Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Administration of the Fifth Community Pharmacy Agreement

Please direct enquiries relating to reports through our contact page.
The audit objective was to assess the effectiveness of the development and administration of the Fifth Community Pharmacy Agreement (5CPA), and the extent to which the 5CPA has met its objectives.
Summary
Introduction
1. The Australian Government provides subsidised medicines to Australians and eligible overseas visitors through the Pharmaceutical Benefits Scheme (PBS). In 2013–14, the PBS subsidised over 210 million prescriptions at a reported cost to government of some $9.15 billion. The Government also subsidised an additional 12.4 million prescriptions in 2013–14 to the veteran community through the Repatriation Pharmaceutical Benefits Scheme (RPBS), at a cost of $397.9 million.
2. Since 1990 the Australian Government has entered into and funded successive five year community pharmacy agreements, at a cost of over $45 billion1, to help maintain a national network of approximately 5460 retail pharmacies as the primary means of dispensing PBS medicines2 to the public. The Government has also used the agreements to fund professional programs, and to establish a funding pool to be drawn on by pharmaceutical wholesalers that can meet specified service standards for supplying PBS medicines to retail pharmacies.
3. The Fifth Community Pharmacy Agreement (5CPA) is the current agreement between the Minister for Health, representing the Commonwealth, and the Pharmacy Guild of Australia (Pharmacy Guild), representing the majority of retail pharmacies currently approved to supply PBS medicines.3 The introduction to the 5CPA states that:
Community pharmacy is an integral part of the infrastructure of the health care system in its role in primary health care through the delivery of the Pharmaceutical Benefits Scheme and related services.
The Fifth Community Pharmacy Agreement
4. To support community access to pharmaceutical services, the 5CPA provides that the Australian Government will deliver some $15.4 billion in funding from 1 July 2010 to 30 June 2015 as follows4:
- $13.8 billion in ‘pharmacy remuneration’5, including various fees for approved pharmacists—the owners of retail pharmacies that dispense PBS and RPBS subsidised medicines to the public6;
- $663 million for several categories of government funded professional programs7; and
- $950 million to be shared among eligible pharmaceutical wholesalers from a Community Service Obligation (CSO) funding pool, an arrangement which generally requires participating wholesalers to be able to supply the full range of PBS items to any retail pharmacy in Australia within 24 hours at an agreed price.
5. One of the key objectives of the 5CPA negotiations was to achieve savings to contribute to the structural repair of the Commonwealth Budget as there had been high cost growth under the 4CPA (an average growth of 9.4 per cent per year) that was due, in part, to a $1.1 billion transitional structural adjustment package (financial assistance) to assist pharmacies adjust to the introduction of Price Disclosure in 2007.8
6. The 5CPA anticipates that the initiatives covered by the agreement will result in $1 billion in government savings.9 The major savings initiatives were:
- cessation of the PBS Online incentive payment ($417.7 million);
- freezing the dispensing fee for two years ($281.5 million);
- cessation of underperforming professional programs ($226.4 million);
- reduction in private hospital pharmacy remuneration ($35.3 million); and
- freezing the CSO Funding Pool for one year ($19.2 million).
7. The 5CPA also references the Australian Government’s Pharmacy Location Rules (Location Rules), which regulate where new pharmacies that dispense PBS prescriptions may open and where existing pharmacies may relocate.
8. Six broad ‘principles and objectives’ are specified in the 5CPA:
i. Ensure a fair Commonwealth price is paid to Approved Pharmacists for providing pharmaceutical benefits while maximising the value to taxpayers by encouraging an effective and efficient community pharmacy network.
ii. Ensure that the Programs are patient-focused and target areas of need in the community including continued improvement in community pharmacy services provided to Aboriginal and Torres Strait Islander people.
iii. Ensure transparency and accountability in the expenditure of the Funds.
iv. Promote the sustainability and efficiency of the PBS within the broader context of health reform and ensuring that community resources continue to be appropriately directed across the health system, while also supporting the sustainability and viability of an effective community pharmacy sector.
v. Maintain a co-operative relationship between the Commonwealth and the Guild.
vi. Ensure the Location Rules work for the benefit of the Australian community including increased access to community pharmacies for the population of rural and remote areas.
9. The 5CPA is a complex multi-part agreement underpinned by a number of further agreements between the Department of Health (Health) and the other entities involved in its administration, including: the Department of Human Services (Human Services); the Pharmacy Guild of Australia10; and Australian Healthcare Associates (AHA). The Pharmacy Guild and AHA are non-government entities.
Administrative arrangements
10. The 5CPA was developed and negotiated by Health, which has overarching responsibility for its administration, and agreed by government. The Pharmaceutical Benefits Division within Health has responsibility for policy advice on all elements of the 5CPA, while the Department of Veterans’ Affairs (DVA) is responsible for policy advice on the RPBS, including RPBS pharmacy remuneration.
11. Until 1 March 2014, Human Services administered most 5CPA professional programs on behalf of Health (valued at $583 million), while the Pharmacy Guild administered some of the smaller programs (valued at $67 million). On 1 March 2014, Health transferred responsibility for the 5CPA professional programs administered by Human Services to the Pharmacy Guild, which now administers all 5CPA professional programs on behalf of Health.11
12. In respect of the 5CPA, the Pharmacy Guild is variously:
- an industry association and advocate acting on behalf of retail pharmacy owners, making representations to government and public inquiries, and conducting public campaigns;
- a publicly funded administrator under the 5CPA, at times acting as the Department of Health’s agent;
- a recipient of Commonwealth grants relating to certain 5CPA professional programs12;
- an owner of business enterprises that sell products and services to pharmacies on a commercial basis—with some products and services relating to 5CPA programs and activities; and
- an advisor to Health, through its co-membership of the overarching 5CPA governance body13 and under its contracts with the department.
13. Human Services processes pharmacy claims for reimbursement of PBS and RPBS dispensing on behalf of Health and DVA respectively, and accesses relevant Health and DVA appropriations for this purpose. The administration of the CSO Funding Pool is outsourced by Health to Australian Healthcare Associates (AHA), a private company based in Melbourne. AHA collects data from participating CSO pharmaceutical wholesalers and calculates their monthly share of the funding pool; with Health paying CSO wholesalers on the basis of AHA’s advice.
14. A summary of the five year budget and staffing for key entities involved in administering the 5CPA, before and after the transfer of 5CPA professional programs from Human Services to the Pharmacy Guild in March 2014, is shown in Table S.1.
Table S.1: Five year budget for 5CPA administration
|
Entity |
2010–15 budget before 1 March 2014 |
2010–15 budget after 1 March 2014 |
||
|
|
Staffing (ASL) |
Budget ($m) |
Staffing (ASL) |
Budget ($m) |
|
Healtha |
237.8 |
30.8 |
239.7 |
31.2 |
|
Human Services |
273.5 |
41.8 |
150.1 |
25.5c |
|
Veterans’ Affairs |
1.0 |
0.1 |
1.0 |
0.1 |
|
Pharmacy Guildb |
- |
29.3 |
- |
31.2 |
Source: Health, Human Services, Pharmacy Guild and Veterans’ Affairs information.
Notes: An entity’s administrative budget may cover a range of administrative costs in addition to staffing, such as ICT, property, legal and miscellaneous costs. ASL is average staffing level.
- See Table 1.7 for Health’s disaggregated annual staffing and administrative expenditure.
- The Pharmacy Guild was unable to provide ASL figures. See Table 5.2 for a detailed breakdown of administrative and program funding as specified in Health contracts with the Pharmacy Guild.
- Human Services’ ongoing budget was reduced by the equivalent of $16.4 million over five years following the transfer of 5CPA professional programs to the Pharmacy Guild. Prior to the transfer of functions, Human Services had expended $14.3 million. Health and the Pharmacy Guild received the unexpended $2.1 million portion of Human Services’ original budget.
Audit objective, criteria and methodology
Audit objective and scope
15. The audit objective was to assess the effectiveness of the development and administration of the Fifth Community Pharmacy Agreement (5CPA), and the extent to which the 5CPA has met its objectives. The audit examined the development and negotiation of the 5CPA by the then Department of Health and Ageing (now the Department of Health), and the administration of the 5CPA by Health. The audit also examined aspects of the 5CPA that were implemented by the Department of Human Services (Human Services) and the Department of Veterans’ Affairs (DVA).
16. While the ANAO did not examine the Pharmacy Guild of Australia’s administration of 5CPA professional programs, the audit refers to aspects of its involvement relating to the development, negotiation and administration of the 5CPA.
17. The Pharmacy Location Rules are not examined in this performance audit. They were considered in 2014 by the report of the National Commission of Audit14 and the draft report of the National Competition Policy Review.15
Criteria
18. To form a conclusion against the audit objective, the ANAO adopted the following high-level criteria:
- the 5CPA provides transparent and accountable remuneration arrangements for the dispensing of Commonwealth pharmaceutical benefits, which achieve value for money, consistent with Government policy;
- the 5CPA’s funding and savings commitments are being met;
- the additional programs and services funded under the 5CPA are managed effectively and provide value for money; and
- the 5CPA performance framework enables an assessment of the extent to which the 5CPA is meeting its objectives.
Methodology
19. The audit methodology included:
- interviewing staff from Health, Human Services and DVA;
- extracting pharmacy claims and payment records from Health and Human Services databases;
- reviewing relevant documentation, including departmental files, briefings, legal advice, program guidelines, monitoring and reporting systems, reviews, evaluations and correspondence;
- consulting stakeholders and peak bodies, including the Pharmacy Guild; and
- reviewing over 100 stakeholder submissions received by the ANAO through its citizen’s input facility.
Overall conclusion
20. The Fifth Community Pharmacy Agreement (5CPA) continues the Australian Government’s policy approach, over 25 years, to enter into a funding agreement with the Pharmacy Guild of Australia (Pharmacy Guild)—representing the majority of retail pharmacy owners—to help maintain a national network of some 5460 retail pharmacies as the primary means of dispensing PBS medicines to the public. The 5CPA also provides for access to patient services that may be delivered by retail pharmacies or consultant pharmacists. The parties have entered into five successive agreements, valued at over $45 billion, since 1990.
21. The $15.4 billion 5CPA provides that the Australian Government will deliver, over five years from 2010 to 2015: $13.8 billion in ‘pharmacy remuneration’, including various fees for approved retail pharmacies; $663 million in funding for professional programs; and a funding pool of $950 million to be drawn on by eligible pharmaceutical wholesalers to provide PBS medicines to pharmacies in a timely manner and at an agreed price. Although actual expenditure on the components of pharmacy remuneration is demand driven—depending on the number of PBS and RPBS medicines prescribed by doctors—the 5CPA commits the Government to delivering a fixed sum of money.16 The 5CPA also states that the initiatives covered by the agreement will result in $1 billion in savings to government.
22. The 5CPA is the head agreement in a complex scheme of legal, financial and administrative arrangements involving both government entities and third parties in its implementation. The 5CPA is underpinned by further agreements, principally between the Department of Health (Health) and the Department of Human Services (Human Services), the Pharmacy Guild of Australia and Australian Healthcare Associates (AHA). The arrangements were developed and negotiated by Health, which has overarching responsibility for the 5CPA’s administration, and agreed by Government.
23. Overall, the Department of Health’s administration of the Fifth Community Pharmacy Agreement has been mixed, and there is a limited basis for assessing the extent to which the 5CPA has met its key objectives, including the achievement of $1 billion in expected savings. The department developed and negotiated a complex agreement and related contracts with the Pharmacy Guild in a timely manner, enabling the 5CPA to be signed by the Health Minister and Pharmacy Guild on 3 May 2010, prior to the expiry of the 4CPA on 30 June 2010. However, a number of key government negotiating objectives for the 5CPA were only partially realised and there have been shortcomings in key aspects of Health’s administration at the development, negotiation and implementation phases.
24. The 5CPA states that the initiatives covered by the agreement will result in $1 billion in savings over the term of the agreement. The 2010–11 Budget Papers17 clarified that the $1 billion in savings is a gross figure, and after taking into account approved additional expenditure of $0.4 billion, net savings were estimated to be $0.6 billion.18 However, ANAO analysis indicates that the net savings estimated before the agreement was signed were closer to $0.4 billion, due to shortcomings in the department’s 5CPA estimation methodology.19 The principal issues relate to: unexplained increases in the baseline cost of professional programs; the application of inappropriate indexation factors; and the treatment of patient co-payments. In particular:
- The baseline budget for 5CPA professional programs in the Commonwealth forward estimates was $638.7 million (before adjusting for the negotiated 5CPA savings and spending measures). However, Health’s records showed that the approved baseline budget for 5CPA professional programs was only $511.6 million, and there was no documentary evidence of authority to increase the 5CPA baseline budget in the forward estimates by $127.1 million.
- The official indexation factors released by the then Department of Finance and Deregulation (Finance)20 were not utilised in estimating 5CPA savings, resulting in an overestimate of 5CPA savings of approximately $43.2 million.
- Health advised, in the course of this audit, that the estimated savings for the 5CPA incorrectly included $42.7 million in co-payments made by patients to pharmacies for the receipt of pharmaceutical benefits. Co-payments are a private contribution to the cost of PBS medicines, which are not a cost to government.
25. Actual pharmacy remuneration (paid by government and patients) in the first four years of the 5CPA aligned closely with the commitment made originally in the 5CPA.21 However, during the life of the agreement there have been two estimates variations (in 2011 and 2013) relating to the cost of one component of pharmacy remuneration—the Premium Free Dispensing Incentive22—which increased the expected cost to government of pharmacy remuneration by $292 million23 and also impacted the level of savings from the 5CPA.
26. In addition to the shortfall in anticipated savings, a number of the Government’s other strategic negotiating objectives were only partially realised, as previously indicated. In this context, the then Government and department considered that the 5CPA offered an opportunity to improve health outcomes and value for money by restructuring pharmacy remuneration arrangements ‘to diminish their link to the price of PBS medicines’. The Commonwealth anticipated doing so by shifting financial incentives from the volume driven sale of medicines to the delivery of value-adding professional services. However, the structure of pharmacy remuneration remained essentially unchanged from the 4CPA to the 5CPA—based on defined mark-ups to the base price of pharmaceuticals and the addition of a variety of fees. Further, key wholesaler and pharmacy mark-ups continued at previous rates.
27. A further Commonwealth negotiating objective, which Ministers considered to be ‘non-negotiable’, related to obtaining access from pharmacies to the full range of PBS data, including information relating to prescriptions that cost less than the general patient co-payment. This information would help the Commonwealth determine actual PBS pharmacy remuneration from all sources, including patients, and the total volume and cost of the PBS to both government and consumers. This objective was partially realised—while the 5CPA made provision for pharmacies to provide certain prescription information from 1 April 2012, it did not make provision for the receipt of cost information.
28. Another key government negotiating objective for the 5CPA was to support information technology systems that are fully interoperable with broader e-health systems. However, the two Prescription Exchange Services (PESs) that were approved by Health for the purpose of downloading electronic prescriptions by pharmacies, did not have systems that were interoperable. Government funding for the Electronic Prescription Fee (EPF) was subsequently re-allocated to pay the PESs directly to make their systems interoperable.
29. Six broad principles and objectives were included in the 5CPA.24 Limited departmental information25, plus shortcomings in Health’s performance reporting and 5CPA evaluation framework, mean that the department is not well positioned to assess whether the Commonwealth is receiving value for money from the agreement overall, or performance against the six principles and objectives. While some aspects of the agreement will be evaluated, the 5CPA evaluation framework does not make provision for reviews of the agreement’s two major financial components—pharmacy remuneration ($13.8 billion) and Community Service Obligation (CSO) payments to pharmaceutical wholesalers ($950 million). Pharmacy remuneration, which lies at the heart of the 5CPA and previous community pharmacy agreements—accounting for some 90 per cent of funding delivered under the current agreement—has not been fully reviewed since 1989.26 There is scope to improve the performance and evaluation frameworks for the 5CPA and the next community pharmacy agreement.
30. In addition to shortcomings in 5CPA costings, performance reporting and the evaluation framework, this audit identified scope for improvement in key aspects of the department’s general administration which covered the 5CPA’s development, negotiation and implementation phases. The key issues relate to: the clarity of the 5CPA and related public reporting; record-keeping; the application of financial framework requirements; risk management; and seeking Ministerial approvals. In particular:
- The 5CPA does not clearly document expected net savings under the agreement, and there is no straightforward means for the Parliament and other stakeholders to be informed of the expected or actual cost of key 5CPA components. Specifically, the agreement does not document that some $2.2 billion of the $13.8 billion that the Commonwealth ‘will deliver’ for pharmacy remuneration is sourced from patient co-payments, which are not a cost to government.27 Similarly, the department’s annual report aggregates the cost of pharmacy remuneration (expenditure on services) with the cost of PBS medicines (expenditure on products), without differentiating between the two types of expenditure.
- There were persistent shortcomings in departmental record-keeping relating to the 5CPA. Health did not keep a formal record of its meetings with the Pharmacy Guild during the 5CPA negotiations, and did not document its subsequent discussions with the Guild on the negotiation of related contracts. Given the significance of the issues under negotiation, the decision not to prepare an official record of discussions was not consistent with sound practice, as shortcomings in record-keeping can affect a government entity’s capacity to discharge advisory, accountability and contract management obligations.
- The department did not assess whether financial framework requirements would apply to Pharmacy Guild officials when making payments of public money pursuant to the administration of 5CPA professional programs, resulting in a risk of non-compliance with legislative requirements. In designing and implementing complex administrative arrangements, it is important to consider relevant resource management requirements at the design stage, so as to avoid potential compliance and reputational risks.
- The 5CPA provides flexibility to re-allocate money between the various professional programs funded under the agreement, subject to Ministerial approval. However, at times the department has re-allocated funds without prior Ministerial approval, including to a $5.8 million communication strategy to be delivered by the Pharmacy Guild. The communication strategy is not a professional program, but was nonetheless funded mainly from professional program allocations.
- Further, Health did not secure Ministerial approval before re-allocating $7.3 million of funding originally approved by Ministers as a component of pharmacy remuneration—the Electronic Prescription Fee (EPF)—to other purposes, including financial assistance paid to Prescription Exchange Services28 and $896 110 to the Pharmacy Guild to increase pharmacies’ understanding, awareness and uptake of EPF. As a consequence, in the first three years of the 5CPA some 80 per cent of EPF payments were not paid to pharmacies but were instead used to fund other activities. While Health advised the ANAO that discussions were held with the Minister’s office, documented evidence to support this was not available.
31. The 5CPA is a substantial agreement that is integral to the parties achieving shared objectives—the maintenance of a national network of retail pharmacies as the primary means of dispensing PBS medicines to the public, and providing professional services to patients. Features of the 5CPA include complexity in policy design and administrative arrangements, and a key lesson of this audit is the importance of identifying and treating risks at the earliest opportunity.29 The successful implementation of complex programs requires active management and a disciplined and co-ordinated approach to managing risks and challenges through the program life cycle—including the development, costing, negotiation and implementation phases. Further, there is a need to ensure that there is appropriate authority for revised positions and outcomes when events do not unfold according to expectations.
32. The ANAO has made eight recommendations aimed at improving the overall administration of the 5CPA and informing the development of the next community pharmacy agreement. Seven recommendations are directed to Health, and relate to: the development of costings; improving the clarity of the next agreement and related public reporting; record-keeping; and improving performance information. A further recommendation directed to Health, Human Services and DVA focuses on improving the accuracy of Health’s calculation of pharmacy remuneration for reporting and evaluation purposes.
Summary of entity responses
33. The proposed audit report was provided to the Department of Health, and extracts were provided to the Department of Human Services, the Department of Veterans’ Affairs, the Department of Finance, the Pharmacy Guild of Australia and MediSecure Limited. Entities’ summary responses are included below and full responses are included at Appendix 1.
Health
34. The Department agrees with the Recommendations of the Report.
35. The Department welcomes the Report as an opportunity to further review the administration and processes for community pharmacy agreements. The Department acknowledges there is scope to realise further improvement in the effective and efficient administration of these agreements and welcomes the recommendations as a platform for ongoing development of future community pharmacy agreements and transparent engagement with the pharmacy sector.
36. I am pleased the Australian National Audit Office (ANAO) acknowledged a number of the significant outcomes of the negotiations and implementation of the Fifth Community Pharmacy Agreement (the Agreement) including, but not limited to, the negotiations having been completed on-time for a 1 July 2010 commencement of the Agreement, a 70% reduction in polypharmacy and 50% reduction in medication errors due to the introduction of the National Residential Medication Chart, and the successful implementation of the Continued Dispensing initiative. Further, the Report accurately notes the complexity of the Agreement and its subsidiary arrangements and the pharmacy sector, as one component interacting with a more complex pharmaceutical sector and a broader health care system.
37. I am also pleased to advise that the Department has already implemented a range of improvements relating to issues identified in this Report and previous draft reports. For example, enhancements to financial modelling processes have been implemented to separately report on the cost impacts to government and patients. This will also enable the Department to revise its public financial reporting to include the cost of each major component of pharmacy remuneration in the future. I also note that risk, probity and legal plans are already in place for the anticipated negotiation of a future agreement along with a recording framework for key decisions throughout the negotiations.
Human Services
38. The Department of Human Services agrees with the findings and conclusions contained in the extract of the report provided. It agrees with ANAO audit Recommendation Number 4 in the extract and will support the Departments of Health and Veterans’ Affairs in their determination of reporting requirements on the cost of the Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme expenditure on products.
Veterans’ Affairs
39. The Department of Veterans’ Affairs (DVA) agrees with the report’s Recommendation Number 4, that the Department of Health (DoH) and DVA work closely with the Department of Human Services (DHS) to develop and refine processes for capturing and reporting data on pharmacy remuneration. DVA has already collaborated successfully with DoH and DHS across a number of key areas. DVA is confident that future projects centred on ensuring the exchange and use of accurate data will result in positive outcomes.
40. In addition to accepting the report’s recommendation, DVA confirms its intent to review the legislative instrument that establishes the Repatriation Pharmaceutical Benefits Scheme (RPBS) in order to clarify pricing arrangements for pharmaceutical benefits. The RPBS is fundamental to DVA’s commitment to ensuring the health and wellbeing of eligible veterans and their dependents and the Department welcomes any recommendations to improve the Scheme’s operation.
Pharmacy Guild of Australia
41. The Pharmacy Guild of Australia (the Guild) welcomes the audit of the Administration of the Fifth Community Pharmacy Agreement (5CPA).
42. As the organisation representing the majority of community pharmacy owners, the Guild has a statutory responsibility under the National Health Act to negotiate with the Commonwealth the remuneration for pharmacies for dispensing PBS medicines. The Guild also plays a key role in the oversight and administration of the professional programmes funded under the community pharmacy agreements, working in partnership with the Department of Health, in consultation with a wide range of industry, consumer and other stakeholders.
43. The Guild takes these responsibilities very seriously. In successive agreements, the Guild has assisted the Commonwealth in facilitating opportunities for pharmacies and pharmacists to play an enhanced role in delivering the objectives of the National Medicines Policy through the provision of an expanding range of professional services, underpinned by nationally accredited quality assurance standards and enabled by leading-edge information technology platforms and systems.
44. The Guild considers that the audit has provided an important opportunity to scrutinise the administration of the 5CPA and will continue to work constructively with the Commonwealth and all stakeholders in ensuring that the next agreement provides maximum benefit to community pharmacy, the pharmacist profession, taxpayers, and, most importantly, the Australian public which relies on these vital PBS medicines and health care services.
MediSecure Limited
45. Our overall comments are that this audit report appears to have been well researched and has brought clarity and simplicity to the complex and convoluted arrangements that constitute the publicly funded support mechanisms for the delivery of medicines to the Australian community. I have worked in this space for 15 years and during that time have not seen a document that is as well researched. We congratulate the Auditor-General’s team on the quality and breadth of the extract we have seen.
Recommendations
|
Recommendation No.1 Paragraph 2.32 |
To clarify the nature of financial commitments entered into by the Australian Government, the ANAO recommends that the Department of Health presents, in key documents, estimated government payments and patient payments for both subsidised and unsubsidised Pharmaceutical Benefits Scheme and Repatriation Pharmaceutical Benefits Scheme medicines. Health response: Agreed. |
|
Recommendation No.2 Paragraph 2.55 |
To provide assurance regarding the basis of costings for the next community pharmacy agreement, the ANAO recommends that the Department of Health applies the relevant forecast indexation factors released by the Department of Finance. Health response: Agreed. |
|
Recommendation No.3 Paragraph 2.78 |
To improve its ability to satisfy accountability requirements and capacity to protect the interests of the Commonwealth in the event of disputes or legal action, the ANAO recommends that the Department of Health:
Health response: Agreed. |
|
Recommendation No.4 Paragraph 3.71 |
To improve the accuracy and transparency of reporting on Australian Government expenditure under the Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme, the ANAO recommends that the Departments of Health, Veterans’ Affairs and Human Services liaise on the collection, recording and sharing of information regarding payments to suppliers, so as to clearly identify the actual cost of medicines and the components of pharmacy remuneration. Health response: Agreed. Veterans’ Affairs response: Agreed. Human Services response: Agreed. |
|
Recommendation No.5 Paragraph 5.30 |
In order to effectively discharge its advisory, accountability and contract management obligations in a timely manner, the ANAO recommends that the Department of Health reviews its record keeping arrangements for the Fifth Community Pharmacy Agreement and the next community pharmacy agreement. Health response: Agreed. |
|
Recommendation No.6 Paragraph 5.67 |
To improve transparency in agreement-making, the ANAO recommends that the Department of Health documents anticipated levels of Australian Government funding for third party administration for the next community pharmacy agreement. Health response: Agreed. |
|
Recommendation No.7 Paragraph 6.12 |
To improve transparency and the quality of program performance reporting, the ANAO recommends that the Department of Health reports annually on the actual cost of each major component of the Fifth Community Pharmacy Agreement and the next community pharmacy agreement, including pharmacy remuneration, CSO wholesaler payments and professional programs. Health response: Agreed. |
|
Recommendation No.8 Paragraph 6.28 |
To inform decision-making and the assessment of outcomes by stakeholders, the ANAO recommends that the Department of Health reviews performance reporting to improve alignment between the next community pharmacy agreement and public reporting against the program objectives, deliverables and KPIs relating to the department’s Program 2.1 and Program 2.2. Health response: Agreed. |
1. Introduction
This chapter outlines the background, scope and objectives of the Fifth Community Pharmacy Agreement (5CPA); the policy, legal and administrative framework relating to the 5CPA; and the audit objective, criteria and methodology.
