/8cpa [accessed 5 June 2024].
12 Department of Health and Aged Care, Strategic Agreement on Pharmacist Professional Practice, available from https://www.health.gov.au/topics/primary-care/what-we-do/strategic-agreement [accessed 5 June 2024].
13 Department of Health and Aged Care, Strategic Agreements with the Medicines Industry, available from https://www.pbs.gov.au/info/general/medicines-industry-strategic-agreement [accessed 18 May 2024].
14 The Department of Health and Aged Care advised the ANAO in June 2024 that as at 20 June 2024 there were 144 legal instruments governing the PBS. The ANAO found that, of the 144 legal instruments identified by Health, some were no longer in force or had been repealed before 20 June 2024, and some instruments were in effect that had not been identified.
15 Sub-delegation means delegating a power that has been delegated to the person making the sub-delegation.
16 The First Assistant Secretary of the Financial Management Division has responsibility for shared functions in common with other programs such as supporting Health’s management of administered funding, which includes PBS funding.
17 Section 90 of the NHA.
18 Subsection 98A(4) of the NHA provides that the chair of the PBRT must be a Deputy President of the Fair Work Commission.
19 Services Australia also provides services to entities on a ‘purchaser-provider’ basis, where the purchasing entity pays for the services through a cost-recovery arrangement. See Auditor-General Report No. 30 2019–20, Bilateral Agreement Arrangements Between Services Australia and Other Entities, ANAO, Canberra, 2020, paragraph 3.120, available from https://www.anao.gov.au/work/performance-audit/bilateral-agreement-arrangements-between-services-australia-and-other-entities [accessed 22 August 2024].
20 The 12 ‘critical elements’ were derived from analysis outlined in Auditor-General Report No. 30 2019–20, Bilateral Agreement Arrangements Between Services Australia and Other Entities, ANAO, Canberra, 2020, paragraphs 2.25 to 2.26, available from https://www.anao.gov.au/work/performance-audit/bilateral-agreement-arrangements-between-services-australia-and-other-entities [accessed 4 May 2024].
21 Neither agreement included documented consideration of expert advice and stakeholder contributions, a critical element that was added to the Bilateral Agreement Framework in January 2024.
22 Department of Health and Aged Care, Corporate Plan 2023–24, Commonwealth of Australia, Canberra, 2023, p. 72, available from https://www.health.gov.au/sites/default/files/2023-12/corporate-plan-2023-24.pdf [accessed 12 May 2024].
23 ibid., p. 73.
24 Additional activities in Health’s 2023–24 corporate plan that fell below its ‘materiality threshold for publishing and reporting against a program performance measure’ were:
- Ensuring patients have access to medicines and professional pharmacy services that support the safe and quality use of medicines through the Seventh Community Pharmacy Agreement, and expanding the range of funded pharmacy programs, including staged supply of opioid dependency treatment medications, to recognise the full scope of practice of pharmacists.
- Supporting and monitoring pharmaceutical wholesalers participating in the Community Service Obligation Funding Pool to ensure all eligible Australians have timely access to PBS medicines, including delivering subsidised PBS units to community pharmacies within agreed timeframes, in a way that supports Australians to access medicines through a reliable domestic supply chain.
- Ensuring continuity of medicines supply through the Minimum Stockholding Requirements designed to help protect Australian patients, pharmacists, and prescribers from the impact of global medicines shortages.
- Monitoring the number and location of PBS suppliers to ensure suppliers are being approved in appropriate locations.
- Supporting the Health Technology Assessment (HTA) Policy and Methods Review to ensure HTA approaches keep pace with advances in health technology.
25 ANAO audits of entity annual performance statements are designed to provide assurance to the Parliament that entities’ annual performance statements comply with the requirements of Division 3 of Part 2‐3 of the PGPA Act. Division 3 of the PGPA Act mandates compliance with relevant requirements of the Public Governance, Performance and Accountability Rule 2014.
26 Auditor-General Report No. 25 2014–15, Administration of the Fifth Community Pharmacy Agreement, ANAO, Canberra, 2015, paragraphs 6.25–6.28, available from https://www.anao.gov.au/work/performance-audit/administration-fifth-community-pharmacy-agreement [accessed 25 May 2024].
27 Auditor-General Report No. 9 2016–17, Community Pharmacy Agreement: Follow-on Audit, ANAO, Canberra, 2016, paragraphs 2.40–2.45, available from https://www.anao.gov.au/work/performance-audit/community-pharmacy-agreement-follow-audit [accessed 25 May 2024].
28 The achievement of targets for PBS 3, PBS 4, PBS 5 and PBS 6 declined in 2023. Services Australia received additional funding in the 2023–24 Budget and Additional Estimates to increase its Average Staffing Level (ASL) by 2,673. The 2024–25 Budget included a further increase of 4,753 ASL for Services Australia.
29 RSA secure identification tokens are used by Health staff to access Services Australia’s ICT system.
30 General risks identified in the plan included: ineffective stakeholder engagement; ineffectively targeted compliance interventions; failure to identify and address underlying and systemic compliance risks; delays and disruptions to the continuity of compliance activities; and inadequate systems and/or data capabilities.
31 Managing out-of-pocket costs for patients is further discussed at paragraphs 3.92 to 3.120.
32 Pharmacy Guild of Australia, 60-day dispensing campaign paused over early 8CPA, 13 September 2023, available from https://www.guild.org.au/news-events/news/forefront/v13n09/60-day-dispensing-campaign-paused-over-early-8cpa [accessed 1 September 2024].
33 Department of Health and Aged Care, Open PBAC consultations, available from https://ohta-consultations.health.gov.au/pbac/ [accessed 7 June 2024].
34 Department of Health and Aged Care, Pharmaceutical Benefits Scheme, available from https://www.pbs.gov.au/pbs/home [accessed 4 June 2024].
35 Services Australia, Services Australia, available from https://www.servicesaustralia.gov.au/ [accessed 4 June 2024].
36 Services Australia, Pharmaceutical Benefits Schedule Item Reports, available from http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp [accessed 4 June 2024].
37 Productivity Commission, Report on Government Services 2024, Part E, Section 10, Commonwealth of Australia, Canberra, 2024, available from https://www.pc.gov.au/ongoing/report-on-government-services/2024/health/primary-and-community-health [accessed 24 June 2024].
38 Australian Public Service Commission, Delivering Great Policy, Commonwealth of Australia, Canberra, 2023, available from https://www.apsacademy.gov.au/aps-craft/strategy-policy-evaluation/delivering-great-policy, [accessed 24 June 2024].
39 Department of the Prime Minister and Cabinet, Australian Government Guide to Policy Impact Analysis, Commonwealth of Australia, Canberra, 2023, p. 4, available from https://oia.pmc.gov.au/sites/default/files/2024-01/australian-government-guide-to-policy-impact-analysis.pdf [accessed 14 August 2024].
40 Subsection 101(3A) of the NHA states that:
the Committee shall give consideration to the effectiveness and cost of therapy involving the use of the drug, preparation or class, including by comparing the effectiveness and cost of that therapy with that of alternative therapies, whether or not involving the use of other drugs or preparations.
PBAC cannot recommend a high-cost medicine which has an alternate therapy unless it has a significant improvement in efficacy or reduction in toxicity.
41 Department of Health and Aged Care, Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee, Canberra, 2016 available from https://pbac.pbs.gov.au/ [accessed 24 June 2024].
42 Department of Health and Aged Care, Procedural guidance for listing medicines on the Pharmaceutical Benefits Scheme, Commonwealth of Australia, Canberra, 2020, available from https://www.pbs.gov.au/industry/listing/procedure-guidance/files/Procedure-guidance-for-listing-medicines-on-the-Pharmaceutical-Benefits-Scheme-v2.5.pdf [accessed 24 June 2024].
43 Department of Health and Aged Care, Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee, Commonwealth of Australia, Canberra, 2016, p. 60, available from https://pbac.pbs.gov.au [accessed 24 June 2024].
44 ANAO selected 18 medicines for analysis as part of this audit. Medicines were selected to assist in testing compliance with the range of pricing mechanisms which are part of the PBS. One medicine was selected for a recently listed (since July 2021) or an older medicine (listed more than five years ago) from each anatomical therapeutic chemical classification. Selection was prioritised based on high costs to the PBS budget and coverage of a range of medicine sponsors. Further information on assessed medicines is in Appendix 6.