Background
1.1 The Australian Government provides subsidised medicines to Australians and eligible overseas visitors through the Pharmaceutical Benefits Scheme (PBS). In 2013–14, the PBS subsidised over 210 million prescriptions at a reported cost to government of some $9.15 billion.30 The Government also subsidised an additional 12.4 million prescriptions in 2013–14 to the veteran community through the Repatriation Pharmaceutical Benefits Scheme (RPBS), at a cost of $397.9 million.31
1.2 Since 1990, the Australian Government and the Pharmacy Guild of Australia (Pharmacy Guild)32 have entered into successive five-year agreements known as community pharmacy agreements. In the context of the agreements, ‘community pharmacies’ are retail pharmacies approved under the National Health Act 1953 (the National Health Act) to supply PBS medicines to the public. The current agreement is the fifth (the 5CPA), and is intended to operate from 2010 to 2015. While the main purpose of the agreements has been to set out remuneration arrangements for the owners of retail pharmacies that dispense PBS prescriptions, the scope of agreements has progressively broadened to establish a range of government funded professional programs (such as medication reviews), and a funding pool for pharmaceutical wholesalers that meet the requirements of the Community Service Obligation (CSO), which generally requires participating wholesalers to be able to supply PBS items to any retail pharmacy in Australia within 24 hours. Community pharmacy agreements have also referenced the Australian Government’s Pharmacy Location Rules (Location Rules), which regulate where new pharmacies that dispense PBS prescriptions may open and where existing pharmacies may relocate.
Table 1.1: The origin of community pharmacy agreements
|
Community pharmacy agreements—background |
|
The Australian Government has reimbursed pharmacy owners for dispensing PBS items to the public since the PBS was first introduced.a From 1953 to 1976, the Minister for Health was empowered under Section 99 of the National Health Act to determine pharmacy remuneration for PBS dispensing. In 1980, the Australian Parliament’s Joint Committee of Public Accounts recommended the establishment of an independent Tribunal to determine pharmacy remuneration for PBS dispensing.b In 1981, the Pharmaceutical Benefits Remuneration Tribunal (the Tribunal) was established under Section 98A of the National Health Act, and operated independently of the Government and the Pharmacy Guild. In 1989, after examining surveys into pharmacies’ dispensing costs, the Tribunal concluded that pharmacy owners were being over-remunerated for dispensing PBS medicines.c The Tribunal decided to change pharmacy remuneration by abolishing the mark-up then applying on PBS medicines, and reducing the dispensing fee. The Pharmacy Guild opposed this decision. The then Minister for Health subsequently negotiated directly with the Pharmacy Guild, and in 1990, entered into the first Community Pharmacy Agreement. The National Health Act was also amended to require the Tribunal to give effect to the terms of any pricing agreementd between the Minister for Health and the Pharmacy Guild (or another organisation representing a majority of retail pharmacy owners approved to supply PBS items).e |
Notes:
- C Sloane, A History of the Pharmaceutical Benefits Scheme 1947–1992, Australian Government Publishing Service, Canberra, 1995, pp. 52–59.
- Joint Committee of Public Accounts, Report 182: Pharmaceutical Benefits Scheme-Chemists’ Remuneration, Australian Government Publishing Service, Canberra, 1980, p. xiii. In this inquiry the Committee examined and reported on the reasons for a significant excess payment by the Department of Health to chemists in respect of their remuneration under the PBS between 1973 and 1980. The Committee also examined the concurrent excess payments made by the Department of Veterans’ Affairs to chemists under the RPBS. The combined total of overpayments was estimated at approximately $253 million.
- Pharmaceutical Benefits Remuneration Tribunal, Data Base Inquiry Final Report, Canberra, 28 August 1989.
- The National Health Act provides that: ‘…where the Minister (acting on the Commonwealth’s behalf) and the Pharmacy Guild of Australia or another pharmacists’ organisation that represents a majority of approved pharmacists have entered into an agreement in relation to the manner in which the Commonwealth price of all or any pharmaceutical benefits is to be ascertained for the purpose of payments to approved pharmacists in respect of the supply by them of pharmaceutical benefits, the Tribunal, in making a determination under subsection 98B(1) while the agreement is in force, must give effect to the terms of that agreement…’, Section 98BAA(1), National Health Act 1953.
- The Tribunal restored the previous mark-up and dispensing fee on 29 December 1989. The reason the Tribunal gave for revoking its previous determination was, among other things, ‘the Tribunal’s concern to ensure as far as possible that the fees fixed are not only fair, just and equitable but are clearly perceived to be so’. Pharmaceutical Benefits Remuneration Tribunal, Determination and Report Fourteenth Inquiry, Canberra, 25 June 1990.
1.3 The overall cost of successive community pharmacy agreements has been over $45 billion. While actual costs of the agreements have not been publicly reported, Health advised the ANAO that the value of community pharmacy agreements (CPAs) was: 1CPA ($3.286 billion); 2CPA ($5.497 billion); 3CPA ($8.804 billion); 4CPA ($12.158 billion). The original estimated cost of 5CPA was $15.384 billion, which Health has since revised to $15.610 billion as reported in November 2014.
Pharmaceutical services
1.4 The use of a medicine is the most common health intervention in Australia.33 Of the 286 million prescriptions dispensed annually from retail pharmacies in Australia34, approximately 269 million (94 per cent) are PBS or RPBS prescriptions.35 Of these 269 million prescriptions, some 208 million (77 per cent) attract a government subsidy, and the rest are priced below the patient co-payment.36
Role of pharmacists
1.5 A pharmacist is a health specialist trained to exercise independent judgement when dispensing medicines and reviewing the use of medicines, in order to ensure that the medicines are safe and appropriate for the patient and that they conform to prescribers’ (generally doctors’) requirements.37 A pharmacist may advise prescribers and patients on the proper use of medicines, and provide primary health care services by educating consumers regarding health promotion and disease prevention.
1.6 Australia’s per capita consumption of certain medicines is among the highest in the developed world38 and access to pharmaceutical services, including appropriate medication management, is of key importance in achieving health outcomes. While medicines can improve quality of life and prevent disability and unnecessary hospitalisation, increasing medicines use is associated with a broad spectrum of medicine related problems.39 Medicine related problems are a direct cost to PBS and RPBS expenditure, and an indirect cost when adverse drug events are undetected or misdiagnosed, and trigger further interventions.40 It has been estimated that over 1.5 million Australians suffer an adverse event from medicines each year.41 Collectively, adverse drug events are responsible for up to 230 000 hospital admissions annually in Australia, costing approximately $1.2 billion per annum.42 In summary, effective medication management has an impact on patient safety and treatment, and the overall effectiveness of government and community spending on medicines and related services.
The pharmacy sector
1.7 The Australian pharmacy sector is highly regulated. State and Territory legislation restricts pharmacy ownership to registered pharmacists, and requires pharmacies to be licensed to operate. At the national level, the Australian Government’s Pharmacy Location Rules43 regulate the location of retail pharmacies approved to dispense PBS subsidised medicines.44
1.8 As at March 2014, there were 28 188 registered pharmacists in Australia.45 About 63 per cent of registered pharmacists work in retail pharmacy with the majority as employees. As at 30 June 2014, there were 5457 retail pharmacies approved under Section 90 of the National Health Act to supply PBS subsidised medicines.46 Section 90 approved pharmacies comprise:
- 5305 commercially operated retail pharmacies; and
- 152 ‘not-for-profit’ Friendly Society pharmacies.47
1.9 In general usage the term ‘community pharmacy’ may refer to:
- a pharmacy located in the community (in contrast to a hospital); or
- a retail pharmacy approved under Section 90 of the National Health Act to supply pharmaceutical benefits48 to the public; or
- the practice of pharmacy by pharmacists who deliver health services in a community setting (in contrast to a hospital setting).
1.10 To avoid confusion, the ANAO has used the term ‘retail pharmacy’ to refer to a pharmacy approved under Section 90 of the National Health Act to supply PBS medicines to the public.
Pharmacy bodies and associations
1.11 The Pharmacy Board of Australia is the national body responsible for registering pharmacists and regulating the pharmacy profession. The Board produces professional codes and guidelines that are mandatory.49 Under assignment from the Board, the Australian Pharmacy Council (APC) is responsible for accrediting pharmacy schools and programs; conducting examinations; and assessing overseas trained pharmacists and international students.
1.12 The professional associations representing the Australian pharmacy profession are shown in Figure 1.1. The largest professional association is the Pharmaceutical Society of Australia (PSA), with over 18 000 members. The Society of Hospital Pharmacists of Australia (SHPA) has the largest number of members practising in hospitals and other health service facilities. The PSA and SHPA have respectively developed extensive professional practice standards, but they are not mandatory.50 Professional Pharmacists Australia represents pharmacists who work as employees of pharmacies. The SHPA and the Australian Association of Consultant Pharmacy (AACP) train and accredit registered pharmacists to perform patient medication reviews.
1.13 The Pharmacy Guild, which signed the 5CPA with the Australian Government, represents the owners of retail pharmacies.51 The Australian Friendly Societies Pharmacies Association (AFSPA) represents not-for-profit pharmacies owned and operated by Friendly Societies.
Figure 1.1: Professional pharmacy bodies and associations
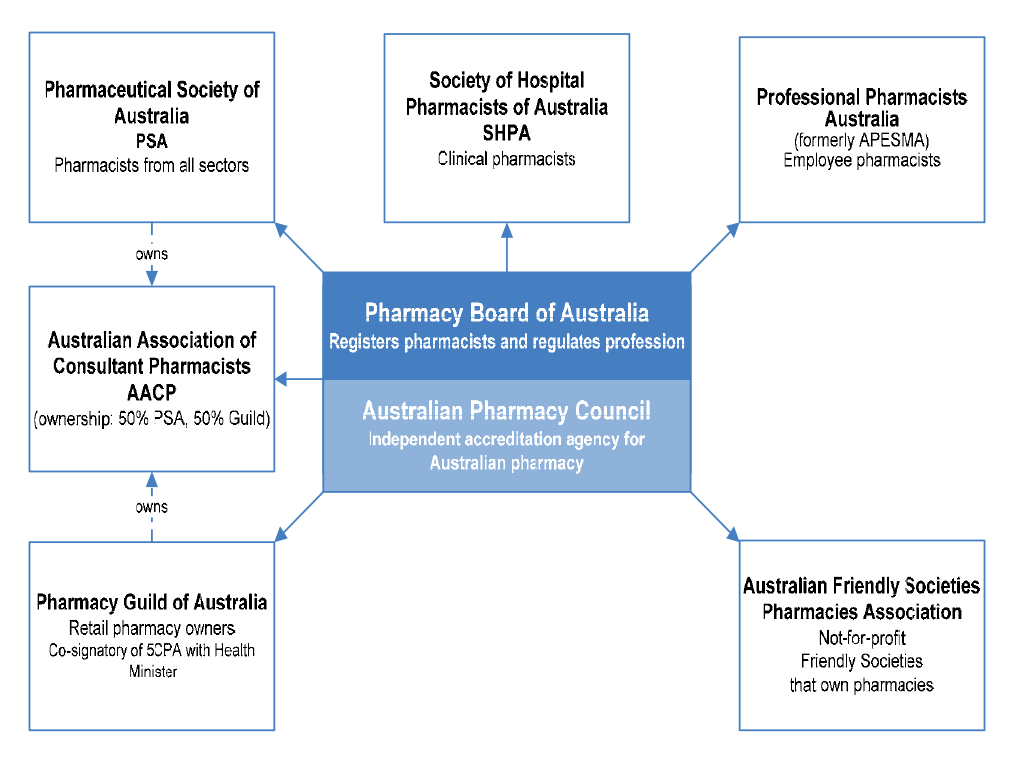
Source: ANAO analysis.
The Fifth Community Pharmacy Agreement (5CPA)
1.14 The Fifth Community Pharmacy Agreement (5CPA), which runs from 1 July 2010 to 30 June 201552, is a complex multi-part agreement. Its structure is outlined in Figure 1.2.
Figure 1.2: Structure of the 5CPA

Source: ANAO analysis of the 5CPA.
1.15 In summary, the 5CPA provides that the Australian Government will deliver $15.4 billion in funding over five years, as follows:
- $13 771.6 million in pharmacy remuneration (excluding the cost of medicines);
- $949.5 million to be shared among eligible pharmaceutical wholesalers that qualify for the CSO Funding Pool; and
- $663.4 million for professional programs.
1.16 Figure 1.3 reproduces the terms of the 5CPA relating to funding.
Figure 1.3: Commonwealth funding under the 5CPA
The Commonwealth will deliver $15.4 billion under the Agreement as set out in the following table:
Table: Funding for elements of the Agreement
|
Element |
$m |
|
Pharmacy remuneration (includes dispensing fee, pharmacy and wholesale mark-up, extemporaneously prepared and dangerous drug fees, premium free dispensing incentive and electronic prescription fee) |
13,771.6 |
|
Programs and services |
386.4 |
|
Additional Programs to support patient services |
277.0 |
|
Community Service Obligation |
949.5 |
|
Total |
15,384.5 |
Source: The Fifth Community Pharmacy Agreement, pp. 2–3.
1.17 The 5CPA was developed and negotiated by the then Department of Health and Ageing (now the Department of Health), and agreed by government. The Minister for Health and the Pharmacy Guild are signatories to the agreement, which was executed on 3 May 2010. The 5CPA’s six ‘principles and objectives’ are shown in Table 1.2.
Table 1.2: Principles and objectives of the 5CPA
|
i |
Ensure a fair Commonwealth price is paid to Approved Pharmacists for providing pharmaceutical benefits while maximising the value to taxpayers by encouraging an effective and efficient community pharmacy network. |
|
ii |
Ensure that the Programs are patient-focused and target areas of need in the community including continued improvement in community pharmacy services provided to Aboriginal and Torres Strait Islander people. |
|
iii |
Ensure transparency and accountability in the expenditure of the Funds. |
|
iv |
Promote the sustainability and efficiency of the PBS within the broader context of health reform and ensuring that community resources continue to be appropriately directed across the health system, while also supporting the sustainability and viability of an effective community pharmacy sector. |
|
v |
Maintain a co-operative relationship between the Commonwealth and the Guild. |
|
vi |
Ensure the Location Rules work for the benefit of the Australian community including increased access to community pharmacies for the population of rural and remote areas. The specific objectives of the Location Rules are to ensure:
|
Source: Fifth Community Pharmacy Agreement, Section 1.2(d).
Key elements of the 5CPA
Pharmacy remuneration ($13.8 billion)
1.18 The 5CPA sets out the remuneration arrangements for ‘approved pharmacists’—the owners of retail pharmacies approved to dispense PBS subsidised medicines. Pharmacy remuneration is the largest financial component of the 5CPA, at a cost of some $11.6 billion to government and $2.2 billion to patients.53 As indicated in Figure 1.3, the 5CPA lists the components of ‘pharmacy remuneration’ as the: wholesale mark-up, pharmacy mark-up, dispensing fee, extemporaneously prepared fee, dangerous drug fee, Premium Free Dispensing Incentive (PFDI) and Electronic Prescription Fee (EPF).54 Further detail on these components is provided in Table 1.3.55
Table 1.3: Components of 5CPA pharmacy remuneration
|
Component as at 1 August 2014 |
Description |
|
Wholesale mark-up: 7.52 per cent capped at $69.94 |
A mark-up added to the ex-manufacturer price for the medicine.a |
|
Pharmacy mark-up: 15 to 4 per cent stepped mark-up bands capped at $70.00 |
A mark-up added to the wholesale price of the medicine.a |
|
Ready Prepared (RP) dispensing fee: $6.76 |
A flat fee paid for dispensing a ready prepared (‘off-the-shelf’) medicine. |
|
Extemporaneously Prepared (EP) dispensing fee: $8.80 |
A flat fee paid for dispensing an extemporaneously prepared medicine (requires ‘compounding’ or some preparation by the pharmacist). |
|
Dangerous drug fee: $2.71 |
A flat fee paid for dispensing a dangerous drug—classified as a ‘controlled drug’ due to its high potential for abuse and addiction. |
|
Premium Free Dispensing Incentive (PFDI): $1.68 |
A flat fee paid for dispensing a premium free PBS medicine that costs the patient no more than the patient co-payment. |
|
Electronic Prescription Fee (EPF): $0.15 |
A flat fee paid for dispensing a PBS/RPBS prescription that is downloaded from an electronic Prescription Exchange Service (PES)—paid on both over co-payment and under co-payment prescriptions. |
Source: ANAO analysis.
Notes:
- The wholesale mark-up and pharmacy mark-up are paid per dispensed maximum quantity for the item as specified in the PBS Schedule.
- This table outlines the pricing structure for Section 85 (National Health Act) General Pharmaceutical Benefits. Different pricing arrangements apply to pharmaceutical benefits supplied under Section 100 (National Health Act) special arrangements, which are outlined in Table 1.4.
PBS dispensing
1.19 In order for a patient to receive a medicine subsidised under the PBS, an authorised prescriber56 must prescribe a medicine that is listed on the PBS Schedule.57, 58 The patient (or their agent) presents the PBS prescription at an approved supplier (usually a retail pharmacy), which dispenses the item and charges the patient any applicable co-payment.59 The patient co-payment reduces the amount the government reimburses the pharmacy.
1.20 Retail pharmacies claim reimbursement for dispensing PBS items from the Department of Human Services (Human Services). Human Services calculates the dispensed price of the medicine, and then deducts any patient co-payment in order to work out the amount owing by the Commonwealth to the pharmacy.60 Different pricing rules apply to different classes of PBS items.
The PBS Schedule
1.21 The PBS Schedule lists over 902 drugs61, available in more than 2335 forms and strengths, and marketed as 5420 differently branded items.62 For pharmacy remuneration purposes, PBS medicines that are dispensed by retail pharmacies may be classified into five main groups, each with different pricing rules, as shown in Table 1.4. The RPBS Schedule is a supplementary list of items (including bandages and dressings) that are available to eligible veterans and their dependents. The RPBS generally adopts PBS pricing arrangements.
1.22 Under the 5CPA, retail pharmacies may also charge additional fees for PBS items that are priced under the patient co-payment. An example of how a common PBS item is priced is included in Appendix 2.
1.23 Table 1.4 summarises the main classes of PBS items dispensed by retail pharmacies. References are to the relevant sections of the National Health Act.
Table 1.4: Main classes of PBS items dispensed by retail pharmacies
|
PBS schedule |
Contains |
|
Section 85 items – general pharmaceutical benefits |
|
|
Section 85 (General schedule) |
The largest group of PBS drugs and the most frequently prescribed. |
|
Prescriber Bag |
About 26 Section 85 drugs provided free of charge to prescribers for emergency use. |
|
Section 100 items – items available under special arrangements |
|
|
Section 100 (Highly Specialised Drugs) |
Drugs for treating chronic conditions, which can only be initiated by a medical specialist. |
|
Section 100 (Efficient Funding of Chemotherapy) |
About 36 drugs for treating cancer, which are administered by infusion or injection. |
|
Section 100 (Remote Aboriginal Health Services) |
Section 85 drugs supplied in bulk to remote area Aboriginal Health Service clinics. |
Source: ANAO analysis.
Note: The Health Minister must declare all PBS items under Section 85 of the National Health Act. However, under Section 100, the Minister may make special arrangements for, or in relation to, providing that an adequate supply of pharmaceutical benefits will be available to persons in defined circumstances. In practice, different pricing arrangements have been implemented for certain groups of drugs supplied under Section 100 arrangements.
Pharmacy Location Rules
1.24 Subject to State and Territory law, any registered pharmacist may open a retail pharmacy. However, if a pharmacy owner wishes to claim reimbursement for dispensing pharmaceutical benefits, the pharmacy owner must first seek Commonwealth approval to supply pharmaceutical benefits from specific premises.63 If successful, the pharmacy owner is granted an approval number, which applies exclusively to those premises. If a pharmacy owner wishes to operate more than one approved pharmacy, they must separately apply for approval for each additional premises.64
1.25 The Location Rules were introduced in the first community pharmacy agreement to address perceived inefficiencies in the retail pharmacy sector and the oversupply and viability of pharmacy services in particular geographic areas.65 The first agreement provided incentive payments for pharmacies to close or merge, and the number of approved pharmacies decreased from around 5600 in 1990 to 4793 in 2003.66 The number of approved pharmacies has since increased to 5457 as at June 2014.67
1.26 The Location Rules set out location based criteria that must be met in order for the Australian Community Pharmacy Authority (ACPA) to recommend approval of a new pharmacy or the relocation of an existing pharmacy.68 Health and ACPA administer the Location Rules, and Human Services administers the pharmacy approvals system on behalf of Health.69
1.27 The Location Rules were considered in 2014 by the National Commission of Audit70 and the National Competition Policy Review.71
CSO Funding Pool ($949.5 million)
1.28 The CSO Funding Pool provides subsidies to eligible pharmaceutical wholesalers that meet the CSO service standards for supplying PBS items to retail pharmacies. The objective of the Funding Pool is to: ‘ensure that arrangements are in place to provide all Australians with ongoing and timely access to all PBS medicines, through community pharmacies’. The Funding Pool was established on 1 July 2006 under the 4CPA, and is administered by Australian Healthcare Associates, a private contractor, on behalf of Health. To access the Funding Pool, pharmaceutical wholesalers must tender for registration and enter into a deed of agreement with Health.
1.29 The CSO service standards include: being able to supply any brand of PBS item to any retail pharmacy in Australia within 24 hours; meeting minimum sales thresholds for low volume items and sales to rural and remote retail pharmacies; and supplying any PBS item at or below the ‘approved price to pharmacist’—which is based on the price agreed between the manufacturer and the government.72
5CPA professional programs ($663.4 million)
1.30 The Australian Government funds the following categories of professional programs under the 5CPA, at a total cost of $663.4 million:
- Pharmacy Practice Incentives and Accreditation ($344 million);
- Medication Management ($163.9 million);
- Rural Support ($107 million);
- Aboriginal and Torres Strait Islander Programs ($28.9 million);
- Research and Development ($10.6 million);
- Medication Continuance73 ($1 million); and
- Other Programs to support patient services (Electronic recording of controlled drugs74 ($5 million); and Supply and PBS Claiming from a Medication Chart in Residential Aged Care Facilities ($3 million)).
1.31 The categories listed above summarise a complex array of publicly funded professional programs and activities, which are illustrated with the other elements of the 5CPA in Figure 1.4.
Figure 1.4: Elements of the 5CPA
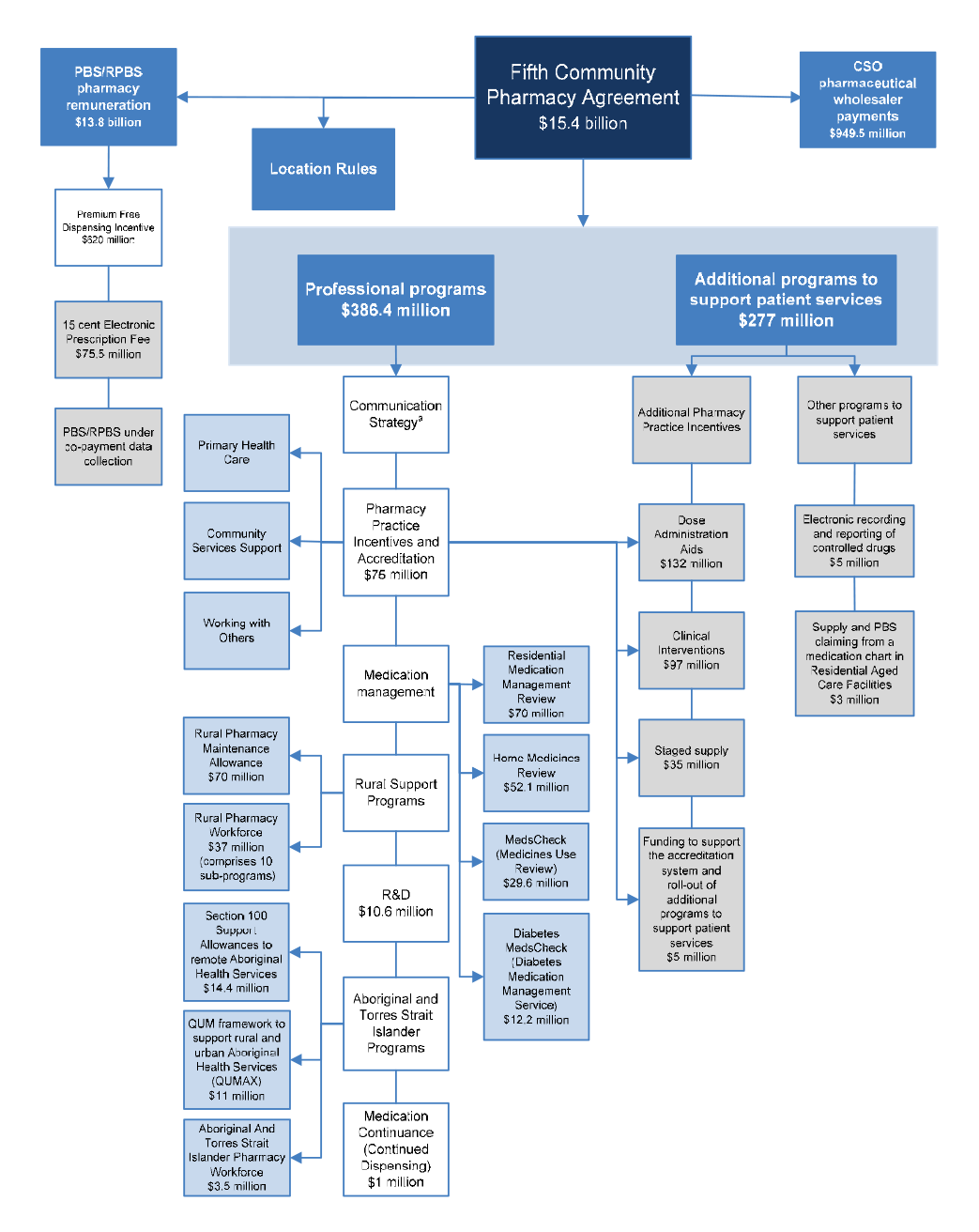
Source: ANAO analysis.
Notes:
- The Communication Strategy (shown above but not mentioned in the 5CPA) was funded by re-allocating $5.8 million from funding for professional programs.
- Grey boxes contain elements of pharmacy remuneration, professional programs and activities funded from new expenditure that was agreed in the 5CPA.