Thirteen of the 18 medicines had new listings on the Schedule between 1 July 2021 and 1 April 2024 and were assessed by the ANAO for compliance with the PBAC guidelines. These medicines were: avatrombopag; budesonide; ciclosporin; dapagliflozin; elexacaftor + tezacaftor + ivacaftor & ivacaftor; eptinezumab; follitropin alfa; molnupiravir; nivolumab; onasemnogene abeparvovec; patiromer; somatropin; and vericiguat.
45 Dapagliflozin, nivolumab, onasemnogene abeparvovec, patiromer and vericiguat were evaluated by PBAC more than once before PBAC made a recommendation for listing on the PBS based on a cost-effective price.
46 A sponsor that does not agree with the PBAC recommended price may also decide not to list the medicine on the PBS.
47 The AEMP is the medicine price agreed or determined between the medicine sponsor and Health. The price is agreed at a specific quantity, concentration and/or volume of the medicine. The AEMP is used to calculate the Commonwealth price, which determines the price pharmacists can charge patients and the costs reimbursed to pharmacists by the Government.
48 Section 85E of the NHA outlines that the minister may enter into deeds of agreement for PBS medicines.
49 Department of Health and Aged Care, Procedural guidance for listing medicines on the Pharmaceutical Benefits Scheme, Commonwealth of Australia, Canberra, 2020, section 8.5.2, available from https://www.pbs.gov.au/industry/listing/procedure-guidance/files/Procedure-guidance-for-listing-medicines-on-the-Pharmaceutical-Benefits-Scheme-v2.5.pdf [accessed 12 August 2024].
50 There are a range of legislative instruments based on whether the medicine is to be placed on the general schedule or various specialised instruments, for example for highly specialised drugs or chemotherapy. The instruments detail the restriction listing of the medicine.
51 A medicine is an ‘exempt item’ if it satisfies the criteria set out in s84H of the NHA. Exempt items are excluded from 15-year anniversary price, first new brand and price disclosure reductions.
52 Lower price offers are submitted by sponsors for brands of medicines. Sponsors of other brands of the medicine are advised of the offer and may either add a brand premium, reduce their brands price, or delist the brand from the Schedule.
53 The NHA was amended in December 2021 to include the statutory price reductions detailed in the strategic agreement with Medicines Australia.
54 The cap applies to brands of a medicine listed as at 1 January 2016. If there was not a brand listed on that date the original AEMP of the first listed brand of the medicine is used.
55 The minister has delegated this to the First Assistant Secretary for the Technology Assessment and Access Division.
56 Appendix 6 provides details of the medicines selected for testing.
57 Section 99ADC of the NHA outlines the price disclosure requirements.
58 Appendix 6 provides details of the medicines selected for testing.
59 Off-script prescribing refers to medical professionals prescribing PBS medicines for conditions outside of those listed in the Schedule.
60 Appendix 6 provides details of this medicine.
61 Medicines may no longer by financially viable to list on the PBS due to post-listing price reductions or increases to manufacturing costs.
62 A ‘supply only’ period enables patients with existing prescriptions to access the medicine through the PBS for a limited time after it is delisted.
63 The review committee is composed of an independent chair, two patient representatives, the chair of PBAC, a clinical/scientific representative, a member nominated by Medicines Australia and a government nominee.
64 Department of Health and Aged Care, Health Technology Assessment Policy and Methods Review – Terms of Reference, available from https://www.health.gov.au/resources/publications/health-technology-asse…
65 Department of Health and Aged Care, Health Technology Assessment Policy and Methods Review – Final report, available from https://www.health.gov.au/resources/collections/hta-review-final-report-collection [accessed 16 September 2024].
66 The Commonwealth price is derived from the AEMP and pharmacy remuneration fees charged for dispensing, administration, wholesale costs, and dangerous drugs.
67 The Pharmacy Guild is a national peak body employers’ organisation for owners of community pharmacies.
68 As part of the 2023–24 Federal Budget the Government announced it would enact legislative changes to enable some medicines to be provided to patients with 60-day prescriptions. This has been implemented in three stages on 1 September 2023, 1 March 2024 and 1 September 2024.
69 Department of Prime Minister and Cabinet, User guide to the Australian Government guide to policy impact analysis, Commonwealth of Australia, Canberra, 2023, available from https://oia.pmc.gov.au/sites/default/files/2023-11/user-guide-to-the-australian-government-guide-to-policy-impact-analysis.pdf [accessed 24 June 2024].
70 The Office of Impact Analysis was known as the Office of Best Practice Regulation prior to November 2022.
71 Department of Health and Aged Care, Post-implementation Review of the Seventh Community Pharmacy Agreement, Commonwealth of Australia, Canberra, 2022.
72 ibid., p. 3.
73 Office of Impact Analysis, 7CPA post implementation review, Department of the Prime Minister and Cabinet, 24 January 2023, available from https://oia.pmc.gov.au/published-impact-analyses-and-reports/7cpa-post-implementation-review [accessed 12 September 2024].
74 Department of the Prime Minister and Cabinet, Negotiation of new Community Pharmacy Agreement (8CPA), Commonwealth of Australia, Canberra, 2024, available from https://oia.pmc.gov.au/published-impact-analyses-and-reports/negotiation-new-community-pharmacy-agreement-8cpa [accessed 24 July 2024].
75 Under the ‘Joint Safety Net’, non-PBS medicines dispensed at outpatient pharmacies at public hospitals can also count towards the PBS Safety Net, further information available from https://www.pbs.gov.au/info/general/faq#WhatistheJointSafetyNet [accessed 30 August 2024]
76 Department of Health and Aged Care, Eighth Community Pharmacy Agreement, Commonwealth of Australia, Canberra, 2024, available from https://www.health.gov.au/topics/primary-care/what-we-do/8cpa [accessed 24 June 2024].
77 The Safety Net card provides patients access to medicines for free (for concession card holders) or $7.70 (for general patients).
78 RISs are required for all submissions to Cabinet. Minor regulatory proposals only require a summary of the policy, overview of impacts and outline of associated costs.
79 The range which can be discounted increases each year in line with CPI.
80 These prices are as of 1 January 2023 and increase annually with CPI. Similar to other discounting practices pharmacies forgo the price difference.
81 In 2022–23 the one dollar discount was applied to 50 million scripts. In 2024 Health advised the government in the context of 8CPA negotiations that removing the one dollar discount had an indicative cost of $190 million to patients and would decrease competition in the pharmacy sector.
82 Auditor-General Report No. 39 2009–10, Medicare Australia’s Administration of the Pharmaceutical Benefits Scheme, ANAO, Canberra, 2010, available from https://www.anao.gov.au/work/performance-audit/medicare-australias-administration-the-pharmaceutical-benefits-scheme [accessed 24 June 2024].
83 ibid., p. 107.
84 Department of Health, Review of Pharmacy Remuneration and Regulation, DoH, Canberra, 2017, p. 10.
85 ibid., p. 9.
86 Department of Health and Aged Care, PBS Expenditure and Prescriptions, Canberra, 2023, available from https://www.pbs.gov.au/info/statistics/expenditure-prescriptions/pbs-expenditure-and-prescriptions [accessed 24 June 2024].
87 Services Australia, Pharmaceutical Benefits Schedule Item Reports, Services Australia, Canberra, 2024, available from http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp [accessed 24 June 2024].
Services Australia, Pharmaceutical Benefits Schedule Groups Statistics Reports, Services Australia, Canberra, 2024, available from http://medicarestatistics.humanservices.gov.au/statistics/pbs_group.jsp [accessed 24 June 2024].
88 As the budget was held in October 2022 there was no MYEFO in 2022–23.
89 Australian Bureau of Statistics, Pharmaceutical Benefits Scheme Supplied Medications, Canberra, 2022, available from https://www.abs.gov.au/articles/pharmaceutical-benefits-scheme-supplied-medications [accessed 12 August 2024].