Legal and financial framework
Pharmacy remuneration
1.32 The PBS is established under Part VII of the National Health Act. As discussed, pharmacy remuneration is payable for PBS dispensing, and most of the components of pharmacy remuneration are built into the ‘Commonwealth price’ of PBS items. The Commonwealth price of PBS items is calculated by reference to determinations made by the Pharmaceutical Benefits Remuneration Tribunal (for Section 85 items), or the Minister for Health (for Section 100 and Prescriber Bag items).75 The payments for these components of pharmacy remuneration are authorised under Section 99 of the National Health Act, and made under Section 137 of the National Health Act, which establishes a special appropriation administered by Health.
1.33 In addition, the 5CPA sets out two additional fees as components of pharmacy remuneration: the Premium Free Dispensing Incentive (PFDI) and the Electronic Prescription Fee (EPF). The Commonwealth relies on provisions in the financial management legislation to authorise payment of these fees.76
1.34 Pharmacy remuneration is also payable for RPBS dispensing. The RPBS is established under Section 91 of the Veterans’ Entitlements Act 1986. The RPBS generally incorporates 5CPA pharmacy remuneration arrangements for the dispensing of items listed on the PBS and RPBS Schedules for eligible veterans and their dependents.77 RPBS pharmacy remuneration is funded through an annual appropriation administered by the Department of Veterans’ Affairs (DVA).
Pharmacy Location Rules
1.35 The Minister for Health determines the Location Rules under Section 99L of the National Health Act (the Act). Division 4B of the Act establishes the Australian Community Pharmacy Authority (ACPA), which considers applications to establish new or relocate existing approved pharmacies in accordance with the Location Rules. The ACPA consists of six members: five appointed by the Minister for Health, including two pharmacists nominated by the Pharmacy Guild and one pharmacist nominated by the Pharmaceutical Society of Australia; and one departmental representative appointed by the Secretary of the Department of Health. The ACPA recommends to the Secretary whether or not to approve a pharmacy.78
CSO Funding Pool
1.36 The Community Service Obligation (CSO) Funding Pool for pharmaceutical wholesalers was established under the 4CPA. The CSO is funded through an annual administered appropriation of the Department of Health. The CSO is an executive scheme, which is not established under legislation. Until 30 June 2014, the legislative authority for CSO payments was the reference to ‘Pharmaceuticals and pharmaceutical services’ in Part 4, Schedule 1AA of the FMA Regulations.79
Professional programs
1.37 The 5CPA professional programs are also executive schemes, which are not established by legislation. The 5CPA programs generally have program guidelines approved by Health, and many of these guidelines are available on the 5CPA website.80 Until 30 June 2014, the legislative authority for 5CPA professional program expenditure was the reference to ‘Community pharmacy and pharmaceutical awareness’ in Part 4, Schedule 1AA of the FMA Regulations.81
Agreements and contracts
1.38 The 5CPA is a complex multi-part agreement underpinned by a number of further agreements between the Department of Health and the other entities involved in its administration, including: the Department of Human Services (Human Services); the Department of Veterans’ Affairs (DVA); the Pharmacy Guild of Australia; and Australian Healthcare Associates (AHA).82 The Pharmacy Guild and AHA are non-government entities.
Administrative arrangements
1.39 The 5CPA was developed and negotiated by Health, which has overarching responsibility for its administration, and agreed by Government. The Pharmaceutical Benefits Division within Health has responsibility for policy advice on all elements of the 5CPA, while DVA is responsible for policy advice on the RPBS, including RPBS pharmacy remuneration.
1.40 Until 1 March 2014, Human Services administered most 5CPA professional programs on behalf of Health, while the Pharmacy Guild administered Rural Support Programs, Indigenous Programs and the Research and Development Program on behalf of Health. On 1 March 2014, Health transferred responsibility for the 5CPA professional programs administered by Human Services to the Pharmacy Guild, which now administers all professional programs.83
1.41 In respect of the 5CPA, the Pharmacy Guild is variously:
- an industry association and advocate acting on behalf of retail pharmacy owners, making representations to government and public inquiries84, and conducting public campaigns85;
- a publicly funded administrator under the 5CPA, at times acting as the Department of Health’s agent;
- a recipient of Commonwealth grants86 relating to certain 5CPA professional programs87;
- an owner of business enterprises that sell products and services to pharmacies on a commercial basis—with some products and services relating to 5CPA programs and activities88; and
- an advisor to Health, through its co-membership of the overarching 5CPA governance body89 and under its contracts with the department.
1.42 Human Services processes pharmacy claims for reimbursement of PBS and RPBS dispensing on behalf of Health and DVA respectively, and accesses relevant Health and DVA appropriations for this purpose. The administration of the CSO Funding Pool is outsourced by Health to Australian Healthcare Associates (AHA), a private company based in Melbourne. AHA collects data from participating CSO pharmaceutical wholesalers and calculates their monthly share of the funding pool, with Health paying CSO wholesalers on the basis of AHA’s advice.
1.43 An outline of 5CPA funding and administrative arrangements from 1 March 2014 is presented in Table 1.5.
Table 1.5: 5CPA funding and administrative arrangements from March 2014
|
5CPA element |
Funding $m |
Policy advice |
Claims processed by |
Paid by |
|
Pharmacy remuneration total |
$13 771.6 |
|
|
|
|
PBS |
|
Health |
DHSa |
DHS |
|
RPBS |
|
DVA |
DHS |
DHS |
|
Location Rules |
|
|
|
|
|
Approval of new pharmacies and relocation of existing pharmacies |
n/a |
Health |
DHS ACPAb |
n/a |
|
Community Service Obligation (CSO) Funding Pool total |
$949.5 |
|
|
|
|
CSO funding for pharmaceutical wholesalers |
$949.5 |
Health |
Australian Healthcare Associates |
Health |
|
Programs and services total |
$386.4 |
|
|
|
|
Pharmacy Practice Incentivesc |
$75.0 |
Health |
Guild |
Guild |
|
Medication Management Programs |
$163.9 |
Health |
Guild |
Guild |
|
Rural Support Programs |
$107.0 |
Health |
Guild |
Guild |
|
Aboriginal and Torres Strait Islander Programs |
$28.9 |
Health |
Guild |
Guild |
|
Research and Development |
$10.6 |
Health |
Guild |
Guild |
|
Medication Continuance |
$1.0 |
Health |
n/a |
n/a |
|
Additional Programs to Support Patient Services total |
$277.0 |
|
|
|
|
Additional Pharmacy Practice Incentivesc |
$269.0 |
Health |
Guild |
Guild |
|
Electronic recording of controlled drugs |
$5.0 |
Health |
n/a |
n/a |
|
Supply and PBS claiming from a medication chart in Residential Aged Care Facilities |
$3.0 |
Health |
n/a |
n/a |
Source: ANAO analysis of Health, Human Services and DVA information.
Notes:
- DHS is the Department of Human Services; DVA is the Department of Veterans’ Affairs.
- ACPA is the Australian Community Pharmacy Authority. ACPA does not process claims but reviews applications and makes recommendations.
- Pharmacy Practice Incentives and Additional Pharmacy Practice Incentives are part of the same program.
1.44 A summary of the five year budget and staffing for key entities involved in the 5CPA’s administration, before and after the transfer of 5CPA professional programs from Human Services to the Pharmacy Guild in March 2014, is shown in Table 1.6.
Table 1.6: Five year budget for 5CPA administration
|
Entity |
2010–15 budget before 1 March 2014 |
2010–15 budget after 1 March 2014 |
||
|
|
Staffing (ASL) |
Budget ($m) |
Staffing (ASL) |
Budget ($m) |
|
Healtha |
237.8 |
30.8 |
239.7 |
31.2 |
|
Human Services |
273.5 |
41.8 |
150.1 |
25.5c |
|
Veterans’ Affairs |
1.0 |
0.1 |
1.0 |
0.1 |
|
Pharmacy Guildb |
- |
29.3 |
- |
31.2 |
Source: Health, Human Services, Pharmacy Guild and Veterans’ Affairs information.
Notes: An entity’s administrative budget may cover a range of administrative costs in addition to staffing, such as ICT, property, legal and miscellaneous costs. ASL is average staffing level.
- See Table 1.7 for Health’s disaggregated annual staffing and administrative expenditure.
- The Pharmacy Guild was unable to provide ASL figures. See Table 5.2 for a detailed breakdown of administrative and program funding as specified in Health contracts with the Pharmacy Guild.
- Human Services’ ongoing budget was reduced by the equivalent of $16.4 million over five years following the transfer of 5CPA professional programs to the Pharmacy Guild. Prior to the transfer of functions, Human Services had expended $14.3 million. Health and the Pharmacy Guild received the unexpended $2.1 million portion of Human Services’ original budget.
1.45 Health’s annual expenditure and staffing for the administration of the 5CPA is shown in Table 1.7.
Table 1.7: Health’s 5CPA annual administrative expenditure and staffing
|
Year |
Before transfer of the 5CPA professional programs on 1 March 2014 |
After transfer of the 5CPA professional programs on 1 March 2014 |
||
|
|
Staffing (ASL) |
Expenditure ($m) |
Staffing (ASL) |
Expenditure ($m) |
|
2010-11 (actual) |
64.0 |
7.2 |
64.0 |
7.2 |
|
2011-12 (actual) |
49.2 |
6.5 |
49.2 |
6.5 |
|
2012-13 (actual) |
45.3 |
6.2 |
45.3 |
6.2 |
|
2013-14 (actual) |
41.5 |
5.7 |
42.1 |
6.0 |
|
2014-15 (budget—indicative) |
37.8 |
5.1 |
39.1 |
5.3 |
Source: Department of Health.
Note: Health’s 2014–15 budget is indicative as at August 2014.
Audit objective, criteria and methodology
Audit objective and scope
1.46 The audit objective was to assess the effectiveness of the development and administration of the Fifth Community Pharmacy Agreement (5CPA), and the extent to which the 5CPA has met its objectives. The audit examined the development and negotiation of the 5CPA by the then Department of Health and Ageing (now the Department of Health), and the administration of the 5CPA by the Department of Health (Health). The audit also examined aspects of the 5CPA that were implemented by the Department of Human Services (Human Services) and the Department of Veterans’ Affairs (DVA).
1.47 While the ANAO did not examine the Pharmacy Guild of Australia’s administration of 5CPA professional programs, the audit refers to aspects of its involvement relating to the development, negotiation and administration of the 5CPA.
1.48 The Pharmacy Location Rules are not examined in this performance audit. They were considered in 2014 by the report of the National Commission of Audit and the draft report of the National Competition Policy Review.
Criteria
1.49 To form a conclusion against the audit objective, the ANAO adopted the following high-level criteria:
- the 5CPA provides transparent and accountable remuneration arrangements for the dispensing of Commonwealth pharmaceutical benefits, which achieve value for money, consistent with Government policy;
- the 5CPA’s funding and savings commitments are being met;
- the additional programs and services funded under the 5CPA are managed effectively and provide value for money; and
- the 5CPA performance framework enables an assessment of the extent to which the 5CPA is meeting its objectives.
Methodology
1.50 The audit methodology included:
- interviewing staff from Health, Human Services and DVA;
- extracting pharmacy claims and payment records from Health and Human Services databases;
- reviewing relevant documentation, including departmental files, briefings, legal advice, program guidelines, monitoring and reporting systems, reviews, evaluations and correspondence;
- consulting stakeholders and peak bodies, including the Pharmacy Guild; and
- reviewing over 100 stakeholder submissions received by the ANAO through its citizen’s input facility.90
1.51 Fieldwork was conducted in Health, Human Services and DVA, in Canberra, Adelaide and Melbourne. This is the first ANAO performance audit of a community pharmacy agreement.91
1.52 The audit was conducted in accordance with ANAO auditing standards at a cost to the ANAO of some $828,541.
Report structure
1.53 The structure of this report is outlined in Table 1.8.
Table 1.8: Structure of the audit report
|
Chapter |
Overview |
|
2. Development of the 5CPA |
Examines the development of the 5CPA, including the planning, costing and negotiation of the agreement. |
|
3. Pharmacy Remuneration |
Examines the implementation of 5CPA pharmacy remuneration arrangements; departmental reporting of pharmacy remuneration; and the actual costs of pharmacy remuneration compared to departmental estimates. |
|
4. Pharmaceutical Services |
Examines dispensing services and 5CPA professional programs and services funded by the Australian Government under the 5CPA. |
|
5. Administration of Professional Programs |
Examines the governance and administrative arrangements for the 5CPA professional programs and services, and Health’s contractual arrangements with the Pharmacy Guild. |
|
6. Reporting and Evaluation |
Examines Health’s reporting and evaluation arrangements for the 5CPA. |
2. Development of the 5CPA
This chapter examines the development of the 5CPA, including the planning, costing and negotiation of the agreement.
Introduction
2.1 The Department of Health (Health) has developed and negotiated successive community pharmacy agreements on behalf of the Australian Government. This chapter examines Health’s processes for planning, costing and negotiating the Fifth Community Pharmacy Agreement (5CPA) with the Pharmacy Guild of Australia (Pharmacy Guild), including:
- planning, with particular reference to the development of a 5CPA costing model by Health;
- the forecasting and reporting of expected 5CPA costs and savings; and
- the negotiation of the 5CPA and outcomes of negotiations.
Planning for the 5CPA
2.2 Health advised the ANAO that there was no clear start date for its planning of the 5CPA, as there was a gradual ‘ramping up’ of activity that started at least 12 months before negotiations with the Pharmacy Guild commenced in July 2009.92 Although there was no documented plan for Health’s development of the 5CPA, Health advised the ANAO that the key steps in the process were:
- background research;
- the development of a forecasting model to estimate the costs of pharmacy remuneration under different scenarios;
- engagement with the department’s senior executive;
- seeking government approval; and
- negotiations with the Pharmacy Guild.
2.3 Health further advised the ANAO that the department engaged more widely beyond the department’s senior executive, including across the department, through an inter-departmental committee, and other stakeholder groups beyond the Pharmacy Guild.93
Background research and analysis
2.4 Health indicated that the 5CPA planning process involved consideration of evaluations of the components of the 4CPA. One evaluation was completed before the 5CPA was signed, and eleven were completed afterwards.94 To fully inform the development of the agreement, the evaluations should desirably have been completed before the agreement was finalised. In this respect, Health advised the ANAO that:
Evaluations and/or reviews undertaken during 4CPA, including those that did not commence until 2010, were still used to inform the construct of 5CPA programs (i.e., how continuing programs may have been implemented). In several instances, the Department used draft reports (if they existed) as a part of the negotiations.
2.5 In October 2008, Health convened a Departmental Workshop Group that canvassed a range of strategic and policy issues related to the development of the 5CPA. In November 2008, Health also convened a Remuneration Working Group to conduct background research for the development of the 5CPA. Following research and analysis of pharmacy’s role in the health care system, Health concluded that the current system of remunerating pharmacies based on the price and volume of medicines that they dispense had significant drawbacks. Health advised the ANAO that:
The results of the Department’s research and analysis were presented and discussed at an Inter-Departmental Committee (IDC) consisting of officers from the Departments of Finance and Deregulation, the Treasury, the Prime Minister and Cabinet and Industry, Tourism and Resources. Following the Department’s research and analysis and the consideration of the IDC, it was concluded that the current system of remunerating pharmacies based on the price and volume of medicines that they dispensed had drawbacks consistent with any fee-for-service or retail model, namely:
- the cost of pharmacy remuneration was driven by factors unrelated to health outcomes for patients, or services provided to patients; and
- retail pharmacies had limited incentive to improve the quality use of medicines, or other professional services to consumers.
2.6 Health did not keep a record of the meetings of the inter-departmental committee that considered these issues in the lead up to the negotiation of the 5CPA.
2.7 Pharmacy remuneration is structured around the price of individual medicines through a complex system of mark-ups and fees95, which do not necessarily relate to the level of professional service required by different patients with different medication regimens. Health’s records indicate that, at the time, the department considered the negotiation of the 5CPA to be an opportunity to improve health outcomes through better utilisation of the professional skills of pharmacists. Health considered that these outcomes could be achieved by restructuring pharmacy remuneration to shift the financial incentives from the volume driven sale of medicines, to the delivery of professional services.
Development of the 5CPA costing model
Limitations of previous costing model
2.8 A key departmental objective in the lead up to the negotiation of the 5CPA was the development of a pharmacy remuneration costing model. Health had experienced difficulties in negotiating the 4CPA partly due to a lack of technical capability.96 According to a departmental report:
Unfortunately, the Department had to rely on an Excel spreadsheet to forecast the costs to government of the various remuneration scenarios put forward during the negotiations of the Fourth Agreement. The Excel spreadsheet could only provide linear forecasting, relied on manual manipulation of data (therefore subject to human error), was dependent on availability of Departmental staff with advanced Excel skills and struggled to manage the large amount of Medicare PBS dispensing data. The Excel spreadsheet was slow and cumbersome. The deficiencies of the Excel spreadsheet were again highlighted in the 2006–07 negotiations of the compensation package to pharmacists under the PBS reforms…
2.9 The shortcomings of Health’s Excel spreadsheet re-emerged in 2006–07 when the Government introduced Price Disclosure for certain PBS items, in order to align their prices with actual market prices, as outlined in Figure 2.1.
Figure 2.1: Price Disclosure
|
Manufacturers may sell PBS medicines to wholesalers and pharmacies at less than the ex-manufacturer price agreed with the Australian Government. In 2007, the growing disparity between the government price and the market price of PBS drugs led to the introduction of mandatory Price Disclosure for some drugs. Under Price Disclosure arrangements, manufacturers are required to report the actual price and sales volumes for certain PBS drugs (generally those off-patent). Under the 2007 Price Disclosure rules, if the weighted average manufacturer discount was 10 per cent or more over a 12 month period, the government price was reduced to the average market price. However, at that time, the reduction in government price did not take effect until 24 to 28 months from the start of data collection.a Price Disclosure produces savings in two ways:
Price disclosure directly affects pharmacy remuneration as pharmacies receive higher remuneration for more expensive medicines because the wholesale mark-up and pharmacy mark-up are generally based on a stepped percentage mark-up of the relevant base price, and are only capped at a flat fee for the highest price bracket. |
Source: ANAO analysis of Department of Health information.
Note:
- Department of Health, Report to the Minister for Health: Review of Funding Arrangements for Chemotherapy Services, DoH, Canberra, October 2013, available from: <http://www.health.gov.au/internet/main/publishing.nsf/Content/chemotherapy-review/$File/review-of-chemotherapy-funding-arrangements.pdf> [accessed 20 March 2014].
2.10 Health had relied on the Excel spreadsheet when negotiating a structural adjustment package (financial assistance) to assist pharmacies transition to the new Price Disclosure arrangements. Health reported the following outcome of negotiating the structural adjustment package with the Pharmacy Guild:
… The Commonwealth and the Guild arrived at a significantly different figure for the dispensing fee adjustment required to achieve the agreed compensation package. A review of the Excel spreadsheet highlighted significant errors in the manual manipulation of PBS dispensing data. The Department therefore agreed with the Guild to rebuild its remuneration model, and recalculate the dispensing fee amount within a six month timeframe.97
2.11 By 2009, Health was also considering options for expanding Price Disclosure to bring the government price for medicines closer to the actual market price. In addition, the 5CPA negotiations were expected to canvass changes to the components of pharmacy remuneration. Consequently, Health needed to develop a costing model in order to accurately forecast:
- the cost of pharmacy remuneration under different scenarios (such as a different professional fee structure); and
- savings from expanded Price Disclosure.98
2.12 Health advised the ANAO that its new costing model, called the Pharmacy Remuneration and Negotiation Cost Information System (PhRANCIS), was developed for 5CPA costings and due to its capabilities it could also be used to forecast savings for different Price Disclosure scenarios.99
Forecasting pharmacy remuneration
2.13 In planning for the 5CPA, Health advised that it employed its new costing model as follows:
PhRANCIS forecast prescription volumes using historical data and derived a base average ex-manufacturer price using the known wholesale mark-up, pharmacy mark-up, dispensing fee and dangerous drug fee applicable at a point in time before the negotiations started. It [PhRANCIS] then used the script forecasts and base ex-manufacturer prices for medicines to apply expected price changes and other remuneration components under various costing scenarios, including Further PBS Reforms [revised Price Disclosure arrangements].
2.14 Under PBS arrangements, public and private hospitals approved under Section 94 of the National Health Act may claim reimbursement for dispensing pharmaceutical benefits. At the commencement of the audit the ANAO sought confirmation from Health that remuneration for hospital pharmacies was not included in 5CPA ‘community pharmacy’ remuneration. Health advised the ANAO that remuneration for s94 hospitals was not contained within the $15.4 billion funding for 5CPA.
2.15 However, the ANAO’s examination of the prescription data used to forecast 5CPA pharmacy remuneration indicated that Health:
- had used prescription data for Section 85 items100 from all approved suppliers (including those dispensed from public and private hospitals); and
- did not include prescription data for Section 100 items101 (such as Highly Specialised Drugs) dispensed from retail pharmacies.
2.16 Health’s inclusion of Section 85 items dispensed from suppliers other than retail pharmacies (including public and private hospitals) overstated forecast remuneration for retail pharmacies under the 5CPA. In contrast, Health’s exclusion of Section 100 items dispensed from retail pharmacies understated forecast remuneration for retail pharmacies from this source.
2.17 In addition, a key Australian Government objective of the 5CPA negotiations was to develop more appropriate remuneration arrangements for chemotherapy infusions. It was anticipated that chemotherapy infusions would be transferred from Section 85 to Section 100 arrangements, with higher dispensing fees. Although PhRANCIS had the capacity to model changes in remuneration for such classes of drugs—and the impact of price disclosure on those drugs—the department did not use PhRANCIS to model changes to remuneration arrangements for chemotherapy infusions.102
2.18 In summary, notwithstanding the limitations of Health’s methodology, the 5CPA costing model enabled Health to forecast prescription volumes, and broadly estimate the costs of pharmacy remuneration for the 5CPA. The 5CPA baseline pharmacy remuneration was forecast by incorporating the expected price reductions that would occur under existing Price Disclosure arrangements (as established in 2007). The 5CPA model was then used to forecast pharmacy remuneration under proposed new Price Disclosure settings (proposed to be implemented in 2010).
2.19 The 5CPA costing model (PhRANCIS) forecasts the total cost of pharmaceutical benefits, including pharmacy remuneration, which is paid jointly by government and by patients. Health advised the ANAO that, in order to identify the cost to government, the total costs forecast by PhRANCIS were manually adjusted to exclude patient contributions.
5CPA costs
Treatment of patient contributions
Advice to Ministers before negotiating the 5CPA
2.20 In the lead up to the negotiation of the 5CPA103, Health advised Ministers that the 4CPA was forecast to provide $11.6 billion104 in Australian Government funding to pharmacies and wholesalers over the five years of its operation to 30 July 2010. In October 2009, Health again advised Ministers that expenses under the 4CPA were expected to cost government $11.6 billion, and that if 4CPA arrangements continued, expenditure for the 5CPA would be just under $16.0 billion.105
2.21 The ANAO analysed Health’s 2009 estimate of the cost to government of the 4CPA and the proposed 5CPA as reported to Ministers, and observed that both estimates included the value of patient co-payments, which are payments made to pharmacies by patients rather than the Australian Government. Patient co-payments are described in Figure 2.2.
Figure 2.2: Sources of funding for pharmaceutical benefits: government and patient contributions
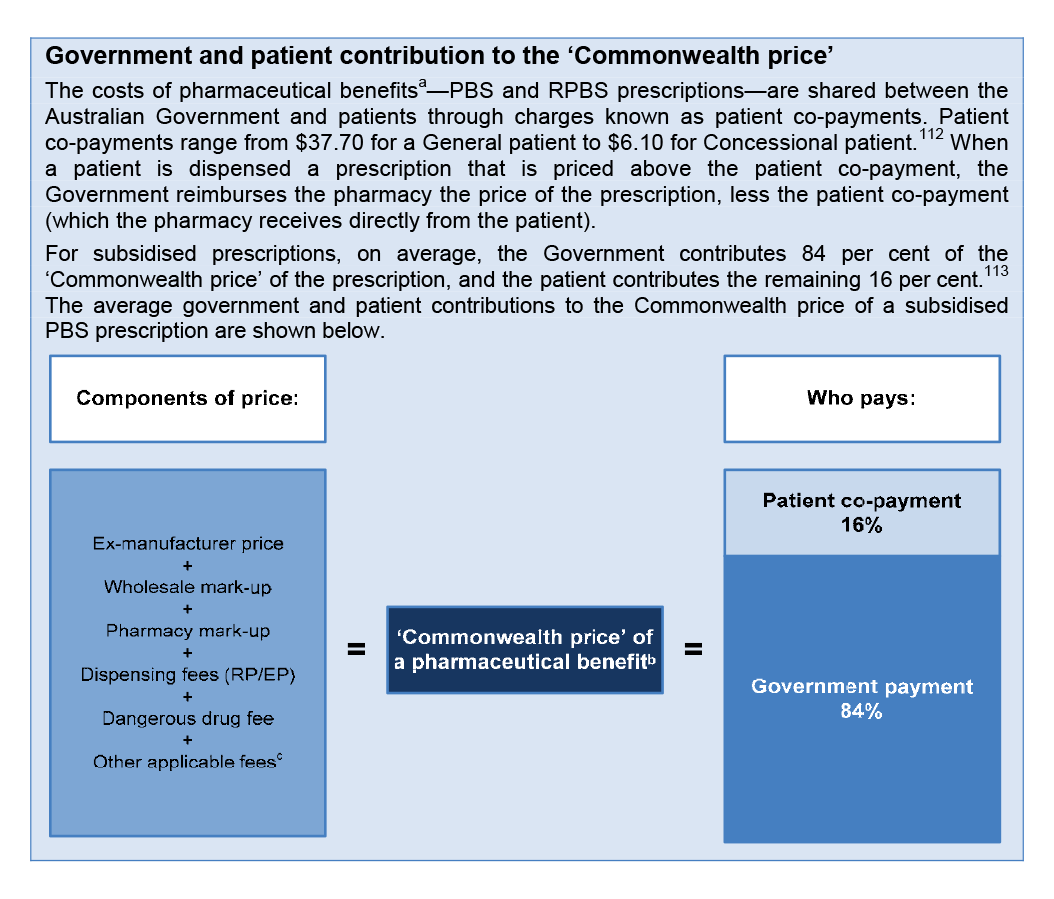
Source: ANAO analysis.
Notes:
- A pharmaceutical benefit is not a benefit payment, but a medicine that is listed on the PBS or the RPBS Schedules. In this report, the ANAO has used the term ‘PBS item’ or ‘RPBS item’ to refer to a pharmaceutical benefit.
- The ‘Commonwealth price’ for a pharmaceutical benefit does not include a range of other fees that are paid to pharmacies in addition to the ‘Commonwealth price’. These are: the Premium Free Dispensing Incentive and the Electronic Prescription Fee (which are included in 5CPA pharmacy remuneration, and paid entirely by the Government); the allowable extra fee; the Safety Net recording fee; brand price premiums, therapeutic group premium and special patient contribution (which are not included in 5CPA pharmacy remuneration, and are paid entirely by patients).
- Other applicable fees may include: a wastage factor for broken packs; a container fee; and a diluent fee. These fees are not included in the 5CPA definition of ‘pharmacy remuneration’.
- Patient co-payments as at 1 January 2015. General patients who qualify for the Safety Net pay the Concessional co-payment, and Concessional patients who qualify for the Safety Net pay nil.
- ANAO analysis of PBS/RPBS prescription data from 2010–11 to 2012¬–13. For prescriptions priced less than the patient co payment, the patient meets the full cost of the prescription.
2.22 Including patient co-payments in cost estimates that Health provided to Ministers in August to October 2009 had the effect of significantly overstating the cost to Government of both the 4CPA and 5CPA. In the case of the 5CPA, the cost to Government was overstated by approximately $2.2 billion.106
Funding obligations set out in the 5CPA
2.23 Although actual expenditure on pharmacy remuneration is demand driven—depending on the number of PBS and RPBS medicines prescribed by doctors—the 5CPA commits the Australian Government to delivering a fixed sum of money as follows:
The Commonwealth will deliver $15.4 billion under the Agreement as set out in the following table … Pharmacy remuneration: $13,771.6 million107
2.24 There is no formal mechanism in place to reconcile actual expenditures on pharmacy remuneration against funding specified in the 5CPA.
2.25 The ANAO analysed Health’s final costing for pharmacy remuneration of $13 771.6 million—as incorporated in the 5CPA—and observed that it included the value of patient co-payments (approximately $2.2 billion). Health advised the ANAO that patient contributions have always been included when remuneration is reported in community pharmacy agreements, and the 5CPA included ‘the total remuneration that it delivers’.108 Health also acknowledged that:
This [approach] could create some confusion about the cost to Government versus the value provided from both Government and patients to pharmacy under the Agreement.
Total pharmacy remuneration delivered by the 5CPA
2.26 As outlined in Figure 1.3 and paragraph 1.18, under the 5CPA the specified components of ‘pharmacy remuneration’ are the: wholesale mark-up, pharmacy mark-up, dispensing fee, extemporaneously prepared fee, dangerous drug fee, Premium Free Dispensing Incentive (PFDI) and Electronic Prescription Fee (EPF). The ANAO analysed the total pharmacy remuneration delivered by the 5CPA. About a quarter of PBS and RPBS prescriptions dispensed by retail pharmacies are paid in full by patients—with no Government subsidy—because they are priced under the patient co-payment.109 However, Health’s estimated $13 771.6 million for 5CPA pharmacy remuneration did not include remuneration received from patients for under co-payment prescriptions.
2.27 On the basis of Health’s advice that the 5CPA (and earlier agreements) included total pharmacy remuneration that it will deliver, this substantial element of remuneration—at least $2.6 billion—could usefully also have been included in the agreement to provide full transparency of total remuneration from the PBS and RPBS. The various sources of pharmacy remuneration are outlined in Figure 2.3.
Figure 2.3: Pharmacy remuneration delivered by the 5CPA—government and patient contributions
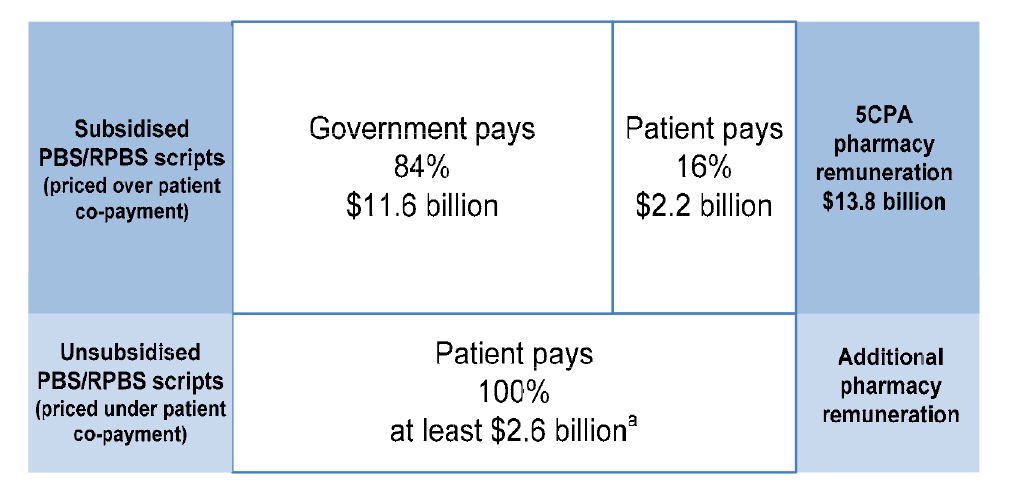
Source: ANAO analysis.
Note:
- The ANAO estimate of pharmacy remuneration for under co-payment scripts does not include discretionary patient charges set out in the 5CPA, such as the allowable extra fee and safety net recording fee.
5CPA costs reported in Commonwealth Budget commentary
2.28 The 2010–11 Commonwealth Budget commentary reported the following 5CPA funding, which included the cost of patient co-payments, notwithstanding the reference in the Budget papers to funding provided by the Government:
The Government and the Pharmacy Guild of Australia have signed a new five-year Community Pharmacy Agreement to commence on 1 July 2010 … The agreement will see the Government provide an estimated $15.4 billion over five years from 2010–11 for community pharmacies (including $3.3 billion in 2014-15). This represents an increase of $3.8 billion over the previous agreement, which ends on 30 June 2010.110
2.29 There would have been benefit, in the interests of clarifying and clearly documenting the nature of financial commitments entered into by the Australian Government, to explicitly identify the contribution made by the Commonwealth and patients respectively to the $15.4 billion funding commitment in the 5CPA.
2.30 In respect of the statement appearing in the Budget papers, Health advised the ANAO that while the cost of patient co-payments was included in the Budget Paper No. 2 commentary, these costs were not factored into the Budget and forward estimates as a Commonwealth expense:
The PBS forward estimates were only amended to reflect the agreed savings from the various components of the 5CPA. To clarify, the forward estimates were therefore not adjusted by $15.4 billion, but only by the net saving position as a result of the agreed changes from the 4CPA arrangements.
There was only one savings component that incorrectly included patient payments, the Freezing of the Dispensing Fee. As previously agreed with DoFD [Department of Finance and Deregulation], patient payments [co-payments] account for approximately 16% of PBS costs. Therefore of the $267 million save from this measure, only $42.7 million was incorrectly included in the PBS forward estimates. As a percentage of the total PBS forward estimates over that same period this equates to approximately 0.1%.
The reference to the 2010-11 Budget uses the terminology ‘The agreement will see Government provide an estimated $15.4 billion over five years …’ As the agreement is negotiated between the Government and the Guild, although patient contributions are included, the Government is still providing the remuneration arrangements.
2.31 In summary, the 5CPA provides that the Commonwealth ‘will deliver’ funding of $15.4 billion, including some $13.8 billion in pharmacy remuneration. In effect, pharmacy remuneration includes $11.6 billion to be paid by Government and $2.2 billion to be paid by patients. In reporting the estimated costs of the 5CPA—in Health’s advice to Ministers; the written 5CPA agreement; and the 2010–11 Commonwealth Budget commentary—there was no indication that some $2.2 billion of the $15.4 billion in funding would be sourced from patients. Further, while Health considered that the 5CPA should include the total amount that it delivers, additional pharmacy remuneration of at least $2.6 billion for unsubsidised PBS medicines (priced under the patient co-payment and paid by patients) are not mentioned in the 5CPA. To clarify the nature of financial commitments entered into by the Australian Government, the department should present, in key documents, estimated government payments and patient payments for both subsidised and unsubsidised PBS and RPBS medicines.
Recommendation No.1
2.32 To clarify the nature of financial commitments entered into by the Australian Government, the ANAO recommends that the Department of Health presents, in key documents, estimated government payments and patient payments for both subsidised and unsubsidised Pharmaceutical Benefits Scheme and Repatriation Pharmaceutical Benefits Scheme medicines.
Health response: Agreed.
Forecasting the cost of 5CPA dispensing fees
2.33 Dispensing fees are the largest component of pharmacy remuneration under the 5CPA, and the ANAO examined Health’s forecast of dispensing fees in its planning for the agreement. The dispensing fee was indexed annually to Wage Cost Index 9 (WCI9)111, and WCI9 is documented as the applicable indexation factor for the 5CPA.112 However, in its 5CPA modelling Health assumed that indexation for the dispensing fee would be 2 per cent per year for the term of the agreement. This indexation rate was significantly higher than the official WCI9 indexation rates as forecast at the time by the Department of Finance and Deregulation (Finance), for use by departments. Applying Finance’s forecast indexation rates would have produced lower dispensing fees under the 5CPA than those forecast by Health, as shown in Table 2.1.
Table 2.1: Comparison of Health’s indexation rates to official forecast
|
Agency |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
2014–15 |
|
Health’s indexation rate:a |
2.0% |
2.0% |
2.0% |
2.0% |
2.0% |
|
Finance’s WCI9 rate:b |
1.6% |
1.5% |
1.6% |
1.6% |
- |
|
Dispensing fees forecast using Health’s indexation rate: |
$6.55 |
$6.68 |
$6.81 |
$6.95 |
$7.09 |
|
Dispensing fees forecast using official WCI9 rate: |
$6.52 |
$6.62 |
$6.73 |
$6.83 |
$6.94 |
Source: ANAO analysis of Health and Finance information.
Notes:
- Health used a 2 per cent per annum indexation rate in its original 21 September 2009 5CPA baseline forecast and subsequent revisions of the 5CPA baseline until November 2009, when the 5CPA baseline was frozen.
- Finance released its forecast WCI9 rates on 17 September 2009. As the last year of Finance’s WCI9 forecast was 2013–14, the ANAO re-applied this rate to 2014–15 to calculate the 2014–15 dispensing fee.
2.34 The ANAO estimates that Health’s use of a two per cent indexation rate to forecast dispensing fees, rather than the WCI9 rates released, overestimated the total costs of dispensing fees by some $95 million in the 5CPA baseline.
2.35 In October 2014, Health advised the ANAO that:
Health did not assume an indexation rate. The indexation rate was approved by DoFD [the Department of Finance and Deregulation] as per Budget Operational Rules; the costs were agreed with DoFD prior to 30 March 2010; DoFD was aware of the change to WC19 and then NOT requested a further update to the 5CPA package … while the WCI9 rates available at the time were not used, Finance approved costings using 2%, so clearly they were correct for Finance costing approval processes.
2.36 In late October 2014, Finance advised the ANAO that:
It is clear from the costing models and signed costing agreements that Finance agreed to costings that included the use of a two per cent indexation factor … the costing model prepared by Health and provided to Finance, a copy of which is attached to the signed costing agreement on file states in the ‘Assumptions’ section that: ‘Indexation for the dispensing fee is assumed to be 2% pa.’ It is unclear why a 2 per cent indexation factor was used.
2.37 In summary, Health’s advice provided to Finance on 23 December 2009 indicated that ‘WCI9 (2%)’ was used in Health’s costings. However, the official WCI9 indexation factors released by Finance ranged from 1.5 to 1.6 per cent per year. In advice to the ANAO, Finance confirmed that while it had agreed to Health costings that included the use of a 2 per cent indexation factor, it was unclear from Finance records why this value was used. Finance suggested that one likely explanation was that the relevant Finance officer accepted the information received from Health on face value.
5CPA savings
Budgetary context of 5CPA savings targets
2.38 One of the key objectives of the 5CPA negotiations was to achieve savings to contribute to the structural repair of the Commonwealth Budget. There had been particularly high cost growth under the 4CPA (growth averaged some 9.4 per cent per year) that was due, in part, to a $1.1 billion transitional structural adjustment package (financial assistance) to assist pharmacies adjust to the introduction of Price Disclosure in 2007.113
2.39 While Ministers originally proposed a savings target over $1 billion, it was not achieved in the 5CPA negotiations, and consequently the target was reduced.114 The 5CPA states that:
The initiatives covered by this Agreement result in $1 billion in savings over the Term of the Agreement against the Commonwealth forward estimates.115
2.40 The 2010–11 Budget Papers116 clarified that the $1 billion in savings is a gross figure, and after taking into account approved additional expenditure of $0.4 billion, net savings were estimated to be $0.6 billion.117 The approved additional expenditure of $0.4 billion arises from additional funding of $285.5 million for professional programs and $82.6 million to encourage the electronic processing of prescriptions by pharmacies.
2.41 The 5CPA savings measures discussed in the following paragraphs are: cessation of the PBS Online incentive ($417.7 million); freezing the indexation of the dispensing fee ($267 million) and the CSO Funding Pool ($19.2 million); and reducing funding for professional programs and services ($226.4 million).118
Cessation of PBS Online incentive ($417.7 million)
2.42 The largest savings measure negotiated for the 5CPA was the cessation of the PBS Online incentive on 30 June 2010, which Health estimated would deliver $417.7 million in savings.119
2.43 The PBS Online incentive was a payment of 40 cents for each prescription that pharmacies claimed through Medicare’s PBS Online system.120 The incentive was one element of a structural adjustment package that was intended to operate from 1 July 2007 to 30 June 2011. The 40 cent incentive was intended to accelerate pharmacies’ take-up of online claiming. The introduction of the incentive resulted in a rapid and almost complete adoption of PBS Online, with over 97 per cent of pharmacies online by 28 August 2009.
2.44 The departmental estimate of $417.7 million savings from ceasing the PBS Online incentive was based on the assumption that this payment would otherwise continue until 30 June 2015. However, departmental records indicated that the PBS Online incentive was a lapsing measure121, and intended to be time limited:
These payments [PBS Online incentive and the Premium Free Dispensing Incentive] were agreed as part of PBS Reform to compensate pharmacy for income they would otherwise lose due to statutory price reductions. The payments were intended to be time limited to provide pharmacy with sufficient time to restructure their businesses as a result of the price reductions. The Government’s commitments to these payments ceases from 1 July 2011 … By 30 June 2011, both incentives will have ensured that the agreed value of the compensation package to pharmacies under PBS Reform has been met.122
2.45 Health advised the ANAO that it included the costs of the PBS Online incentive in the last four years of the 5CPA (from 2011–12 to 2014–15) as a saving because the PBS Online incentive was ongoing in the department’s Pharmaceutical Benefit Scheme (PBS) forward estimates.123
Dispensing fee indexation freeze ($267 million)
2.46 Under the 4CPA the dispensing fee was indexed annually to Wage Cost Index 9 (WCI9). The second largest savings measure for the 5CPA was the freezing of the dispensing fee at $6.42 for two years, which Health estimated would deliver $267.1 million in savings.124
2.47 In examining Health’s costing of the indexation freeze, the ANAO observed that the costing that Health provided to Ministers in April 2010 was not based on the forecast indexation rates that Finance released to agencies in March 2010. Finance’s forecast indexation rates, and those used by Health, are shown in Table 2.1.
2.48 Health advised the ANAO that:
…the WCI9 indexation figures used for the 5CPA costing reflect the agreed indexation amounts from the Department of Finance and Deregulation at the time the costing needed to be finalised, in order to sign the 5CPA by the date requested by Government. This is an issue of timing of the 5CPA needing to be effectively signed off, with figures included, prior to receiving the updated WCI9 figures in March 2010.
2.49 In light of Health’s advice, the ANAO examined the costing agreement between Health and Finance for the dispensing fee freeze, dated 19 January 2010. The forecast WCI9 indexation rates, which were available to Health for the purpose of preparing its costings, had been released by Finance on 17 September 2009. As shown in Table 2.2, the forecast indexation rates released by Finance to agencies in September 2009 differed from the two per cent indexation rate used by Health to cost the dispensing fee freeze.
Table 2.2: Health and Finance forecast indexation rates—September 2009 WCI9
|
Agency |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
2014–15 |
|
Health (January 2010): |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
|
Finance (September 2009): |
1.6 |
1.6 |
1.5 |
1.6 |
- |
Source: ANAO analysis of Health and Finance information.
2.50 The ANAO estimated that a consequence of not applying the September 2009 indexation rates released by Finance was that the savings from freezing the dispensing fee were overestimated by approximately $38.7 million for the 5CPA.
2.51 Overall, the estimated saving to government of the proposed dispensing fee freeze under the 5CPA was overestimated by approximately $81.4 million125—some 30 per cent of the total saving of $267.1 million—due to the combined effect:
- using higher estimates for indexation than the WCI9 indexation figures provided by Finance (which the ANAO estimates overstated savings by approximately $38.7 million);
- including the patient co-payment in its calculations (which Health advised overstated savings by approximately $42.7 million)126; and
- using forecast prescription volumes that include public hospital dispensing (which does not attract a dispensing fee).127
CSO Funding Pool indexation freeze ($19.2 million)
2.52 The CSO Funding Pool is also indexed by WCI9. Health estimated that savings of $19.2 million would be delivered by not applying indexation to the CSO Funding Pool for the first year of the 5CPA.128 However, Health again applied a two per cent indexation factor rather than the forecast WCI9 to calculate these savings. The ANAO estimates that Health’s use of incorrect indexation rates overestimated the savings from freezing the CSO Funding Pool by approximately $4.5 million, or some 23 per cent of the total estimated savings of $19.2 million.
2.53 Health advised the ANAO that the CSO costing was approved by Finance at the time. However, as noted previously, while Finance has advised that it agreed to costings prepared by Health which included a two per cent per annum indexation rate across the five years of the 5CPA, this figure did not correspond to Finance’s official WCI9 forecast indexation figures for that period, and there is no documented basis as to why Finance agreed to the change. This experience indicates that there is scope for all entities involved in the planning of the next community pharmacy agreement to document the basis of their costings.
2.54 Further, the repeated application of indexation factors that did not correspond to those released by Finance, indicates that Health should in future exercise care to use the official forecast indexation factors to inform the preparation of its costings.
Recommendation No.2
2.55 To provide assurance regarding the basis of costings for the next community pharmacy agreement, the ANAO recommends that the Department of Health applies the relevant forecast indexation factors released by the Department of Finance.
Health response: Agreed.
Reduced funding for professional programs ($226.4 million)
2.56 The third largest savings measure for the 5CPA was a reduction in funding for professional programs compared to the 4CPA. This measure was reported as delivering $226.4 million in net savings in Budget Paper No. 2, Budget Measures 2010–11.
2.57 Health advised the ANAO that the maximum expenditure on professional programs is capped and the budget is fixed. Accordingly, the baseline cost of professional programs funded under the 5CPA (before being adjusted for any proposed savings or spending measures) should equal the baseline cost of professional programs funded under the 4CPA, adjusted for any authorised ongoing change that may have occurred during the 4CPA. To determine whether this was the case, and whether the intended savings were realised, the ANAO examined Health’s reporting of the baseline costs for 4CPA and 5CPA professional programs, as shown in Table 2.3.
Table 2.3: Comparison of baseline funding for professional programs
|
Professional programs |
Year 1 $m |
Year 2 $m |
Year 3 $m |
Year 4 $m |
Year 5 $m |
Total $m |
|
4CPA budget as reported October 2009 |
168.0a |
100.0 |
100.0 |
100.0 |
100.0 |
568.0 |
|
5CPA baseline cost adjusted for new funding approved during 4CPA |
102.7 |
102.2 |
102.2 |
102.2 |
102.2 |
511.6 |
|
5CPA baseline cost as reported October 2009 |
120.0 |
120.0 |
120.0 |
120.0 |
120.0 |
600.0 |
|
5CPA baseline cost as reported April 2010 |
127.7 |
127.7 |
127.7 |
127.7 |
127.7 |
638.7 |
Source: ANAO analysis of Health information.
Note:
- Health advised the ANAO that $68 million was carried over from the 3CPA to the first year of the 4CPA.
2.58 The 4CPA professional program budget was $568 million, which comprised $500 million in 4CPA baseline funding and $68 million that was carried over from the 3CPA. During the 4CPA, the baseline increased by some $2 million per year to fund new programs. In October 2009, Health advised Ministers that the baseline for the 5CPA was $600 million, with no explanation as to why $88.4 million (an additional $17.7 million per year) had been added to the 5CPA baseline. In April 2010, Health further advised Ministers that the 5CPA baseline cost for professional programs was $638.7 million, with no explanation of the further $38.7 million increase.
2.59 In summary, there was an unexplained increase of $127.1 million (comprising $25.4 million per year) in the baseline funding provided by the Government for 5CPA professional programs. Health was unable to provide evidence of Ministerial authority for this significant increase in baseline costs.
Summary of forecast 5CPA savings
2.60 In summary, the ANAO examined the potential savings from the 5CPA savings measures, taking into account the:
- correct values for WCI9 indexation as forecast by Finance at the time Health prepared its costings;
- inclusion of patient co-payments in the savings from freezing the dispensing fee as per Health’s advice to ANAO129; and
- unexplained increases in the baseline cost of 5CPA professional programs.
2.61 The ANAO estimates that a more accurate estimate of the net savings from proposed 5CPA measures was approximately $397 million rather than $610 million, as reported in the Budget papers.130
Regulatory Impact Statement for the Location Rules
2.62 Health was required to prepare a Regulatory Impact Statement (RIS) as part of its advice to Ministers on the proposed 5CPA because the negotiation of the 5CPA would canvass the regulation of pharmacies through the continued application of the pharmacy Location Rules. The Office of Best Practice Regulation (OBPR) assessed the RIS as inadequate because it did not:
- adequately identify the problem;
- provide an objective assessment of options;
- provide an adequate impact analysis; and
- outline consultation in an adequate manner.
2.63 The OBPR noted that the Location Rules are a restriction on competition, and that the RIS did not demonstrate that the benefits of Location Rules outweighed the costs. Consequently, the OBPR reported the proposal as non-compliant in its 2009–10 Best Practice Regulation Report:
The Department of Health and Ageing (DHA) did not comply in full with the best practice regulation requirements in 2009–10. DHA did not prepare an adequate RIS at the decision-making stage for the proposal to retain pharmacy location rules. DHA is required to commence a post-implementation review of the pharmacy location rules within one to two years of the implementation of the decision.131
2.64 In the absence of an adequate RIS, Health was required to undertake a Post Implementation Review (PIR) to examine, among other things, the effectiveness of the regulation in meeting its objectives. According to OBPR guidance, agencies should not require more than three months to complete a PIR.132 If the regulation is particularly large in scope or in its impacts, then six months may be more adequate. However, the PIR was not finalised until October 2014, over four years after the decision to retain the Location Rules.
2.65 In May 2014, Health advised the ANAO that:
[The] Department did commence the PIR within the two year period as recommended by the OBPR. OBPR did not request the PIR from the Department until September 2012 and the Department provided an initial draft PIR in late 2012. OBPR has since been provided with a further three draft PIRs, incorporating their feedback and responding to their comments. OBPR has also directly assisted with the drafting of the PIR on two occasions. The most recent draft PIR was submitted to OBPR on 8 February 2014. In OBPR’s response to the Department on 14 March 2014 OBPR acknowledged that the PIR largely meets the requirements but that the Government’s regulation requirements had changed since the PIR was drafted and further (additional) information was now required. The Department has been in discussions with OBPR since December 2012 regarding the PIR, with both parties needing to clarify various issues. General agreement has been reached with only some minor amendments to be finalised. It is not accurate to attribute time delays necessarily to the Department.
2.66 On 25 November 2014 the OBPR reported on its website that Health had in October 2014 completed a PIR on the 2010 decision to renew the Location Rules.133
Consultation with key stakeholders
2.67 In respect to stakeholder consultation undertaken in developing the 5CPA, Health advised the ANAO that:
From July 2009–January 2010, consultation was undertaken with SHPA [Society of Hospital Pharmacists], UQ [University of Queensland], NPSA [National Pharmaceutical Services Association], PSA [Pharmaceutical Society of Australia], CHF [Consumers Health Forum], Medicines Australia and other stakeholders seeking input and proposals relating to a future Fifth Agreement.
In addition, the CHF was contracted to undertake consumer consultation under a project titled “Fifth Community Pharmacy Agreement Consumer Consultation Project”. The project commenced in January 2010 and findings throughout the consultation were used to inform directions in negotiations. For example the outcomes of the consumer consultation workshop associated with this project were delivered to Health in March 2010.
Consumer consultation
2.68 The department engaged the Consumer Health Forum (CHF) to provide consumer input to the 5CPA in December 2009, and CHF submitted to Health a draft project plan for the proposed community consultation on 12 January 2010. However, the main elements of the 5CPA were agreed in principle on 24 December 2009, through an exchange of letters between the Minister for Health and the Pharmacy Guild.134
2.69 The CHF consulted widely with consumer representatives through teleconferences, a national workshop and meetings, in accordance with the project plan approved by Health. While participants in the national workshop provided useful information from a health consumers’ perspective, a number expressed concern that consumer input would not have significant impact on the 5CPA, given that it had already been agreed in principle.135 The CHF submitted its final report to Health on 27 May 2010, some 24 days after the 5CPA was signed by the Health Minister and Pharmacy Guild on 3 May 2010.
2.70 The CHF advised the ANAO that:
Following CHF’s submission of contract reports [to Health], there was limited discussion of the 5CPA. CHF undertook (in collaboration with a number of other pharmacy and health groups including the Society of Hospital Pharmacists, APESMA [now Professional Pharmacists Australia] and the Public Health Association) to initiate a senate inquiry into the Community Pharmacy Agreements (CPAs), which was unsuccessful. We also jointly wrote to the [then] Minister conveying our concerns about the lack of consultation with consumer and pharmacy groups at the negotiation stage of the Agreements. The Minister referred this correspondence to the Department, which then conducted two meetings with these groups in late 2010 … The discussions centred on the extent to which these groups were excluded from discussions on CPAs and a place at the table for discussions on the 6CPA. There has been ongoing criticism in the pharmacy press about the lack of consultation on the 6CPA and it seems very little has changed in the approach that the Department takes to these arrangements. As a consumer organisation, these arrangements seemed highly inappropriate and out of step with requirements for transparency and accountability of government agreements, particularly those involving billions of taxpayer dollars.