90 Parliamentary Budget Office, 2020–21 Medium-term fiscal projections, Commonwealth of Australia, Canberra, 2020, available from https://www.aph.gov.au/-/media/05_About_Parliament/54_Parliamentary_Depts/548_Parliamentary_Budget_Office/Reports/2020-21/2020-21_Med-term_fiscal_projections/2020-21_Medium-term_fiscal_projections_PDF.pdf [accessed 12 August 2024], Table 3.1.
91 Horizon scanning is the identification of new and emerging technologies that may impact the supply of healthcare. It often occurs between one to three years before the technology becomes available.
92 The National Health (Supply of Pharmaceutical Benefits–Under Co-payment Data and Claims for Payment) Rules 2022 are made by the minister under subsection 99AAA(8) of the NHA.
93 Expenditure excludes electronic prescription fee payments and claim number excludes claims yet to be closed by approved suppliers. Services Australia, Annual Report 2022–23, available from https://www.servicesaustralia.gov.au/annual-report-2022-23?context=22 [accessed 20 June 2024].
94 In the case of approved suppliers that are pharmacists, in practice, these are pharmacy owners rather than the pharmacist or technician performing the pharmaceutical services at a pharmacy or hospital.
95 Services Australia deducts the sum of 47 cents plus GST in respect of each supply of a PBS medicine for which payment is sought.
96 In 2022–23 the ANAO found that all key controls underpinning the automated system for PBS payments were operating as intended.
97 Auditor-General Report No. 39 2009–10, Medicare Australia’s Administration of the Pharmaceutical Benefits Scheme, ANAO, Canberra, 2010, paragraph 4.102, available from https://www.anao.gov.au/work/performance-audit/medicare-australias-administration-the-pharmaceutical-benefits-scheme [accessed 11 December 2024].
98 The advice stated that the 65-day timeframe arises from subsections 6(3) and 6(4) of the National Health (Supply of Pharmaceutical Benefits–Under Co-payment Data and Claims for Payment) Rules 2022 which provide for certification to be done within 30 days of the end of a period not exceeding 35 days:
6(3) The information must be given to the Chief Executive Medicare in relation to pharmaceutical benefits supplied by the approved supplier during a period not exceeding 35 days, unless the Chief Executive Medicare is satisfied that the approved supplier was unable, through circumstances outside the approved supplier’s control, to comply with that requirement.
6(4) The information must be given to the Chief Executive Medicare not more than 30 days after the last day of the period in respect of which previous information in relation to supplies of pharmaceutical benefits was given by the approved supplier, unless the Chief Executive Medicare is satisfied that the approved supplier was unable, through circumstances outside the approved supplier’s control, to comply with that requirement.
99 Strategic Performance Measure 3 is limited to social security and welfare payments included in program agreements between Services Australian and the Department of Social Services and manually and automatically processed health provider claims under the Medicare Benefits Scheme.
100 Strategic Performance Measure 5 is limited to social security and welfare payments included in program agreements between Services Australian and the Department of Social Services, child support registrations, emergency payments, and manually and automatically processed health provider claims under the Medicare Benefits Scheme.
101 PBS 1 has a target of 30 seconds for average speed of answer for calls to PBS Authorities telephony line. PBS 6 has a target of 82 per cent or more of written requests processed within five working days (electronic/self-serve) or 10 working days (complex).
102 Strategic Performance Measure 5 does not include data on the timeliness of PBS claims, see paragraph 4.28.
103 This project is part of the ‘Strengthening Medicare’ funding package announced in the 2023–24 Budget. A total of $69.7 million over four years was allocated for health delivery modernisation including the development of new digital health services.
104 Services Australia assumed responsibility for claims processing from its predecessor (the Department of Human Services) in June 2019.
105 Other results for a claim are possible (registered, closed and reviewed); however, these comprise a small proportion of the overall claims processed (‘registered’, 1629, 1.12 per cent; ‘closed’, 369, 0.25 per cent; ‘reviewed’, 19, 0.01 per cent). These results are larger in proportion than rejected claims (0.0069 per cent), though they do not represent finalised states of claims.
106 As discussed in paragraph 4.70, Services Australia has not achieved performance targets for PBS Safety Net processing since September 2023.