Consultations with professional organisations
2.71 Although the Pharmaceutical Society of Australia (PSA) is not a signatory to the 5CPA, it is the largest professional association of pharmacists in Australia, and its role is recognised in the 5CPA as follows:
The parties understand that the Pharmaceutical Society of Australia, whilst not a signatory to this Agreement, will be an active participant in those areas of this Agreement that are related to professional practice.136
2.72 A peak pharmacists body advised the ANAO that in respect to the development and negotiation of the 5CPA, it considers that:
There is little transparency … For example, this lack of transparency was evident in the secrecy surrounding the development of initial proposals that were subsequently negotiated between the Guild and DoHA [Health]. The outcome of these secret negotiations was announced on Christmas Eve 2009 by the then Minister for Health and Ageing as the ‘major components’ of an agreed 5CPA with funding of $15.1 billion (at that time). After several representations, [this body] was offered a belated opportunity to present proposals to the Department and the Guild for consideration for inclusion in 5CPA and during early 2010 [this body] drafted proposals and provided the evidence-bases for a Clinical Interventions program and a Medicines Use Review program. At its own expense, [this body] also ensured that experts were made available to DoHA to discuss more detailed aspects of the Clinical Interventions program. [This body] also made its views known that the funding allocated to some individual 5CPA programs (such as Home Medicine Reviews) was inadequate.
2.73 Other peak bodies that made submissions to the ANAO regarding the 5CPA also expressed concerns that the processes for developing and negotiating the 5CPA did not provide an opportunity for effective engagement prior to it being finalised.137
Negotiations with the Pharmacy Guild
Negotiation process and record keeping
2.74 Health’s negotiating team consisted of three departmental executives, and several support staff. The head of the negotiation team advised the ANAO that there was extensive engagement with Health’s senior executive throughout the negotiations.
2.75 Health advised the ANAO that the negotiations involved ‘dozens of meetings’ with the Pharmacy Guild, which were conducted face to face, by email, telephone and teleconference. However, Health also advised that it had not kept a formal record138 of its meetings and discussions with the Pharmacy Guild. Health advised the ANAO that for the duration of the negotiations, it did not:
- keep a record of its meetings with the Pharmacy Guild;
- take minutes of meetings; or
- prepare agreed notes of what had been discussed.
2.76 Given the significance of the issues under negotiation, the decision not to prepare an official record of discussions was not consistent with sound practice and potentially limits the department’s capacity to satisfy accountability requirements and protect the Commonwealth’s interests. Appropriate record keeping enables government entities to discharge accountability and advisory obligations to Government and the Parliament, and to contribute effectively to audit139, FOI and administrative review processes. It also enables departments to protect the Commonwealth’s interests in the event of disputes140 and legal action.
2.77 The department should maintain an adequate record of the negotiation of the next community pharmacy agreement. In view of the approach to record keeping adopted in negotiating the 5CPA, Health should also review its internal guidance on appropriate record keeping practices relating to the negotiation of significant contracts and agreements.
Recommendation No.3
2.78 To improve its ability to satisfy accountability requirements and capacity to protect the interests of the Commonwealth in the event of disputes or legal action, the ANAO recommends that the Department of Health:
- maintains an adequate record of the negotiation of the next community pharmacy agreement and related contracts; and
- reviews its internal guidance on record keeping for the negotiation of significant contracts and agreements.
Health response: Agreed.
Risk management
2.79 Systematic risk management practices enable entities to be confident that implementation has been designed to achieve government objectives most effectively.141
2.80 Notwithstanding the complexity and high value of the agreement, Health records indicate that in its preparations for the 5CPA negotiations and implementation, the department did not: develop a risk management plan; develop a probity plan or consult with a probity advisor; complete specific conflict of interest declarations for members of its negotiation team; or develop a strategic implementation plan.142
2.81 Health advised the ANAO that:
No written risk management plan specifically for 5CPA negotiations [was] prepared; however, the risks associated with the negotiations were actively managed through regular communication with the Departmental Executive and Minister’s Office. Risk management actions were undertaken before and during the negotiation process, such as consultation with a range of interested parties to ensure their views were considered. In addition, the CHF were separately contracted to provide advice on the consumer perspectives for the negotiation and implementation.143
2.82 Health advised the ANAO that the negotiation team sought legal advice on issues ‘as required’—arranged through the department’s Legal Services Branch—but not regarding probity issues.
2.83 In respect of the management of potential conflicts of interest, Health advised the ANAO that it relied on routine declaration of interest processes for its staff involved in the negotiations, rather than solicit declarations specifically for the negotiation process.
2.84 Health advised the ANAO that a framework has been put in place to record key decisions made in the course of negotiations for a future agreement.
Government objectives in the 5CPA negotiations
2.85 Ministers set nine objectives for negotiating the 5CPA, including a savings target over five years of significantly more than $1 billion.144, 145
2.86 The then Government and department also considered that the 5CPA offered an opportunity to improve health outcomes and value for money by restructuring pharmacy remuneration arrangements ‘to diminish their link to the price of PBS medicines’. The Commonwealth anticipated doing so by shifting financial incentives from the volume driven sale of medicines to the delivery of value-adding professional services.
2.87 Some of the other negotiating objectives for the 5CPA included:
- better quality care and patient access to care in pharmacies and other health care settings, by investing in more effective and cost-effective health services provided by pharmacies and pharmacists;
- reformed programs that focus on improving medication-related services to patients;
- support for information technology systems that leverage investment from the pharmacy sector and are fully interoperable with broader e-health systems, including current and prospective National eHealth Transition Authority standards;
- access to the full range of PBS data, including prescriptions that cost less than the general patient co-payment—considered to be a ‘non-negotiable’ objective—which would help the Commonwealth determine actual PBS pharmacy remuneration from all sources, including patients, and the total volume and cost of the PBS to both government and consumers; and
- appropriate remuneration for pharmacies for chemotherapy infusions.
Revised 5CPA negotiation objectives
2.88 On 14 December 2009, Health provided an update on the 5CPA negotiations to Ministers. Health advised that the Pharmacy Guild had identified some $1 billion in potential savings, including savings from private hospital pharmacy and the Safety Net, but opposed changes to the structure of pharmacy remuneration. In addition, the Guild sought to retain the transitional 4CPA structural adjustment package that was due to expire on 30 June 2011.146
2.89 While the Pharmacy Guild had identified some $1 billion in potential savings, it also sought additional funding of: $277 million147 in compensation for the effects of revised Price Disclosure arrangements; and $75.5 million for the processing of electronic prescriptions and funding for software vendors, to integrate a range of agreement–related elements into existing dispensing software for pharmacies.148
2.90 Ministers agreed that the Health Minister should pursue savings over five years relating to both the 5CPA and revised Price Disclosure arrangements, but would not offer compensation for the flow-on effects of the revised Price Disclosure arrangements.149 However, Ministers did agree to consider some additional payments to pharmacies for delivering improved professional health services.
2.91 Ministers also agreed that the Health Minister develop a revised negotiating package incorporating the Government’s position and taking into account the Pharmacy Guild’s offer. If further negotiations were successful, the Health Minister would write to seek Ministers’ agreement to the final negotiated package, with costs to be agreed by Finance before exchanging a Letter of Intent with the Pharmacy Guild.
2.92 On 23 December 2009, Health advised the Health Minister that the proposed 5CPA would deliver $1 billion in gross savings, and that costings for the savings were currently being agreed with Finance. On that basis, Health recommended that the Health Minister sign the Letter of Intent with the Pharmacy Guild, which the Minister signed on 24 December 2009.
2.93 The ANAO requested that Health provide documentation of relevant Finance approvals and correspondence seeking Ministers’ agreement to the final negotiated package before the Letter of Intent was exchanged with the Pharmacy Guild. Health advised the ANAO that relevant documentation did not exist.150 The department further advised, in February 2015, that:
the core issue is that no formal written advice directing the Department to proceed with the associated Minute and Letter of Intent exists. While authority was given verbally, the Department acknowledges that better practice would have been to record this in a file note. However, any implication that the Department acted without Government knowledge or authority is incorrect.
The Report has only considered the Department’s advice that such a request was made, and that the Minister was satisfied in exercising their decision by virtue of signing the Letter of Intent. The Department accepts that in future, formal written advice should be obtained for record-keeping purposes to ensure an audit trail of any requests made can be furnished.
As evidenced by records considered by the Report, the Fifth Agreement negotiations were dynamic and often compressed in order to meet required timeframes. The Department remains of the view that in all instances it sought to be responsive to Government priorities and objectives. The above is an example of an area for improvement in the future and accordingly, the Department maintains its agreement to Recommendations 3 and 5 in the proposed Report that relate to record-keeping.
2.94 In the absence of Health documentation, the ANAO also examined Finance records relating to the 5CPA. Finance records indicate that while various 5CPA savings measures were agreed by Finance between 13 January 2010 and 7 April 2010, none were agreed prior to the signing of the Letter of Intent on 24 December 2009, and some were agreed over three months afterwards. The largest savings measure—cessation of the PBS Online Incentive payment ($417.7 million)—was not agreed by Finance until 1 April 2010. Finance provided its first assessment of the cessation of the PBS Online incentive to Health in a facsimile dated 12 March 2010. In that message, Finance indicated that it had not provided input on costs for this savings measure:
This costing relates to a proposal agreed by SPBC [Strategic Priorities Budget Committee] on 14 December 2009 as part of the 5CPA. Agreeing to costs post a decision by SPBC is a deviation from usual process where costs are agreed by Finance prior to the proposal being considered for decisions by SPBC (or ERC) [Expenditure Review Committee]. The SPBC decision accepted this savings proposal and provided authority to the Minister for Health and Ageing to use it as part of their negotiations, hence, Finance has no input in relation to the policy.
2.95 It is not uncommon for Ministers, in the context of government decision-making processes, to request additional follow-up action, and it is incumbent on departments to implement appropriate systems to ensure that such requests are actioned. The ANAO has recently commented on other instances where departments have failed to do so151, and there would be benefit in Health reviewing its internal processes relating to the implementation of action requested by Ministers through Cabinet processes.
Outcome of negotiations
2.96 Ministers noted the outcome of the 5CPA negotiations on 9 April 2010 and agreed to the savings measures and funding arrangements proposed for the agreement, including estimated savings to government of $1 billion, and new spending measures of $0.4 billion. The largest new spending measures were: $269 million for pharmacies that registered for Pharmacy Practice Incentives, which required accreditation with the Pharmacy Guild’s Quality Care Pharmacy Program (QCPP); and $75.5 million for an Electronic Prescription Fee for pharmacies to offset the cost of downloading electronic prescriptions from a Prescription Exchange Service.152 The 5CPA was signed on 3 May 2010 by the Health Minister and the Pharmacy Guild.
2.97 A number of the Commonwealth’s strategic negotiating objectives for the 5CPA were only partially realised. Although the net forecast savings to Government were approximately $0.6 billion, due to shortcomings in Health’s costing methodology, the expected savings were more likely to be approximately $0.4 billion.
2.98 The Government considered that the 5CPA offered an opportunity to improve health outcomes and value for money by restructuring pharmacy remuneration arrangements ‘to diminish their link to the price of PBS medicines’. The Commonwealth anticipated doing so by shifting financial incentives from the volume driven sale of medicines to the delivery of value-adding professional services. However, the structure of pharmacy remuneration remained essentially unchanged from the 4CPA to the 5CPA—based on defined mark-ups to the base price of pharmaceuticals and the addition of a variety of fees. Further, key wholesaler and pharmacy mark-ups continued at previous rates.
2.99 A further Commonwealth negotiating objective, which Ministers considered to be ‘non-negotiable’, related to obtaining access from pharmacies to the full range of PBS data, including information relating to prescriptions that cost less than the general patient co-payment. As discussed, this information would help the Commonwealth determine actual PBS pharmacy remuneration from all sources, including patients, and the total volume and cost of the PBS to both government and consumers. This objective was partially realised—while the 5CPA made provision for pharmacies to provide certain prescription information from 1 April 2012, it did not make provision for the receipt of cost information.
2.100 A key negotiating objective of the 5CPA was to implement more appropriate pharmacy remuneration for chemotherapy infusions. However, the negotiation outcome required additional consideration and government funding some 18 months after the 5CPA was signed.153
2.101 Another key negotiating objective of the 5CPA was to support information technology systems that are fully interoperable with broader e-health systems. However, the two Prescription Exchange Services (PESs) that were approved by Health for the purpose of downloading electronic prescriptions by pharmacies, did not have systems that were interoperable. Government funding for the Electronic Prescription Fee was subsequently re-allocated to pay the PESs directly to make their systems interoperable.
2.102 In respect of the ‘non-negotiable’ Commonwealth objectives that were not met, Health advised the ANAO in February 2015 that:
in any negotiation, objectives may or may not be fully realised for a variety of factors. While it is therefore correct that not all the negotiating objectives agreed by Government prior to the commencement of the negotiations were met—it is reasonable to assert that nonetheless, Government was satisfied sufficient objectives were realised through their agreement to the final package of measures at the conclusion of negotiations, as approved by the Cabinet.
Pharmacy Guild contracts
2.103 After the 5CPA was signed, Health entered into a range of contracts with the Pharmacy Guild relating to the agreement. These included:
- a Multi Schedule Funding Agreement with twelve Schedules that provided a total of $3.4 million in funding to the Guild to ‘develop strategic direction and planning’ for 5CPA programs;
- a Deed for Multi Schedule Funding with 23 Schedules that provided a total of $79 million funding to the Guild to make program payments and provide administrative services;
- a contract that provided $2.7 million to the Guild to provide accreditation data for the Pharmacy Practice Incentives program; and
- variations to 4CPA contracts that provided $12 million to the Guild to continue making program payments and administering 4CPA programs in the first year of the 5CPA.
2.104 Health also entered into eight contracts with a Pharmacy Guild related entity known as Fred IT Group154, to provide funding of up to $22.9 million. Two of these eight contracts are for services relating to the 5CPA.
2.105 In common with the approach adopted for the earlier 5CPA negotiations, Health did not document its discussions and negotiations with the Pharmacy Guild relating to the negotiation of the contracts.
Conclusion
2.106 The department developed and negotiated a complex agreement and related contracts with the Pharmacy Guild in a timely manner, enabling the 5CPA to be signed by the Health Minister and the Guild on 3 May 2010, prior to the expiry of the 4CPA on 30 June 2010. However, a number of key government negotiating objectives for the 5CPA were only partially realised and there were shortcomings in key aspects of Health’s administration at the development and negotiation phases.
2.107 Health advised that its planning for the 5CPA involved reviewing evaluations of components of the 4CPA. One evaluation was completed before the 5CPA was signed and eleven were completed afterwards.
2.108 The 5CPA committed the Commonwealth to delivering funding of $15.4 billion—including $13.8 billion in pharmacy remuneration for PBS and RPBS dispensing, comprising $11.6 billion to be paid by Government and $2.2 billion to be paid by patients. In reporting the estimated costs of the 5CPA—in Health’s advice to Ministers; the written 5CPA agreement; and the 2010–11 Commonwealth Budget commentary—there was no indication that some $2.2 billion of the $15.4 billion in funding would be sourced from patients. Further, while Health considered that the 5CPA should include the total amount that it delivers, additional pharmacy remuneration of at least $2.6 billion for unsubsidised PBS medicines (priced under the patient co-payment and paid by patients) are not mentioned in the 5CPA. To clarify the nature of financial commitments entered into by the Australian Government, the department should present, in key documents, estimated government payments and patient payments for both subsidised and unsubsidised PBS and RPBS medicines.
2.109 Clause 1.2(e) of the 5CPA provides that: ‘The initiatives covered by this Agreement result in $1 billion in savings over the Term of the Agreement against the Commonwealth forward estimates.’ The 2010-11 Budget Papers clarified that the $1 billion in savings is a gross figure, and after taking into account approved additional expenditure of $0.4 billion, net savings were estimated to be $0.6 billion. The difference of $0.4 billion results from additional funding of $285.5 million for professional programs and $82.6 million to encourage the downloading of electronic prescriptions by pharmacies.
2.110 ANAO analysis indicates that the net savings estimated before the agreement was signed were closer to $0.4 billion (rather than $0.6 billion) due to shortcomings in the department’s 5CPA estimation methodology. The principal issues relate to: unexplained increases in the baseline cost of professional programs; the application of inappropriate indexation factors; and the treatment of patient co-payments. In particular:
- The baseline budget for 5CPA professional programs in the Commonwealth forward estimates was $638.7 million (before adjusting for the negotiated 5CPA savings and spending measures). However, Health’s records showed that the approved 5CPA baseline budget for professional programs was only $511.6 million, and there was no documentary evidence of authority to increase the 5CPA baseline budget in the forward estimates by $127.1 million.
- The official indexation factors released by the then Department of Finance and Deregulation (Finance) were not utilised in estimating 5CPA savings, resulting in an overestimate of 5CPA savings of approximately $43.2 million. The repeated application of indexation factors that did not correspond to those released by Finance, indicates that Health should in future exercise care to use the official forecast indexation factors to inform the preparation of its costings.
- Health advised, in the course of this audit, that the estimated savings for the 5CPA incorrectly included $42.7 million in co-payments made by patients to pharmacies for the receipt of pharmaceutical benefits. Co-payments are a private contribution to the cost of PBS medicines, which are not a cost to government.
2.111 In addition to the shortfall in anticipated savings, a number of the Commonwealth’s other strategic negotiating objectives for the 5CPA were only partially realised. In particular:
- The then Government and department considered that the 5CPA offered an opportunity to improve health outcomes and value for money by restructuring pharmacy remuneration arrangements ‘to diminish their link to the price of PBS medicines’. The Commonwealth anticipated doing so by shifting financial incentives from the volume driven sale of medicines to the delivery of value-adding professional services. However, the structure of pharmacy remuneration remained essentially unchanged from the 4CPA to the 5CPA—based on defined mark-ups to the base price of pharmaceuticals and the addition of a variety of fees. Further, key wholesaler and pharmacy mark-ups continued at previous rates.
- A negotiating objective which Ministers considered to be ‘non-negotiable’, related to obtaining access from pharmacies to the full range of PBS data, including information relating to prescriptions that cost less than the general patient co-payment. This information would help the Commonwealth determine actual PBS pharmacy remuneration from all sources, including patients, and the total volume and cost of the PBS to both government and consumers. This objective was partially realised—while the 5CPA made provision for pharmacies to provide certain prescription information from 1 April 2012, it did not make provision for the receipt of cost information.
- The Government wished to support information technology systems that were fully interoperable with broader e-health systems. However, the two Prescription Exchange Services (PESs) that were approved by Health for the purpose of downloading electronic prescriptions by pharmacies, did not have systems that were interoperable. As discussed in Chapter 3, Government funding for the Electronic Prescription Fee (EPF) was subsequently re-allocated to pay the PESs directly to make their systems interoperable.
- The Government also wished to implement more appropriate pharmacy remuneration for chemotherapy infusions, with any additional funding to be settled within the 5CPA funding envelope. However, the negotiation outcome for chemotherapy remuneration required additional consideration and a further $82 million in government funding some 18 months after the 5CPA was signed.
2.112 The ANAO identified persistent shortcomings in departmental record-keeping relating to the 5CPA. In December 2009, Ministers had agreed that the Health Minister would write to seek the agreement of Ministers to the final 5CPA negotiated package, with costs agreed by Finance, before exchanging a Letter of Intent with the Pharmacy Guild. The ANAO requested that Health provide documentation of relevant Finance approvals and correspondence seeking the agreement of Ministers to the final package negotiated with the Pharmacy Guild. Health advised the ANAO that relevant documentation did not exist.
2.113 Health recommended that the Health Minister sign the Letter of Intent with the Pharmacy Guild on the basis that costings for the savings were currently being agreed with Finance, and the Minister signed the Letter of Intent on 24 December 2009. Department of Finance records indicate that while various 5CPA savings measures were agreed by Finance between 13 January 2010 and 7 April 2010, none were agreed prior to the signing of the Letter of Intent in December 2009, and some were agreed over three months afterwards.
2.114 The department also advised the ANAO that during the 5CPA negotiations, it did not keep a formal record of its meetings with the Pharmacy Guild and did not document its subsequent discussions with the Guild on the negotiation of contracts relating to the 5CPA. Given the significance of the issues under negotiation, the decision not to prepare an official record of discussions was not consistent with sound practice, as shortcomings in record keeping can affect a government entity’s capacity to discharge advisory, accountability and contract management obligations. The department should maintain an adequate record of the negotiation of the next community pharmacy agreement. In view of the approach to record keeping adopted in negotiating the 5CPA, Health should also review its internal guidance on appropriate record keeping practices relating to the negotiation of significant contracts and agreements.
2.115 Notwithstanding the complexity and high value of the agreement, Health records indicate that in its preparations for the 5CPA negotiations and implementation, the department did not: develop a risk management plan; develop a probity plan or consult with a probity advisor; complete specific conflict of interest declarations for members of its negotiation team; or develop a strategic implementation plan.
3. Pharmacy Remuneration
This chapter examines the implementation of 5CPA pharmacy remuneration arrangements; departmental reporting of pharmacy remuneration; and the actual costs of pharmacy remuneration compared to departmental estimates.
Introduction
3.1 Australian Government funding of pharmacy remuneration for dispensing pharmaceutical benefits has been a core element of successive community pharmacy agreements. The agreements have supported a pharmacy business model for the delivery of pharmaceutical services that has been in place since the start of the Pharmaceutical Benefits Scheme (PBS) in the 1950s. The provision of ‘pharmacy remuneration’ is the largest financial component of the 5CPA, accounting for $13.8 billion (90 per cent) of the total value of the 5CPA.155 Actual spending on pharmacy remuneration is demand driven, as it depends on the number of PBS and RPBS prescriptions dispensed by pharmacies, and the price of medicines dispensed.156
3.2 This chapter examines the:
- implementation of 5CPA pharmacy remuneration arrangements;
- implementation of the two most recent components of pharmacy remuneration—the Premium Free Dispensing Incentive and the Electronic Prescription Fee; and
- actual 5CPA pharmacy remuneration compared to Health’s estimated costs.
Implementation of pharmacy remuneration arrangements
Role of the 5CPA in setting pharmacy remuneration
3.3 The 5CPA specifies that the components of ‘pharmacy remuneration’157 are the: wholesale mark-up, pharmacy mark-up, dispensing fee, extemporaneously prepared fee, dangerous drug fee, Premium Free Dispensing Incentive (PFDI) and Electronic Prescription Fee (EPF).158 Apart from the PFDI and EPF, the components of pharmacy remuneration are part of the dispensed price159 of a PBS item. The method for working out the dispensed price of a PBS item determines how much remuneration a pharmacy receives for dispensing the item. Under the National Health Act, the Pharmaceutical Benefits Remuneration Tribunal (the Tribunal) and the Minister for Health are responsible for determining the method for calculating the dispensed price for different classes of PBS items.
3.4 For pharmacy remuneration purposes, PBS medicines may be classified into five main groups as shown in Table 3.1. This table references the relevant sections of the National Health Act.
Table 3.1: Main classes of PBS items dispensed by retail pharmacies
|
PBS schedule |
Contains |
|
Section 85 items – general pharmaceutical benefits |
|
|
Section 85 (General Pharmaceutical Benefits) |
The largest group of PBS drugs (about 750) and the most frequently prescribed. |
|
Prescriber Bag items |
About 26 Section 85 drugs provided free of charge to prescribers for emergency use. |
|
Section 100 items – items provided under special arrangements |
|
|
Section 100 (Highly Specialised Drugs) |
Drugs for treating chronic conditions, which can only be initiated by a medical specialist. |
|
Section 100 (Efficient Funding of Chemotherapy) |
About 36 drugs for treating cancer, which are administered by infusion or injection.e |
|
Section 100 (Remote Aboriginal Health Services Program) |
Section 85 drugs supplied to remote area Aboriginal Health Service clinics. |
Source: ANAO analysis
Notes to Table 3.1:
- Blue shaded rows: remuneration arrangements set out in the 5CPA.
- Grey shaded rows: remuneration agreed in negotiations (as advised by Health) but not mentioned in the 5CPA.
- Retail pharmacies may also dispense Human Growth Hormone, Botulinum Toxin, and drugs for treating opiate dependence under Section 100 arrangements.
- The Health Minister must declare all PBS items under Section 85 of the National Health Act. However, under Section 100 of the National Health Act, the Minister may make special arrangements for providing PBS medicines in specified circumstances.
- Health advised the ANAO that the s100 (Efficient Funding of Chemotherapy) instrument also covers the supply and remuneration arrangements for a significant number of ‘related’ chemotherapy medicines that are not necessarily administered by infusion or injection, for example anti-nauseates.
3.5 Remuneration arrangements for four classes of PBS items were discussed in the 5CPA negotiations. However, the remuneration arrangements for only two of these classes are mentioned in the agreement. These are:
- Section 85 (General Pharmaceutical Benefits) items—referred to in clause 10 of the 5CPA; and
- Section 100 (Highly Specialised Drugs) – referred to in clause 13 of the 5CPA.
3.6 Other remuneration arrangements discussed in the 5CPA negotiations, but not mentioned in the agreement, related to:
- the dispensing of chemotherapy infusions (later known as Section 100 (Efficient Funding for Chemotherapy) items);
- the fee paid to pharmacies that supply PBS items in bulk to remote Aboriginal Health Services (known as the Section 100 (Remote Aboriginal Health Services Program)); and
- remuneration for PBS dispensing in private hospitals.160
3.7 In April 2010, Health advised Ministers that agreement had been reached with the Pharmacy Guild on remuneration arrangements for dispensing chemotherapy infusions, and also the fee paid to pharmacies that supply PBS items in bulk to remote Aboriginal Health Services. However, the Pharmacy Guild subsequently disputed that agreement had been reached on remuneration arrangements for dispensing chemotherapy infusions in view of the proposed changes to Price Disclosure. The Guild advised the ANAO that:
EFC [Efficient Funding of Chemotherapy] arrangements were, and remain, a separate budget measure unrelated to the 5CPA, as evidenced by there being no reference, directly or indirectly, to those arrangements in the 5CPA document as signed in May 2010. No amendments were made, or suggested to be made, to that Agreement upon the introduction of the revised chemotherapy arrangements in November 2011. The origin of these chemotherapy arrangements was a 2008 Budget measure, announced without reference to the prevailing 4CPA and without consultation with the Guild.
3.8 As summarised in Table 3.2, the supply of chemotherapy infusions was subsequently examined in a Senate inquiry, which reported in May 2013, and the Government announced an additional $82 million per year for dispensing chemotherapy infusions in November 2014.161, 162
Table 3.2: The Efficient Funding of Chemotherapy initiative
|
The Efficient Funding of Chemotherapy |
|
A key Australian Government objective of the 5CPA negotiations was to provide appropriate remuneration for pharmacies that supply chemotherapy infusions. The Efficient Funding of Chemotherapy (EFC) arrangements were negotiated in the context of the 5CPA but were not formalised in the agreement. The question of what Health had agreed with the Pharmacy Guild in the 5CPA negotiations was subsequently disputed by the Pharmacy Guild.a The preparation and supply of chemotherapy infusions is a specialist area of pharmaceutical production, with fewer than sixty pharmacies supplying 80 per cent of chemotherapy infusions funded under the PBS.b In 2012, some 18 months after the commencement of the 5CPA, pharmacies that supplied chemotherapy infusions claimed that their operations were not viable under the new EFC arrangements. In response, the then Government announced an additional $30 million in interim funding to increase the fees paid to pharmacists for preparing and dispensing chemotherapy medicines while a review of chemotherapy arrangements was undertaken.c A Senate committee also examined the issued, followed by a six month departmental review. Although revised funding arrangements for chemotherapy were meant to be settled within the fiscal parameters of the 5CPA negotiations, on 30 November 2013, the incoming Government announced that an additional $82 million per year would be provided for chemotherapy infusions.e |
Notes:
- Senate Community Affairs References Committee, Supply of chemotherapy drugs such as Docetaxel May 2013, available from: <http://www.health.gov.au/internet/main/publishing.nsf/Content/ chemotherapy-review/$File/appendix-b.pdf> [accessed 8 April 2014].
- Department of Health, Report to the Minister for Health: Review of Funding Arrangements for Chemotherapy Services, Canberra, October 2013, available from: <file:///F:/Chemotherapy%20review-of-chemotherapy-funding-arrangements%20Departmental%20report%20October%202013.pdf> [accessed 8 March 2014].
- Department of Health, Minister for Health, The Hon Tanya Plibersek MP, Media Release, 14 May 2013, <https://www.health.gov.au/internet/budget/publishing.nsf/Content/ 7F69704E8AAB2B89CA257CA0003FF562/$File/healthmedia02.pdf> [accessed 15 June 2014].
- Senate Community Affairs References Committee, Supply of chemotherapy drugs such as Docetaxel May 2013, [Internet].
- Department of Health, Minister for Health, The Hon Peter Dutton MP, Media Release, 30 November 2014, <http://www.health.gov.au/internet/ministers/publishing.nsf/Content/ 2F788ED052CA9347CA257C35001A1ED3/$File/PD019.pdf > [accessed 15 June 2014].
3.9 For the avoidance of doubt, there would be merit in including in the written agreement all matters relating to pharmacy remuneration that are agreed in the context of negotiating the next community pharmacy agreement.163
Legal framework for paying 5CPA pharmacy remuneration
3.10 The legal framework applying until 30 June 2014 for Commonwealth payments relating to the components of pharmacy remuneration for dispensing Section 85 (General schedule) items and Section 100 (Highly Specialised Drugs), as set out in the 5CPA, is shown in Figure 3.1.
Figure 3.1: Legal framework for paying 5CPA pharmacy remuneration until 30 June 2014
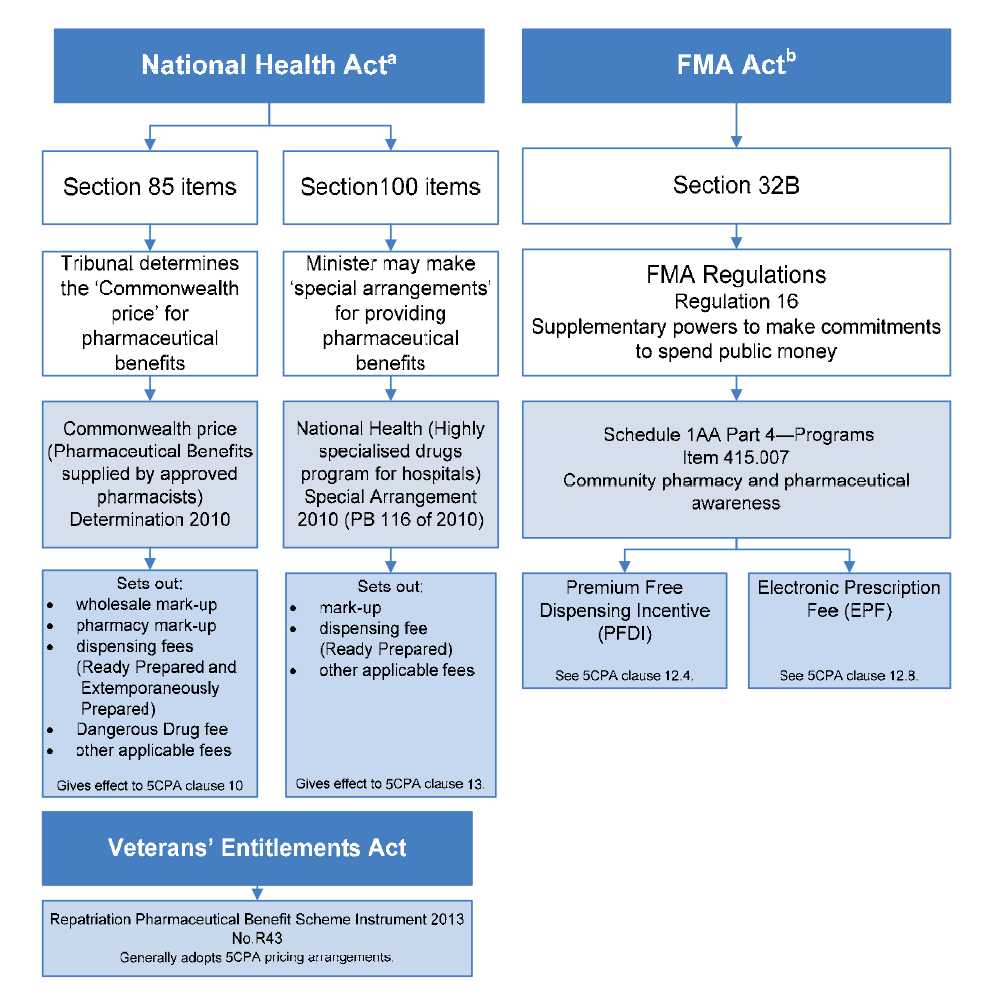
Source: ANAO analysis of the National Health Act 1953, Veterans’ Entitlements Act 1986, Financial Management and Accountability Act 1997 and Financial Management and Accountability Regulations 1997.
Notes to Figure 3.1:
- Payments made under the National Health Act are a special appropriation under Section 137.
- Payments made under the FMA Regulations for Commonwealth programs with a non-statutory basis are an annual administered appropriation. The FMA Act and Regulations were in operation until 30 June 2014—see footnote 81 in Chapter 1.
- Payments for 5CPA professional programs were also made under the FMA Regulations Schedule 1AA, Part 4—Programs, Item 415.007; and payments for the CSO Funding Pool were made under Item 415.008.
3.11 After the 5CPA was signed, the then Minister for Health made a determination for the pricing of Section 100 (Highly Specialised Drugs), giving effect to clause 13 of the agreement.164 The Minister also made separate determinations to implement the following items, which were discussed in the context of the 5CPA negotiations but not mentioned in the 5CPA:
- remuneration for dispensing chemotherapy infusions—Section 100 (Efficient Funding for Chemotherapy) Special Arrangement 2011;
- an increase in the fee paid to pharmacies that supply medicines in bulk to remote Aboriginal Health Services—Section 100 (Remote Aboriginal Health Services Program) Special Arrangements Instrument 2010; and
- restructuring of remuneration for private hospital pharmacies—National Health (Pharmaceutical benefits supplied by private hospitals) Determination 2010.
3.12 In addition, the Pharmaceutical Benefits Remuneration Tribunal165 made a new determination for the pricing of Section 85 (General schedule) items, giving effect to clause 10 of the Agreement.166 However, the Tribunal’s determination does not mention the Premium Free Dispensing Incentive (PFDI) and Electronic Prescription Fee (EPF), which are listed as components of pharmacy remuneration in the 5CPA.
3.13 The legislative instruments made under the National Health Act to give effect to payments for pharmacy remuneration as specified in the 5CPA are listed in Table 3.3. These legislative instruments were lodged with the Federal Register of Legislative Instruments, as required by the Legislative Instruments Act 2003 and published on the Australian Government ComLaw website.167
Table 3.3: Legislative instruments authorising payments of 5CPA pharmacy remuneration payments
|
Legislative Instrument |
Made by |
Date of effect |
|
Commonwealth Price (Pharmaceutical Benefits supplied by approved pharmacists) Determination 2010 |
Pharmaceutical Benefits Remuneration Tribunal (PBRT) |
1 July 2010 |
|
National Health (Highly specialised drugs program for private hospitals) Special Arrangements Instrument 2010 (PB 64 of 2010) |
Minister for Healtha |
1 July 2010 |
|
National Health (Highly specialised drugs program for hospitals) Special Arrangement 2010 (PB 116 of 2010) |
Minister for Healtha |
1 Dec 2010 |
Source: ANAO analysis of legislation.
Note:
- These instruments are made by the Minister for Health or delegate under the National Health Act.
3.14 Health advised the ANAO that the Premium Free Dispensing Incentive and the Electronic Prescription Fee (EPF) are ‘non-statutory schemes’, and the authority for their payment (until 30 June 2014) was provided by an item in Schedule 1AA of the Financial Management and Accountability Regulations 1997 (FMA Regulations), ‘Community pharmacy and pharmaceutical awareness’, which had the objective ‘to provide funding to improve access to medicines and pharmacy services’.168
3.15 In February 2010, when considering its options for implementing the EPF169, Health sought advice from the Australian Government Solicitor (AGS), which advised in March 2010 on possible Constitutional risks involved in paying the EPF as a stand-alone payment outside the National Health Act. AGS indicated that an alternative approach was to include the EPF as a component of the Commonwealth price of a pharmaceutical benefit. This approach would help avoid doubt as to the Constitutional basis for making EPF payments.170
3.16 Health did not advise Ministers on these legal issues when seeking approval in April 2010 to include the EPF proposal in the 5CPA. Further, Health did not implement the EPF or the Premium Free Dispensing Incentive as components of the Commonwealth price for pharmaceutical benefits, notwithstanding the advice received from the AGS. For the avoidance of doubt, there would be merit in Health reviewing the legislative basis for paying these components of pharmacy remuneration.171
3.17 Health advised the ANAO that:
In light of the most recent Williams versus the Commonwealth of Australia case Health are reviewing all programs, not just those under the CPA [Community Pharmacy Agreement].
Payments for dispensing Repatriation Pharmaceutical Benefits Scheme (RPBS) items
3.18 The RPBS is not mentioned in the 5CPA. However, the Department of Veterans’ Affairs (DVA) advised the ANAO that the RPBS has adopted 5CPA pharmacy remuneration arrangements. The relevant RPBS legislative instrument specifies that the dispensing fee payable to community pharmacists for the supply of RPBS items shall be the fee payable under the PBS for the supply of similar items.172
3.19 The RPBS legislative instrument makes no reference to wholesale or pharmacy mark-up—both of which are part of the 5CPA pharmacy remuneration arrangements. Unlike the legislative instruments for the PBS, the RPBS does not set out a method for working out the price of RPBS items for the purpose of paying pharmacists. Section 21 of the RPBS authorises payments to pharmacists as follows:
(1) In respect of each Pharmaceutical benefit provided to an Eligible Person under this Scheme, the Commission will accept financial responsibility for:
(a) subject to (b) all of the dispensed price but the co-payment that would be payable by the person if the person were a concessional beneficiary; or …
(b) if the safety net applies to the person, all of the dispensed price.
3.20 The ANAO notes that the RPBS pays pharmacists on the basis of the ‘dispensed price’. However, a definition of ‘dispensed price’ is not included in the RPBS legislative instrument or the RPBS Explanatory Notes to the RPBS Schedule. In March 2014 DVA confirmed, in advice to the ANAO, that the term ‘dispensed price’ is not defined for the purposes of the RPBS.
3.21 In the course of this audit DVA advised the ANAO that it would review the legislative instrument that establishes the RPBS to clarify the pricing arrangements for RPBS pharmaceutical benefits.
Administrative arrangements for pharmacy remuneration
3.22 Health and DVA are responsible for negotiating the prices of PBS items and RPBS items, respectively, with an item’s sponsor173, and maintaining a record of those prices. With input from DVA, each month Health compiles a file containing the ex-manufacturer price and ‘price to pharmacist’ (the notional wholesale price) for each item on the PBS and RPBS Schedules.174 The Department of Human Services (Human Services) uses this information to process and pay the claims of pharmacy owners for PBS and RPBS dispensing.
3.23 The process for recording and transmitting PBS and RPBS pricing information that is used to calculate pharmacy remuneration by Human Services is shown in Figure 3.2.
Figure 3.2: Transmission of PBS and RPBS pricing information
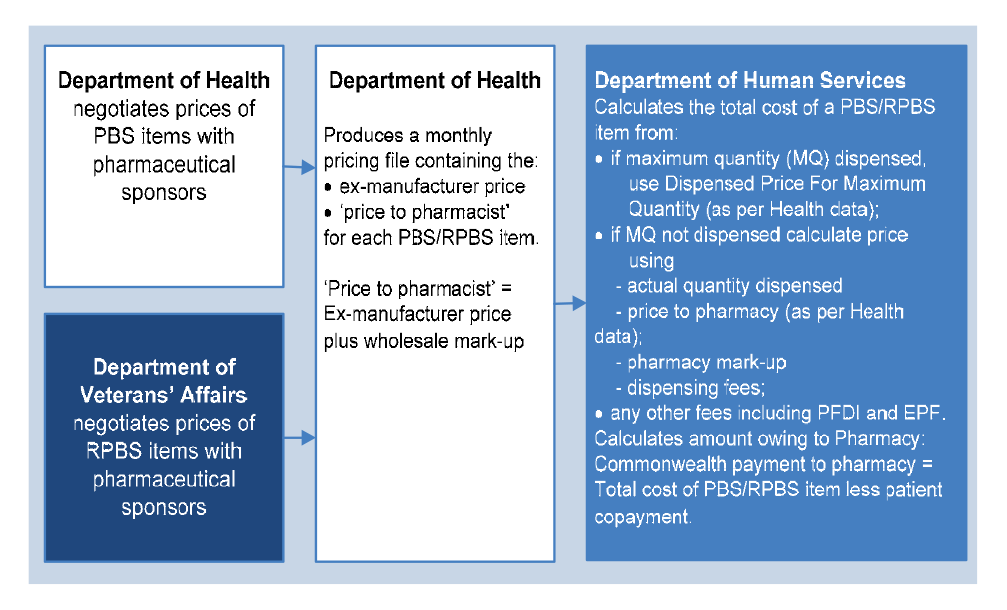
Source: ANAO analysis and Human Services advice.
Notes: a. The ex-manufacturer price and ‘price to pharmacist’ (notional wholesale price) is based on the maximum quantity for each item.
b. Health advised the ANAO that for Section 100 items with no wholesale mark-up, the amount recorded in the ‘price to pharmacist’ field is the same as the ex-manufacturer price.
3.24 When the 5CPA took effect on 1 July 2010, the then Medicare Australia (Medicare) administered the PBS and RPBS on behalf of the Australian Government. Medicare’s administrative responsibilities for the PBS were formalised in a Memorandum of Understanding (MoU) and a Business Practice Agreement (BPA) with the then Department of Health and Ageing, signed in May 2009. On 1 July 2011, Medicare was integrated into the Department of Human Services175, and in November 2012, the MoU and BPA were replaced by a Bilateral Heads of Agreement and a Business Agreement between Human Services and Health.
3.25 Under the current Business Agreement with Health, Human Services will, among other things:
- assess, process and pay claims under the PBS;
- perform functions relating to community pharmacy agreements; and
- be responsible for implementing any changes to the Schedule of Pharmaceutical Benefits, including undertaking any systems development work and using the Schedule of Pharmaceutical Benefits in the assessment of claims.176
3.26 Human Services relies on Health and DVA to provide accurate and complete information about the prices of PBS and RPBS items. The ANAO requested that Health and DVA provide a list of ex-manufacturer prices and corresponding wholesale prices177 for PBS and RPBS items as at 1 July 2010 (the date on which the 5CPA took effect), in order to confirm that the wholesale mark-up agreed under the 5CPA had been implemented from the commencement of the 5CPA. Health and DVA provided lists containing the wholesale prices as at 1 July 2010, but were unable to provide the corresponding ex-manufacturer prices as at that date.
3.27 Health advised the ANAO that the department’s inability to provide a list that contained both the ex-manufacturer price and the wholesale price as at 1 July 2010 was because, at that time, there was no legal requirement to use the ex-manufacturer price as the basis of calculating pharmacy remuneration.178 While the wholesale mark-up was specified in the 5CPA, the legislative arrangements that implemented pharmacy remuneration under the 5CPA did not refer to the wholesale mark-up until 1 October 2012, over two years after the 5CPA commenced on 1 July 2010.
3.28 Health further advised the ANAO of problems related to the calculation of government reimbursement for certain suppliers of pharmaceutical benefits:
Since December 2012 Health has provided (as integers) all prices for packs and maximum quantities at all price points within the supply chain. These are calculated by Health working forward from the negotiated price ex-manufacturer. DHS continue to select a price in the midpoint (price to pharmacists) and calculate back from that figure to determine the correct reimbursement. Inevitably this has the potential to cause rounding errors when reimbursing for some supply situations other than pharmacy. This also complicates the process of changing the pricing structure. Health considers it essential that Health set all prices at all levels and remove the need for any downstream user of the data (including DHS) to perform pricing calculations. It is outside the remit of the DHS, or anyone other than Health to determine pricing.
3.29 Human Services advised the ANAO that:
while PBS pricing information is set by Health, the department, as the agency delivering the PBS, has responsibility to make PBS pricing calculations in accordance with Commonwealth legislation, specifically the Commonwealth price determination authorised under paragraph 98B(1)(a) of the National Health Act 1953.
3.30 In summary, Health considered that Human Services’ system for calculating reimbursement for certain suppliers had the potential to cause rounding errors. However, Human Services advised the ANAO that while Health provides the prices for single packs and maximum quantities179 for PBS and RPBS items to Human Services, these prices do not necessarily correspond to what is actually dispensed. For example, a prescriber may prescribe 1.5 packs or two packs where the maximum quantity is three packs. Consequently, Human Services must perform a pricing calculation (using the price for a single pack and applying the appropriate scaling or wastage factors) in order to more accurately determine the correct reimbursement to suppliers.
3.31 At present, the system used to calculate reimbursement for the suppliers of pharmaceutical benefits is imprecise and would benefit from review to assess its effectiveness in facilitating the accurate processing and payment of PBS and RPBS claims. To provide assurance that approved suppliers are accurately reimbursed for dispensing pharmaceutical benefits, there would also be benefit in Human Services and Health reviewing departmental use of pricing information for reimbursing approved suppliers of pharmaceutical benefits.
Components of pharmacy remuneration
3.32 The most frequently dispensed PBS and RPBS items are general pharmaceutical benefits (Section 85 items)180, which account for approximately 99.4 per cent of retail pharmacy PBS and RPBS dispensing.181 The ANAO examined the pricing structure for Section 85 items since the first community pharmacy agreement.182 In summary, the pricing structure for pharmacy remuneration has become increasingly complex under successive agreements. For instance, the number of different levels of pharmacy mark-up has increased six-fold, and a complex mix of fees and incentives has been introduced. The following section examines the implementation of the two most recently introduced components of pharmacy remuneration: the Premium Free Dispensing Incentive (PFDI) and the Electronic Prescription Fee (EPF).183
Implementation of the Premium Free Dispensing Incentive
3.33 On 1 August 2008, as part of the structural adjustment package (financial assistance) negotiated for Price Disclosure, a Premium Free Dispensing Incentive (PFDI) of $1.50 per prescription was introduced for dispensing premium-free substitutable184 PBS items. The purpose of the incentive was to encourage greater use of generic medicines.185 Although the PFDI was originally scheduled to remain in place only until 30 June 2011, it continued under the 5CPA, indexed to WCI9.
3.34 In the context of the proposed 5CPA negotiations in October 2009, Health advised Ministers that pharmacists receive the PFDI for dispensing a substitutable premium-free medicine in place of a medicine with a patient-paid premium. However, in 2008 Health had implemented the incentive more broadly, and the PFDI was paid on all substitutable premium-free PBS brands, whether or not the pharmacist had actually substituted a premium-free medicine in place of one with a premium. As at 1 December 2014, approximately 4500 PBS branded items (some 83 per cent of all PBS branded items) automatically attract the PFDI payment whenever they are dispensed.186 187
3.35 Pharmacists may substitute a premium free PBS brand only when a brand with a price premium is prescribed, and the prescriber does not tick a box on the prescription that specifies ‘brand substitution not permitted’. Consequently, premium free brands are frequently dispensed without any substitution undertaken by the pharmacist, as outlined in Figure 3.3.
Figure 3.3: Payment of the Premium Free Dispensing Incentive (PFDI)
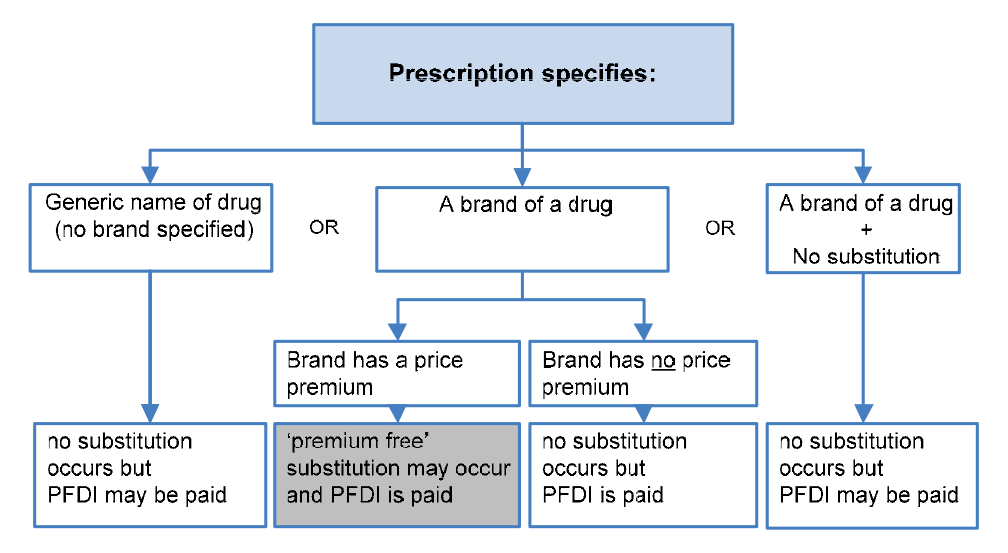
Source: ANAO analysis.
Note: Grey box indicates the only circumstances where pharmacists may substitute a ‘premium free’ PBS medicine for one with a price premium.
3.36 The PFDI could have been better targeted by restricting the incentive to the actual substitution of premium free PBS brands by pharmacists, as described by Health in its October 2009 advice to Ministers. While the original 5CPA forecast cost for the PFDI payment was $620 million, in January 2011, some six months after the commencement of the 5CPA, Health increased the budget estimate for PFDI by $226 million. In January 2013, Health increased the budget estimate for PFDI by a further $120 million, of which $66 million related to the period of the 5CPA. Since the commencement of the 5CPA, the PFDI has had two budget variations totalling $292 million, some 47 per cent higher than originally budgeted in the 5CPA funding envelope. More selective targeting of the PFDI would have helped contain costs for this component of pharmacy remuneration.
Implementation of the Electronic Prescription Fee (EPF)
3.37 Electronic prescriptions are prescriptions that are generated electronically by prescribers and uploaded to a Prescription Exchange Service (PES). If a pharmacy subscribes to a PES, the pharmacy can download the details of a patient’s prescription from the PES instead of manually entering prescription details into the pharmacy’s database. There are two PES providers: eRx (a wholly owned subsidiary of FRED IT, which is part-owned by the Pharmacy Guild188) and MediSecure Limited189 (supported by the Royal Australian College of General Practitioners).
3.38 In December 2009, Health advised Ministers that a key feature of the Pharmacy Guild’s platform in negotiations for the 5CPA was the introduction of government funding for:
- a per prescription payment to pharmacies to cover the cost of processing each electronic prescription dispensed ($75.5 million); and
- software vendors190 to integrate a range of agreement-related elements into existing dispensing software for pharmacies ($13 million).
3.39 While Ministers did not approve funding for software vendors to integrate agreement-related elements into existing dispensing software, Ministers did approve $75.5 million in Government funding for a 15 cent per prescription payment to pharmacies (known as the Electronic Prescription Fee) for each PBS/RPBS prescription downloaded from a PES.191 The purpose of the 15 cent fee was to offset a 25 cent charge to pharmacies by the PESs for providing the service. In seeking approval for this measure, Health advised Ministers that the Pharmacy Guild had agreed that the Electronic Prescription Fee (EPF) would be paid only to pharmacies for downloading e-prescriptions192, and that it was a re-allocation of funding that would otherwise have been spent on other aspects of pharmacy remuneration.
3.40 As Human Services makes EPF payments on behalf of Health, the ANAO compared Human Services’ and Health’s records of EPF payments for alignment. Over the first three years of the 5CPA, Health’s records showed significantly higher EPF payments ($9.1 million) than Human Services ($1.8 million), as shown in Table 3.4.
Table 3.4: Electronic Prescription Fee payments 2010–2013
|
|
2010–11 |
2011–12 |
2012–13 |
Total |
|
Department of Health |
||||
|
Total EPF payments |
$1 194 000 |
$1 880 183 |
$6 045 871 |
$9 120 053 |
|
Department of Human Services |
||||
|
15 cent payments to pharmacies |
$200 000 |
$500 000 |
$1 107 563 |
$1 807 563 |
|
EPF payments not paid to pharmacies |
$994 000 |
$1 380 183 |
$4 938 308 |
$7 312 491 |
|
Proportion of EPF funds not paid to pharmacies |
83% |
73% |
82% |
80% |
Source: ANAO analysis of Health and Human Services records.
3.41 As shown in Table 3.4, in the first three years of the 5CPA, only $1.8 million of $9.1 million in EPF payments were for the 15 cent incentive payment to pharmacists, as had been agreed by Ministers. The ANAO therefore examined the purposes of other payments made by Health with the unspent funds of $7.3 million shown in Table 3.4.
3.42 In December 2011, the Health Secretary received advice that there had been a low take-up of the EPF due to incompatibility between the two PES providers, and that EPF funds had been used for other purposes:
The underspend was used for two purposes: to fund a communication strategy for prescribers and dispensers and thereby improve take up of the initiative, and to continue manual processing of claims by DHS-Medicare at the request of the Guild, whose dispensing software was not ready for full online claiming by the anticipated 30 June 2011 … Market forces are a disincentive to PES [Prescription Exchange Service] interoperability with one of the two PES operators eRx, part owned by the Guild and supported by the majority of pharmacies due to its links with the Guild and FRED IT. On the other hand, Medisecure is supported by the RACGP [Royal Australian College of General Practitioners] and has a greater take up with GPs but lesser with pharmacies. Consequently there are a large number of scripts (around 80 per cent of those uploaded according to NEHTA) [National e-Health Transition Authority], that are not being downloaded due to the PES preferences of the different clinical areas, and the failure of the two PES providers to interact and share information … Advice from NEHTA is that, eRx has previously rejected MediSecure approaches to establish interoperability between the two providers.
3.43 In December 2011, Health sought Ministerial approval to re-allocate EPF funds to:
- provide financial support to the two PES providers (eRx and MediSecure) to make their systems interoperable;
- pay software vendors to align their products with interoperable PES providers; and
- increase EPF payments until July 2013.
3.44 The then Health Minister did not approve this proposal193, and in January 2012 Health submitted a revised proposal to the new Health Minister194, seeking approval to: re-allocate EPF funds to provide financial assistance through a ‘vendor panel arrangement’ to the two PES providers, to upgrade their systems to address compatibility issues. Health’s revised proposal to the Minister involved:
- an offer of financial assistance of ‘possibly’ $500 000 to each PES provider to implement compatibility initiatives between the two PES systems; and
- increasing the EPF payment from 15 cents to 20 cents.
3.45 In February 2012, the new Health Minister agreed to the department’s revised proposal. However, Health did not advise the new Health Minister that Ministers had previously approved EPF funding on the basis of departmental advice that the Pharmacy Guild had agreed that the $75.5 million funding was to be paid only to pharmacies for downloading e-prescriptions, and that the 15 cent per prescription fee was intended to offset the 25 cent PES charge to pharmacies.
3.46 Subsequently the department commissioned a study into the financial and commercial viability of the PES providers. The study indicated that both PES providers were, at that time, working through a range of financial issues and would welcome a capital contribution. Health did not advise the Minister of the study.195
3.47 In June 2012, the department advised the Pharmacy Guild that Health had:
- offered the two PES providers a total of $1.32 million to address interoperability issues;
- offered the PES providers a payment of 85 cents per prescription196 from 1 July 2012 to 31 December 2012, and 35 cents per prescription197 from 1 January 2013 to 30 June 2013 (providing up to $8 361 460 to the PES providers); and
- agreed to a Pharmacy Guild proposal to provide $896 110 to the Guild to increase pharmacies’ understanding, awareness and uptake of EPF.
3.48 While the Health Minister had agreed to provide financial assistance to the two PES providers based on Health’s advice of ‘possibly $500 000’ per PES and an increase in the Electronic Prescription Fee to 20 cents, the department’s advice to the Health Minister did not mention paying the Electronic Prescription Fee directly to the PESs, instead of pharmacies (as had originally been agreed by the Pharmacy Guild). Further, the department’s contracts provided $660 000 per PES and temporarily increased the Electronic Prescription Fee to $1.00, (including an 85 cent payment direct to the PESs), which provided additional payments of up to $8.4 million to the two PESs in 2012–13. In summary, departmental contracts provided up to $9 681 460 in total for the two PESs.
3.49 Notwithstanding the significant financial assistance provided to PESs in 2012–13, which was intended to increase EPF take-up by pharmacies, in August 2013, Health advised its Minister that almost 70 per cent of pharmacies were not processing e-scripts as part of their normal business practice. Health asked the Minister to note new EPF arrangements that the department and the Pharmacy Guild proposed to put in place, which involved:
- providing a $2000 incentive payment for each pharmacy reaching a minimum rate of e-script scanning by March 2014; and
- supporting PES operators to ensure that all pharmacy dispensing software products could effectively download e-scripts for dispensing.198
3.50 Health advised the ANAO that:
… the new Government elected to hold off re-approving commencement of ePSI [Electronic Prescription Scanning Incentive]. Subsequently it was agreed to modify and propose a two-target approach with March 2014, and September 2014.
3.51 In summary, while the Australian Government had originally approved $75.5 million in funding for a 15 cent EPF incentive payment to pharmacies, in the first three years of the 5CPA, 80 per cent of EPF payments were not paid to pharmacies but were instead used to pay:
- the two PES providers to make their proprietary software compatible with each other (to enable the transfer of electronic prescriptions between the two systems);
- software vendors to align their proprietary software with PES providers (to enable pharmacists to download eligible electronic prescriptions);
- the Pharmacy Guild to undertake promotional activities to support improved uptake of the EPF; and
- Human Services to manually process EPF claims, at the request of the Pharmacy Guild.
3.52 The scope and complexity of the 5CPA and related arrangements, which the parties intended to achieve multiple objectives, makes it necessary to periodically adjust policy and administrative settings in light of experience. The 5CPA therefore provides flexibility to re-allocate funds between the various 5CPA professional programs and services. However, the EPF is not a professional program but a component of pharmacy remuneration. The use of EPF funding for alternative purposes was a significant departure from the purpose for which Ministers originally approved the $75.5 million EPF initiative, and was not provided for in the 5CPA. Further, Health did not advise the Health Minister on a range of issues, including: its decision to temporarily199 increase the incentive payment to pharmacies from 15 cents to $1.00, and to use the 85 cent difference to provide financial assistance to IT service providers; and that a study it had commissioned indicated that both PES providers were, at that time, working through a range of financial issues.
Departmental reporting of pharmacy remuneration
3.53 An objective of the 5CPA is to:
ensure transparency and accountability in the expenditure of the Funds.200
3.54 Each year, Health and DVA report on the cost to government of the PBS and RPBS in their respective annual reports.201 However, neither department reports the actual costs of pharmacy remuneration (essentially a fee for service) separately from the cost of medicines (a product).202 Health advised the ANAO that:
While it is acknowledged that annual reports do not separate this information [pharmacy remuneration], it should be made clear that such information is internally available to Health and can be used as required for policy, planning or reporting purposes.
Pharmacy remuneration budget outcomes
Budget for the components of 5CPA pharmacy remuneration
3.55 The components of pharmacy remuneration are listed in the 5CPA as the: wholesale mark-up; pharmacy mark-up; dispensing fee; extemporaneously prepared and dangerous drug fees; Premium Free Dispensing Incentive; and Electronic Prescription Fee. As the 5CPA does not detail the funding for each of these components, the ANAO examined departmental records to identify the budgeted costs of the components of 5CPA pharmacy remuneration, as shown in Table 3.5.203
Table 3.5: Budgeted components of 5CPA pharmacy remuneration
|
Component |
5CPA budgeta |
Per cent of 5CPA budget |
|
Ready Prepared dispensing fees |
$7 174 725 087 |
52.1 |
|
Extemporaneously Prepared dispensing fees |
$2 552 712 |
0.02 |
|
Pharmacy mark-up |
$3 564 215 354 |
25.9 |
|
Wholesaler mark-up |
$2 260 873 584 |
16.4 |
|
Dangerous drug fees |
$74 154 479 |
0.5 |
|
Premium Free Dispensing Incentive |
$619 673 207 |
4.5 |
|
Electronic Prescription Fee |
$75 428 000 |
0.5 |
|
Total 5CPA pharmacy remuneration |
$13 771 622 423 |
100.0 |
Source: ANAO analysis of Health information.
Note:
- Budget figures are aggregate predicted costs over the five years of the 5CPA in 2010 dollars.
- The number of forecast subsidised prescriptions predicted for the five year term of the 5CPA was: 1 078 212 248 prescriptions.
3.56 Dispensing fees and pharmacy mark-up together account for 78 per cent of the budgeted cost of 5CPA pharmacy remuneration. The wholesale mark-up accounts for 16.4 per cent of ‘pharmacy remuneration’ as defined in the 5CPA.204 205
Estimated versus actual pharmacy remuneration
3.57 Health does not monitor, review or report on actual 5CPA pharmacy remuneration. The ANAO therefore estimated the actual costs of pharmacy remuneration for the first four years of the 5CPA.206
3.58 As at 30 June 2014, actual pharmacy remuneration delivered under the 5CPA was $10 907 million, compared to forecast remuneration of $10 831 million. Actual and forecast remuneration align closely, with an overall variance of approximately 0.7 percent over the budgeted cost.
3.59 While some components of remuneration are under budget (for example, dispensing fees are $116 million or 2 per cent under the budget estimate), this is offset by the Premium Free Dispensing Incentive, which is $180 million or 37 per cent over the budget estimate.
3.60 A comparison of actual 5CPA pharmacy remuneration with the original 5CPA budget for the period 2010–11 to 2013–14 is presented in Table 3.6.
Table 3.6: Total pharmacy remuneration by component 2010 to 2014
|
Component |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
Total |
Variation per cent |
|
Forecast prescriptionsa |
||||||
|
Budget |
205 422 108 |
210 504 321 |
215 645 005 |
220 780 789 |
852 352 223 |
|
|
Actual |
199 287 136 |
205 796 475 |
208 093 891 |
220 538 509 |
833 716 011 |
-2.2% |
|
Dispensing Fees |
||||||
|
Budget |
$1 378 475 188 |
$1 354 692 647 |
$1 419 498 321 |
$1 480 766 129 |
$5 633 432 285 |
|
|
Actual |
$1 271 572 352 |
$1 333 238 578 |
$1 388 327 794 |
$1 524 710 222 |
$5 517 848 947 |
-2.1% |
|
Pharmacy mark-up |
||||||
|
Budget |
$709 333 733 |
$698 506 613 |
$695 881 716 |
$716 881 606 |
$2 820 603 668 |
|
|
Actual |
$707 781 197 |
$729 020 117 |
$681 813 879 |
$684 799 594 |
$2 803 414 786 |
-0.6% |
|
Wholesale mark-up |
||||||
|
Budget |
$446 399 226 |
$441 432 593 |
$442 830 942 |
$456 490 031 |
$1 787 152 792 |
|
|
Actual |
$466 388 519 |
$477 749 501 |
$443 221 403 |
$450 022 890 |
$1 837 382 313 |
2.8% |
|
Dangerous Drug Fee |
||||||
|
Budget |
$14 043 671 |
$14 656 088 |
$15 019 105 |
$15 228 447 |
$58 947 311 |
|
|
Actual |
$14 057 006 |
$15 514 145 |
$17 117 105 |
$19 592 216 |
$66 280 472 |
12.4% |
|
Premium Free Dispensing Incentive |
||||||
|
Budget |
$116 187 000 |
$119 789 000 |
$123 502 000 |
$127 331 000 |
$486 809 000 |
|
|
Actual |
$134 961 239 |
$146 018 788 |
$176 079 042 |
$209 384 547 |
$666 443 616 |
36.9% |
|
Electronic Prescription Fee |
||||||
|
Budget |
$1 257 000 |
$3 622 000 |
$14 757 000 |
$24 129 000 |
$43 765 000 |
|
|
Actual |
$1 194 000 |
$1 880 183 |
$6 045 871 |
$6 562 453 |
$15 682 507 |
-64.2% |
|
Total |
||||||
|
Budget |
$2 665 695 818 |
$2 632 698 941 |
$2 711 489 084 |
$2 820 826 214 |
$10 830 710 057 |
|
|
Actual |
$2 595 954 314 |
$2 703 421 312 |
$2 712 605 094 |
$2 895 071 921 |
$10 907 052 641 |
0.7% |
Source: ANAO analysis of Health records and Human Services over co-payment prescription processing data as at 30 June 2014.
Note:
- ‘Forecast prescriptions’ refers to the predicted number of subsidised prescriptions over the five years of the 5CPA.
Actual cost of pharmacy remuneration
3.61 To assess the actual costs of pharmacy remuneration under the 5CPA, the ANAO examined PBS and RPBS prescription payment data, which is produced by Human Services and provided to Health. Figure 3.4 illustrates the process for exchanging data relating to the costs of pharmaceutical benefits.
Figure 3.4: Pharmacy remuneration data flow
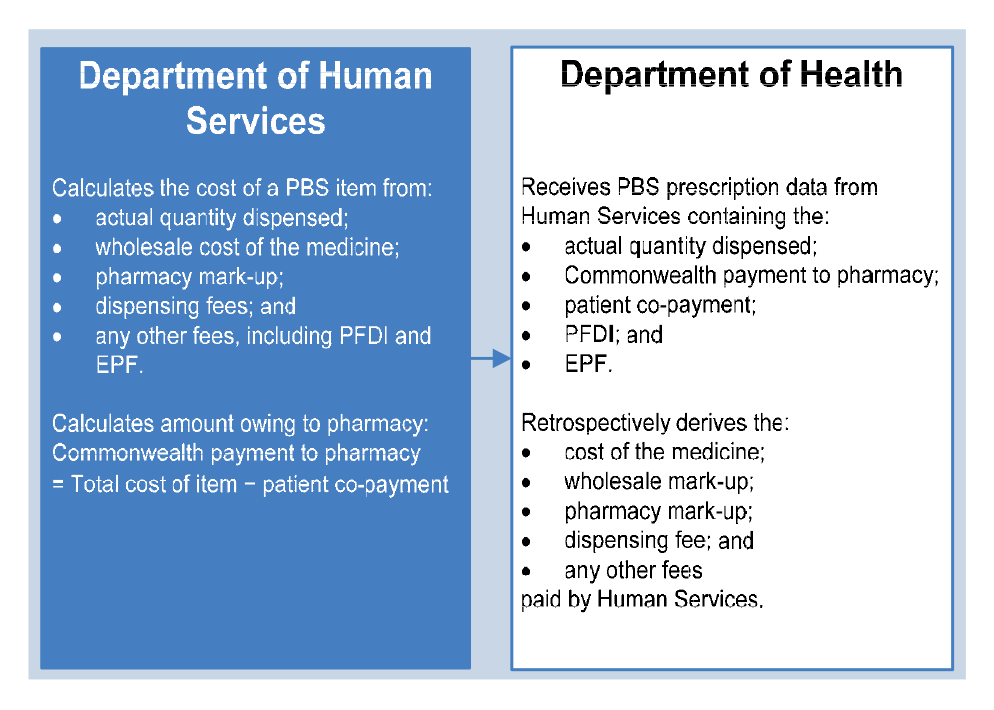
Source: ANAO analysis.
Note: The wholesale cost of a medicine, also known as the ‘price to pharmacist’, is the notional amount that wholesalers charge pharmacies for supplying PBS medicines using the 5CPA mark-up rates. The notional wholesale cost of a medicine is equal to the ex-manufacturer price plus the wholesale mark-up.
3.62 The prescription data provided by Human Services to Health is an output of the pharmacy payments process administered by Human Services, and includes, among other things, the:
- Commonwealth payment to the pharmacy;
- patient co-payment;
- Premium Free Dispensing Incentive (PFDI, if applicable); and
- Electronic Prescription Fee (EPF, if applicable).
3.63 Health advised the ANAO that while the prescription data received from Human Services includes two components of pharmacy remuneration (PFDI and EPF), it does not receive the ex-manufacturer cost of the medicine, and the other components of pharmacy remuneration (the wholesale mark-up; pharmacy mark-up; dispensing fee; and dangerous drug fee). As data for only two components of pharmacy remuneration is received from Human Services, Health uses an algorithm to retrospectively derive the other cost components.
3.64 In the past, Health has been able to derive the cost components for most PBS and RPBS prescriptions.207 However, since the commencement of the 5CPA in 2010–11, the ANAO observed that there were a growing number of prescriptions for which Health had not been able to retrospectively derive the cost components (specifically, the ex-manufacturer cost of the medicine and most components of pharmacy remuneration).
3.65 Using Human Services data (specifically, the Commonwealth payment to the pharmacy and the patient co-payment), the ANAO calculated the total value of PBS and RPBS prescriptions where Health had not been able to retrospectively derive a wholesale price. The ANAO then repeated the calculation using whatever data Health had on the cost components for these prescriptions. The results are shown in Figure 3.5.
3.66 For 2013–14, the ANAO calculated that the actual cost of prescriptions (based on Human Services data) was $459 million higher than the sum of the cost components as derived by Health. Some 87 per cent ($399 million) of the $459 million discrepancy related to Health being unable to derive the cost components for chemotherapy infusions.208
3.67 Health advised the ANAO that the current system of Health retrospectively deriving PBS/RPBS pharmacy remuneration data from information provided by Human Services for the purposes of Health reporting involves duplication of some automated processes, and results in less complete and reliable results than if the department received the actual data to the level of granularity that Human Services used to make payments.
Figure 3.5: Total cost of prescriptions where Health was unable to derive a wholesale cost
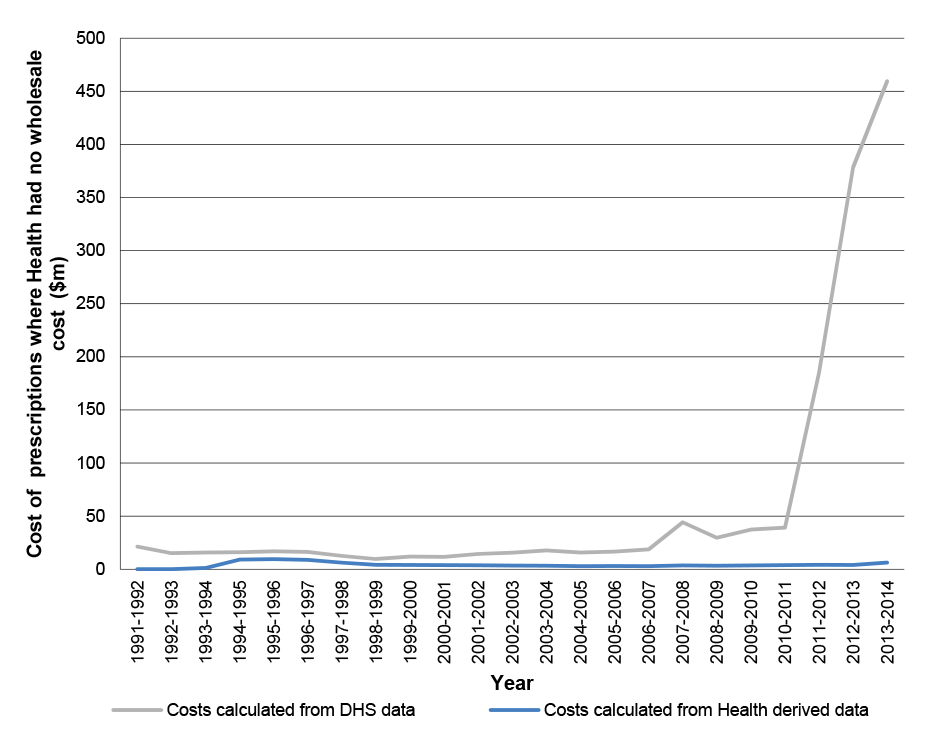
Source: ANAO analysis of Health data
3.68 Revised remuneration arrangements for dispensing chemotherapy infusions were introduced on 1 December 2011, which involved the use of a complex pricing formula to enable payment to be made on the most efficient combination of chemotherapy vials. In seeking approval for the revised remuneration arrangements—a key objective of the 5CPA negotiations—Health advised Ministers that the performance of the new arrangements would be assessed against whether the measure delivers the forecast level of savings. However, as Human Services does not provide the components of pharmacy remuneration for chemotherapy infusions to Health, and Health has been unable to derive this information, it has not been possible for Health to accurately assess the achievement of savings for this measure.
3.69 In summary, Health has been unable to identify actual expenditure on the components of pharmacy remuneration for a growing number of prescriptions subsidised by the Australian Government under the 5CPA.209 Further, in Health’s annual report, expenditure on pharmacy remuneration is embedded in aggregated annual reporting on the cost of the PBS, which does not differentiate between: the cost of PBS medicines (expenditure on products); and government payments for pharmacy remuneration (expenditure on services).
3.70 Health and DVA should work with Human Services to develop more accurate data on actual pharmacy remuneration as a basis for improved internal and external reporting on PBS and RPBS expenditure.
Recommendation No.4
3.71 To improve the accuracy and transparency of reporting on Australian Government expenditure under the Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme, the ANAO recommends that the Departments of Health, Veterans’ Affairs and Human Services liaise on the collection, recording and sharing of information regarding payments to suppliers, so as to clearly identify the actual cost of medicines and the components of pharmacy remuneration.
Health response: Agreed.
Veterans’ Affairs response: Agreed.
3.72 The Department of Veterans’ Affairs (DVA) agrees with the report’s recommendation number 4, that the Department of Health (DoH) and DVA work closely with the Department of Human Services (DHS) to develop and refine processes for capturing and reporting data on pharmacy remuneration. DVA has already collaborated successfully with DoH and DHS across a number of key areas. I am confident that future projects centred on ensuring the exchange and use of accurate data will result in positive outcomes.
Human Services response: Agreed.
3.73 The department agrees with this recommendation. The department will support the Departments of Health and Veterans’ Affairs in their determination of reporting requirements on the cost of Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme expenditure on products.
Pharmacy remuneration across the retail pharmacy network
3.74 The introduction to the 5CPA states that:
Community pharmacy is an integral part of the infrastructure of the health care system in its role in primary health care through the delivery of the Pharmaceutical Benefits Scheme and related services.
3.75 Three of the six 5CPA objectives relate to the financial viability and sustainability of the retail pharmacy network:
i. ensure a fair Commonwealth price is paid to Approved Pharmacists for providing pharmaceutical benefits while maximising the value to taxpayers by encouraging an effective and efficient community pharmacy network;
iv. promote the sustainability and efficiency of the PBS within the broader context of health reform and ensuring that community resources continue to be appropriately directed across the health system, while also supporting the sustainability and viability of an effective community pharmacy sector;
vi. ensure … a commercially viable and sustainable network of community pharmacies dispensing PBS medicines.210
3.76 Health is not well positioned to assess whether these key 5CPA objectives are being met, as the department’s understanding of the cost structures and business models of retail pharmacies is limited, notwithstanding the operation of successive community pharmacy agreements since 1990, and a long-term relationship with the Pharmacy Guild. Health informed a Senate Committee in 2013 that:
There is a single bucket out of which community pharmacy remuneration is paid and negotiated and agreed. Some of it is normal dispensing fees. Some of it is premium free dispensing … Part of the challenge that any government department has is that in the funding model for those things is that we pay a rolled-up price to the retail end of pharmacy for a drug. We do not pay manufacturers, suppliers, wholesalers and everyone separately. We have a formula that wraps up a rolled-up single price that we pay to the retail end of pharmacy. We have no commercial relationships at all with anyone else. So what actually happens down the supply chain is completely a black box. We do not know what the business models are. We do not know what the cost structures are. Until we started measuring, we do not know what discounts are enjoyed. We rely on the organisations such as the Guild to represent the cost structures of their members in this context, and they are able to do so in knowledge of what is coming in terms of policy in this case but also in knowledge of what discounts are being received and would be subject to price disclosure.211
3.77 Further, Health advised the ANAO that:
Health does know PBS statistics and that PBS remuneration can make up between 40 to 80 percent of a pharmacy’s income. However on the issue of business costs such as rents, staffing and other overheads, this is private business data. Pharmacies may also enter into other commercial arrangements with wholesalers and manufacturers. Health does not know on an individual commercial basis the cost structures and business models.
Pharmacy remuneration under the 5CPA 2012–13 to 2013–14
3.78 As Health does not monitor, review or report on actual 5CPA pharmacy remuneration, the ANAO examined Human Services data to assess the average remuneration per pharmacy under the 5CPA, and the distribution of remuneration across the pharmacy network.
3.79 Human Services has received data on under co-payment prescriptions from 1 April 2012, and consequently full year data for under co-payment and over co-payment prescriptions is only available for 2012–13 and 2013–14 (the third and fourth years of the 5CPA). The ANAO analysis related to retail pharmacies that were approved to trade for the full year212, and dispensed at least 1000 prescriptions per year.213
3.80 The average remuneration per pharmacy under the 5CPA is shown in Table 3.7.
Table 3.7: Average annual pharmacy remuneration under the 5CPA
|
|
2012–13 |
2013–14 |
|
Estimated over co-payment remuneration |
$2 703 383 523 |
$2 890 093 281 |
|
Estimated under co-payment remuneration |
$512 773 899 |
$602 247 486 |
|
Total pharmacy remuneration |
$3 216 157 421 |
$3 492 340 767 |
|
Number of pharmacies trading full year |
5289 |
5371 |
|
Average remuneration per pharmacy |
$608 084 |
$650 222 |
|
Number of prescriptions |
268 889 420 |
290 994 936 |
|
Average remuneration per prescription |
$11.96 |
$12.00 |
Source: ANAO analysis of Human Services and Health data.
Notes:
- Remuneration is estimated from PBS and RPBS over and under co-payment prescription data.
- Remuneration does not include discretionary charges applying to under co-payment prescriptions paid by patients.
- Remuneration is estimated as at Human Services processing date (not date of supply).
3.81 The ANAO estimates that average annual pharmacy remuneration under the 5CPA increased from $608 084 per year in 2012–13, to $650 222 per year in 2013–14.
3.82 The ANAO also examined the distribution of pharmacy remuneration under the 5CPA across the network, and found that there was significant variation, as show in Figure 3.6. In 2013–14, out of a total cohort of 5371 retail pharmacies, 150 pharmacies (2.8 per cent) received less than $100 000 in remuneration, while 941 pharmacies (17.5 per cent) received over $1 million in remuneration.
Figure 3.6: Distribution of under and over co-payment pharmacy remuneration 2012–13 and 2013–14
Source: ANAO analysis of departmental data.
Conclusion
3.83 Pharmacy remuneration for dispensing pharmaceutical benefits has been a core element of successive community pharmacy agreements. The 5CPA specifies seven components of pharmacy remuneration, which account for some 90 per cent ($13.8 billion) of the total value of the $15.4 billion 5CPA.
3.84 In April 2010, Health advised Ministers that agreement had been reached with the Pharmacy Guild on pharmacy remuneration arrangements. However, a number of remuneration arrangements discussed in the 5CPA negotiations were not included in the agreement, including the remuneration for dispensing chemotherapy infusions, and the fee paid to pharmacies that supply PBS items in bulk to remote Aboriginal Health Services. The Pharmacy Guild subsequently disputed whether agreement had been reached in respect of remuneration arrangements for dispensing chemotherapy infusions, resulting in a Parliamentary inquiry and the provision of additional Commonwealth funding of $82 million per year. For the avoidance of doubt, there would be merit in including all matters relating to pharmacy remuneration that are agreed in the context of negotiating the next community pharmacy agreement, in the written agreement.
3.85 There is an obligation on administering departments to effectively support Ministers, through their advice, on the design and implementation of complex and financially material schemes such as the 5CPA. In the context of the proposed 5CPA negotiations, Health advised Ministers in October 2009 that pharmacists received a financial incentive for substituting generic medicines in place of medicines with a brand price premium, which was intended to be a means of reducing the cost of medicines to the community. The department’s original cost estimate for continuing the incentive in the 5CPA was some $620 million. However, contrary to the advice provided to Ministers, under the 4CPA Health had implemented the financial incentive more broadly, and in many instances the incentive was paid where a pharmacist had not actually substituted an item. This less targeted approach contributed to the department’s budget estimate for the incentive being later increased to $912 million, some 47 percent higher than originally advised to Ministers.
3.86 On the basis of Health’s advice, Ministers also agreed to new 5CPA funding of $75.5 million for a 15 cent incentive payment to pharmacies for downloading electronic prescriptions, a measure known as the Electronic Prescription Fee (EPF). However, in the first three years of the 5CPA, some $7.3 million (amounting to 80 per cent) of EPF payments were not paid to pharmacies but were used instead to pay for other activities, including financial assistance to the two Prescription Exchange Service (PES) providers to upgrade their systems, and $896 110 to the Pharmacy Guild to increase pharmacies’ understanding, awareness and uptake of EPF. In February 2012, the then Health Minister had agreed to provide financial assistance to the two PES providers based on Health’s advice of ‘possibly $500 000’ per PES and an increase in the EPF to 20 cents. However, the department provided $660 000 per PES and temporarily increased the EPF to $1.00 to provide the financial assistance, without Ministerial approval. While 15 cents continued to be paid to pharmacies, the additional 85 cents was paid to the two PES providers. While Health advised the ANAO that discussions were held with the Minister’s office, documented evidence to support this was not available.
3.87 The 5CPA provides for the re-allocation of funds between the various 5CPA professional programs and services. However, the EPF is not a professional program but a component of ‘pharmacy remuneration’ under the 5CPA, and the use of EPF funding for other purposes was a departure from the original purpose for which Ministers approved the $75.5 million EPF measure. Further, Health did not advise its Minister, before the provision of government financial assistance to the two PES providers, that a study commissioned by the department had indicated that, at that stage, the PES providers were working through a range of financial issues.
3.88 One of the objectives of the 5CPA is to ensure transparency and accountability in the expenditure of 5CPA funds. However, Health does not monitor, review or separately report the actual costs of pharmacy remuneration. At present, pharmacy remuneration is embedded in aggregated annual reporting on the cost of the PBS and RPBS (expenditure on products); and annual reporting on the cost of 5CPA professional programs. Health and DVA should work with Human Services to develop more accurate data on actual pharmacy remuneration as a basis for improved internal and external reporting on PBS and RPBS expenditure.
4. Pharmaceutical Services
This chapter examines dispensing and professional programs and services funded by the Australian Government under the 5CPA.
Introduction
4.1 The Australian Government funds a range of services under the 5CPA:
- the dispensing of PBS and RPBS medicines through retail pharmacies ($13.8 billion);
- professional programs that deliver services to patients through either consultant pharmacists or retail pharmacies (such as the Medication Management programs);
- programs that support the pharmacy network and develop the pharmacy workforce (such as the Rural Support programs)214; and
- development projects (such as the development of a standardised medication chart for residential aged care facilities).
4.2 This chapter examines the different types of services funded under 5CPA arrangements; how they are designed and delivered; and their delivery of health outcomes.
Dispensing services
4.3 Part 2 of the 5CPA sets out how the price for PBS items is to be calculated for the purpose of making Commonwealth payments to pharmacy owners for the provision of dispensing services for PBS medicines. The 5CPA makes the following reference to dispensing services:
In agreeing to a Commonwealth price for a particular medicine the Commonwealth includes allowances for: … the handling and storage of medicines by the pharmacy; and the pharmacist’s specialised skills in dispensing the medicines.215
4.4 Part 3 of the 5CPA specifies four additional types of payments to pharmacy owners related to dispensing. These are:
- Premium Free Dispensing Incentive—paid for dispensing a PBS item that does not have a patient premium;
- Electronic Prescription Fee—paid for dispensing an electronic prescription for a PBS or RPBS item that is downloaded from a Prescription Exchange Service;
- payments for dispensing s100 Highly Specialised Drugs; and
- ‘Additional Charges’—discretionary charges which pharmacists may apply. The relevant service is not specified in the 5CPA.
4.5 While the 5CPA commits the Commonwealth to delivering $13.8 billion over five years in pharmacy remuneration, the agreement generally does not specify the nature, quality or extent of services to be provided by retail pharmacies in return for pharmacy remuneration.
4.6 In the absence of specific performance indicators in the 5CPA, the ANAO asked if Health had identified:
- the services that retail pharmacies deliver in return for 5CPA pharmacy remuneration; and
- the number of people using dispensing services under the 5CPA.
4.7 Health advised the ANAO that there was no further information other than that contained in the 5CPA.
Service standards for dispensing
4.8 Health further advised that the quality and extent of dispensing services are governed through Pharmacy Board requirements and State and Territory legislation, which are intentionally not included in the 5CPA.
4.9 Further, under the National Health Act, the approval of a pharmacy owner to supply pharmaceutical benefits is subject to a range of conditions.216 In August 2007, the Minister for Health determined certain conditions of approval for pharmacists approved to supply PBS medicines.217 The Ministerial Determination (the Determination) sets out approval conditions relating to the:
- professional conduct of approved pharmacists;
- pharmacy practice standards in supplying pharmaceutical benefits; and
- continuing education in pharmacy competency standards.
4.10 The ANAO’s review of the 2007 Health Minister’s Determination indicated that Health had not taken steps to initiate an update to reflect: the introduction in September 2011 of the Pharmaceutical Society of Australia’s (PSA) Code of Ethics for Pharmacists; and the PSA’s June 2010 Professional Practice Standards.
4.11 In the course of this audit, relevant amendments were made to the Minister’s Determination, with effect from 1 December 2014.218 As the Australian Government is the largest purchaser of dispensing services in Australia, Health should maintain the currency of relevant Ministerial Determinations following periodic changes to professional standards.
The number of people using subsidised dispensing services
4.12 Health advised the ANAO that it has not monitored or reported on the number of people using dispensing services under the 5CPA or previous community pharmacy agreements.
4.13 Using prescription claims data, the ANAO calculated the number of people receiving subsidised dispensing services219 from retail pharmacies under the 5CPA and the two previous agreements.220 In summary, over the last 11 years, approximately 9 million people per year have received subsidised dispensing services from retail pharmacies under successive community pharmacy agreements, ranging from 8.9 million people in 2007–08 to 9.5 million people in 2013–14. The results are shown in Figure 4.1.
Figure 4.1: Number of people receiving subsidised dispensing services
Source: ANAO analysis of DHS and Health data.
4.14 The ANAO also calculated the average number of subsidised PBS and RPBS medicines dispensed per person under community pharmacy agreements. In summary, from 2002–03 to 2013–14 the average number of subsidised medicines dispensed from retail pharmacies increased from 18 to 23 items per person per year.221 The results are shown in Figure 4.2.222
Figure 4.2: Number of subsidised prescriptions dispensed per person per year
Source: ANAO analysis of DHS and Health data.
Professional programs
4.15 In addition to PBS and RPBS dispensing services, the Australian Government funds the following categories of professional programs under the 5CPA, at a total cost of $663.4 million:
- Pharmacy Practice Incentives and Accreditation (PPIs) ($344 million);
- Medication Management programs ($163.9 million);
- Rural Support programs ($107 million);
- Aboriginal and Torres Strait Islander programs ($28.9 million);
- Research and Development ($10.6 million);
- Medication Continuance ($1 million); and
- Other Programs to support patient services:
- Electronic recording and reporting of controlled drugs ($5 million); and
- Supply and PBS claiming from a medication chart in Residential Aged Care Facilities ($3 million).
4.16 The 5CPA professional program budgets and actual expenditure in the first four years of the 5CPA are shown in Appendix 7. The deliverables (the services or outputs that a program is intended to deliver) for 5CPA professional programs are shown in Appendix 8. The 5CPA professional programs are reviewed in the following paragraphs.
Pharmacy Practice Incentives and Accreditation ($344 million)
4.17 Under the 5CPA, the Pharmacy Practice Incentives and Accreditation (PPI) program and its six priority areas account for $344 million (52 per cent) of all funding for professional programs. PPI is intended to ‘ensure that patients receive the highest quality of care, information, advice and services through a robust quality framework’.223
4.18 To be eligible for PPI payments, a retail pharmacy must:
- agree to publicly display and comply with the Community Pharmacy Service Charter and Customer Service Statement224;
- register for one or more of the six PPI priority areas, which comprise: Primary Health Care; Community Services Support; Working with Others; Staged Supply; Dose Administration Aids and Clinical Interventions; and
- be accredited by an approved pharmacy accreditation program.
4.19 Under the current PPI business rules, the sole approved pharmacy accreditation program is the Quality Care Pharmacy Program (QCPP), which is owned and operated by the Pharmacy Guild.225 Health advised the ANAO that while other national pharmacy accreditation bodies would be approved, QCPP is the only one currently in existence.
4.20 In effect, the 5CPA provides funding through the Pharmacy Practice Incentive and Accreditation program, to support the QCPP accreditation system in two ways: directly, by payments to the Pharmacy Guild (discussed below); and indirectly, by requiring pharmacies to be QCPP accredited in order to receive PPI payments. In December 2011, Health entered into a contract with the Pharmacy Guild that provided funding of up to $2.7 million, primarily for:
- further development of the QCPP database;
- maintenance and expansion of the QCPP database server and ongoing server costs; and
- assessment, data entry and quality checking of PPI data and the QCPP assessment checklist.
4.21 Health has also entered into separate contracts totalling $3.9 million with the Pharmacy Guild for PPI administration and development more generally.
|
Australian Government funding to promote QCPP The Australian Government provided significant financial support in the 3CPA and 4CPA to promote QCPP accreditation by providing subsidies and incentives to retail pharmacies, and funding promotional activities. In the 3CPA, pharmacies were paid $7500 for accreditation and $2500 for registration/re-accreditation. The Pharmacy Guild was paid $7.5 million to administer the Pharmacy Development Program, which provided $50 million to ‘incentivise’ QCPP uptake. In the 4CPA, pharmacies were paid $3000 to $5000 per year to offset the costs of gaining and maintaining accreditation. The Pharmacy Guild was funded to design and run a Change Management Strategy, to assist pharmacies transition to the second edition of the QCPP. Under the 4CPA, the QCPP received funding of $75.8 million, and the Practice Change Incentive Program received funding of $10.3 million. |
4.22 The QCPP manual indicates that the program is a quality management system, containing 18 elements (topics) relating to a retail pharmacy’s business and professional operations.226 Three of the QCPP elements relate directly to the delivery of professional pharmacy services. The Pharmacy Guild advised that:
The remaining 15 elements are not directly associated with the delivery of professional services, however, they do ensure that accredited community pharmacies maintain the business structures and frameworks to make sure they operate in accordance with legislative and professional guidelines, and maintain rigorous processes within a corporate governance framework. Hence the additional 15 elements promote a quality management framework that supports the delivery of all services within community pharmacy.
4.23 The PPI program guidelines state that:
PPI payments are to be made for the:
- demonstrated delivery of agreed quality Services to patients that are designed to improve their quality use of medicines; and
- demonstrated achievement of defined outcomes, as set out in this document, in relation to the delivery of quality Services to patients.227
4.24 The ANAO examined the detailed PPI program requirements for each of the six PPI priority areas, to assess how the overarching requirements for making PPI payments under the PPI program—demonstrated delivery of agreed services to patients and demonstrated achievement of defined outcomes—are implemented.
4.25 As illustrated in Table 4.1, in respect of two PPI priority areas—Dose Administration Aids and Clinical Interventions—payments are made quarterly on the basis of the number of patient services self-reported by the pharmacy and the pharmacy’s prescription volume.
4.26 However, in respect to the four remaining PPI priority areas—Staged Supply, Primary Health Care, Community Services Support and Working With Others—the program guidelines indicate that payments are not made on the basis of actual patient services delivered, but as a flat annual amount depending on whether participating pharmacies have been accredited.228
Table 4.1: Pharmacy Practice Incentives payments 2010–14
|
PPI element |
2010–11 |
2011–12 |
2012–13 |
2013-14 |
|
Dose Administration Aids |
||||
|
Start-up incentive payment |
$1800 |
- |
- |
- |
|
Periodic two to four monthly payments. |
- |
$150 (base) + $1.92 to $3.01 per service + $0.67 to $0.99 per 10 scripts |
$150 (base) + $0.57 to $1.80 per service + $0.04 to $0.61 per 10 scripts |
$0 (base) + $1.55 to $2.23 per service + 0.35 to $0.65 + per 10 scripts |
|
Clinical Interventions |
||||
|
Start-up incentive payment |
$4550 |
- |
- |
- |
|
Periodic two to four monthly payments. |
- |
$150 (base) + $10.54 to $12.68 per service + $0.34 to $0.69 per 10 scripts |
$150 (base) + $1.01 to $3.77 per service + $0.14 to $0.31 per 10 scripts |
$0 (base) + $2.44 to $6.36 per service + $0.25 to $0.38 per 10 scripts |
|
Staged Supply |
||||
|
Start-up incentive payment |
$1720 |
- |
- |
- |
|
Annual payment |
- |
$1000 |
$1000 |
$1000 |
|
Annual balancing payment |
- |
$423.17 |
$0 |
$0 |
|
Primary Health Care |
||||
|
Annual payment |
$3900 |
$850 |
$850 |
$850 |
|
Annual balancing payment |
|
$219.26 |
$0 |
$0 |
|
Community Services Support |
||||
|
Annual payment |
$3900 |
$850 |
$850 |
$850 |
|
Annual balancing payment |
|
$219.26 |
$0 |
$0 |
|
Working With Others |
||||
|
Annual payment |
$3900 |
$850 |
$850 |
$850 |
|
Annual balancing payment |
|
$219.26 |
$0 |
$0 |
Source: Health and Human Services internal information.
Note: Payments are shown against the year that they accrued—actual payments may have been made in the following year. The total funding for these payments is $344 million over the five year 5CPA.
4.27 Health’s key performance indicator (KPI) for the PPI program is a proxy measure229 focusing on the percentage of community pharmacies participating in the program—that is, claiming payments—and the department is not well placed to assess program effectiveness or alignment with Objective 2 of the 5CPA.230 The department’s 2013-14 annual report indicated that 93 per cent of community pharmacies participated in one or more components of the PPI program.
Clinical Interventions ($97 million)
4.28 Clinical Interventions, one of six PPI priority areas, receives $97 million in funding under the 5CPA. Issues relating to the PPI Program Guidelines were raised by Human Services in February 2013, following a compliance assessment of four pharmacies claiming Clinical Interventions payments under the 5CPA. Human Services231 reported to Health that:
Two pharmacies … aggressively promote Clinical Interventions with their staff. Pharmacists are encouraged to conduct Clinical Interventions associated with companion selling of complementary medicines, for example, blood glucose machines or recommending probiotics accompany antibiotic supply … It appears from the interviews conducted that the guidelines as to what constitutes a Clinical Intervention may be open to interpretation. This has resulted in pharmacies claiming Clinical Interventions that may not be in line with the intent of the Pharmacy Practice Incentive Program. However, in reviewing the guidelines there is no indication that this is a compliance issue and in all but one of the pharmacies assessed, recoveries will not be sought in this instance. You may wish to ‘tighten up’ the Pharmacy Practice Incentive guidelines …
4.29 Professional pharmacists’ associations represented on the Programs Reference Group, an advisory body established under the 5CPA, have also raised issues about the operation of this component of the PPI Program. The peak bodies advised the ANAO that:
Many employee pharmacists have reported to us that they have a KPI included in their job description that requires them to complete a certain rate of Clinical interventions per 100 scripts dispensed. In certain circumstances this has encouraged pharmacists who are below their KPI for Clinical Interventions to ‘make-up’ interventions.
… the professional pharmacy service programs defined in recent CPAs [Community Pharmacy Agreements] have, and are, being developed using a piecemeal approach rather than describing the services required to meet therapeutic goals for individual consumers. Funding systems that break down a pharmacist’s professional service into multiple small activities (such as ‘provision of CMI’ [Consumer Medicine Information] and ‘clinical interventions’) and apportioning token fees to these activities undermines the professional role of pharmacists and prevents an appropriate fee for service for a holistic pharmacist service.
In the Clinical Interventions program, for example, the guidelines only require that pharmacies report numbers of interventions, and not the type of interventions made, a situation that is highly unsatisfactory when it comes to evaluating the impact of 5CPA programs.
4.30 The PPI Guidelines define a clinical intervention as:
a professional activity undertaken by a pharmacist directed towards improving quality use of medicines and resulting in a recommendation for a change in a patient’s medication therapy, means of administration or medication-taking behaviour.
4.31 The PPI Guidelines definition of Clinical Interventions did not exclude the practice that Human Services described in its 2013 report as ‘companion selling of complementary medicines’. More fundamentally, the PPI Clinical Interventions program has been questioned by a number of stakeholders in their representations to the ANAO, on the basis that many such services could reasonably be expected to be provided as part of standard pharmacy dispensing.232 While the design of the 5CPA is a matter for government and the department, stakeholder submissions to the ANAO relate to value for money under the 5CPA, and the operation of the Clinical Intervention component may warrant attention in the context of developing the next community pharmacy agreement.
Medication Management Programs ($164 million)
4.32 Medication management programs are government funded pharmacist reviews of a patient’s medications that are aimed at preventing, detecting and resolving medication related problems. The program rationale for providing a medication management service is the high prevalence of medication related illness in the community.233 It has been estimated that over 1.5 million Australians suffer an adverse event from medicines each year.234 Collectively, adverse drug events are responsible for up to 230 000 hospital admissions annually in Australia, costing approximately $1.2 billion per annum.235
4.33 Two different kinds of medication management programs are funded under the 5CPA:
- in-home medication management reviews:
- Residential Medication Management Reviews (RMMRs, $70 million) conducted by an accredited pharmacist in the patient’s residential aged care facility, after referral from the patient’s GP; and
- Home Medicines Reviews (HMRs, $52.1 million) conducted by an accredited pharmacist in the patient’s home, after referral from the patient’s GP;
- in-pharmacy medication reviews:
- MedsCheck ($29.6 million), a review of a patient’s medicines by registered pharmacists; and
- Diabetes MedsCheck ($12.2 million), a review of a type 2 diabetes patient’s medicines, monitoring devices, education and self-management by registered pharmacists.
4.34 In-home medication management reviews (RMMRs and HMRs) are a collaborative service conducted by an accredited pharmacist236 in consultation with a patient’s general practitioner (GP). The pharmacist provides a comprehensive assessment to the patient’s GP, and facilitates the involvement of other relevant health professionals. The effectiveness of HMRs in improving patient outcomes in the Australian community has been demonstrated in a range of independent studies.237 In addition, a range of cost-effectiveness reviews have indicated that when appropriately targeted, HMRs may produce a net saving to the health system, as well as improved quality of life for patients.238
4.35 In contrast to in-home medication reviews, the in-pharmacy medicine reviews (MedsChecks and Diabetes MedsChecks) conducted by registered pharmacists do not require specialised postgraduate training or GP referral. There is no program requirement to provide a written report of the medicine review, and the review is not required to be conducted in collaboration with the patient’s GP or other health professionals. In-pharmacy reviews are a new service implemented under the 5CPA, and Health provided more limited evidence for the effectiveness of such services, and no cost-effectiveness analysis.239 The Pharmacy Guild advised the ANAO that there is strong evidence to support the effectiveness of in-pharmacy medication reviews.240
4.36 HMRs received an allocation of $52.1 million (or 7.9 per cent) of the 5CPA professional programs budget. Over the course of the agreement the annual budget for HMRs declined from $12.79 million (in the second year of the 5CPA) to $8.6 million (in the third year of the 5CPA)241, and a cap of 20 HMRs per month was placed on service providers. The budgetary problems that emerged in the third year of the 5CPA, and stakeholder concerns relating to the administration of these programs are examined in Chapter 5.242
Rural Support Programs ($107 million)
4.37 The Rural Support Programs are intended to ‘strengthen and support the rural pharmacy workforce, providing increased access to quality pharmacy services for patients residing in rural and remote regions of Australia’.243 There are two main programs, and one has ten components.244
4.38 The largest program is the $70 million Rural Pharmacy Maintenance Allowance (RPMA), paid in recognition of the additional costs of maintaining a pharmacy in rural and remote areas of Australia. The RPMA, paid over five years, is the only payment that directly subsidises the operations of eligible rural and remote pharmacies.245 On 1 March 2014, the administration of the RPMA was transferred from Human Services to the Pharmacy Guild.
4.39 The viability of pharmacies in rural and remote areas potentially affects patient access to PBS and RPBS medicines because there may be only one pharmacy servicing a large geographic area.
4.40 The second rural support program is the $37 million Rural Pharmacy Workforce Program (RPWP), which is intended to maintain and improve access to quality retail pharmacy services in rural and remote communities and strengthen and support the rural pharmacy workforce. Health advised the ANAO that:
… the Department is of the view that there are 11 activities under the RPWP, including Administration support provided by the Guild. The particular activities are listed below:
- Rural Pharmacy Workforce Programs Administration
- Rural Pharmacy Scholarship Scheme
- Rural Pharmacy Scholarship Mentor Scheme
- Rural Pharmacy Liaison Officer Program
- Rural Pharmacy Intern Incentive Allowance
- Rural Pharmacy Post-Intern Incentive Allowance
- Rural Intern Training Allowance
- Rural and Remote Continuing Pharmacy Education Allowance
- Emergency Locum Service
- Rural Pharmacy Student Placement Allowance
- Administrative Support to Pharmacy Schools.
… to rural pharmacy each of these is considered a discrete scheme, irrespective of funding arrangements implemented between the Department and the Guild.
4.41 In 2010, Health commissioned a consultancy firm to evaluate the 4CPA Rural Pharmacy Programs. The evaluation included the Rural Pharmacy Maintenance Allowance, the Rural Pharmacy Workforce Program, and three other rural allowances that were discontinued after the 4CPA concluded in 2010.246 The evaluation reported in November 2010, prior to the implementation of Expanded and Accelerated Price Disclosure.247
4.42 The evaluation indicated that the Rural Pharmacy programs addressed an important need, namely a workforce shortage in some rural and remote areas, and challenges in retaining the workforce in these areas. However, the evaluation also found that while the Rural Pharmacy initiative was successful in achieving its operational targets, the available data was not sufficient to determine whether the initiative had a direct impact on workforce outcomes. The same problem was identified in a prior evaluation of these programs under the 3CPA, which recommended the establishment of a workforce database to redress this issue. This recommendation was not actioned.
4.43 The earlier evaluation also noted that there were opportunities to simplify the programs’ structure to improve operational efficiency, and stakeholder views on scope for improvement. In particular stakeholders observed that:
There is no apparent overarching mechanism that ensures that these programs, and other strategies outside of these programs that also share the broad aim of improving access that people living in rural and remote areas have to community pharmacy services, are integrated and adequately linked. Further, there does not appear to be a mechanism that identifies areas of workforce shortage and ensures a policy response to these priority areas.
4.44 The 4CPA evaluation also indicated that there was no process or structure that allowed the collation of workforce data to assess workforce shortages and to monitor the impacts of program funding. This, in turn, was considered to have limited the sector’s capacity to identify strategic priorities and respond to need.
4.45 Health advised the ANAO that under the 5CPA:
Whilst DoH has not undertaken a formal review of the [Rural Pharmacy Workforce] programme, the programme managers closely monitor the programme data against the program objectives. It should also be noted that under the Fourth Agreement, Rural Pharmacy Workforce Programmes were evaluated … Data collected under Rural Programs, including the Student Placement Support Allowance and Administrative Support to Pharmacy Schools can be used in future evaluations of this initiative.
4.46 Departmental records indicated that Health had not collected or used such data strategically during its development of the 5CPA.248
Aboriginal and Torres Strait Islander Programs ($29 million)
QUMAX and s100 Support Allowance
4.47 The 5CPA funds three programs that are targeted to support Indigenous people. The s100 Pharmacy Support Allowance funds pharmacists to provide support and quality use of medicines services to Aboriginal Health Services (AHSs) in remote areas.249 Similarly, in rural and urban locations, QUMAX250 funds pharmacists to provide quality use of medicines services to Aboriginal Community Controlled Health Services (ACCHSs).
4.48 The Aboriginal and Torres Strait Islander Programs and the Rural Pharmacy Workforce Programs (discussed in the previous section) had the least developed public reporting arrangements, with some programs and activities not reported on at all, or being reported on an ad hoc basis through press releases issued by the Pharmacy Guild. The Pharmacy Guild advised the ANAO that:
The Guild’s reporting requirements on these programs are to the Department of Health as per contracting arrangements. The Guild has met every reporting deliverable to Health. It is the responsibility of the Department to report publicly as it does with other programs such as the medication management programs.
4.49 In 2011, a Senate Committee inquired into the effectiveness of special arrangements for the supply of PBS medicines to remote area Aboriginal Health Services.251 The inquiry made ten recommendations, including that:
- program flexibility be implemented to give remote area AHSs greater access to the services of a pharmacist by AHSs engaging a pharmacist directly, or in collaboration with other stakeholders; and
- Health develop a process for integrating existing programs (such as the s100 support allowance and QUMAX) and publish a clear policy and program logic to show how these programs will work together.252
4.50 In 2010, a 4CPA review of Indigenous Pharmacy programs also noted that stakeholders agreed that it would be preferable for AHSs to directly employ pharmacists.253 There is limited evidence of the department considering the Senate Committee’s recommendations for better targeted and more efficient programs. Three stakeholder organisations, including the Pharmacy Guild, have expressed concern that there has not been a government response to the inquiry.254
Aboriginal and Torres Strait Islander Workforce Program
4.51 The Aboriginal and Torres Strait Islander Workforce Program amalgamated two separate 4CPA programs. The program comprises the Aboriginal and Torres Strait Islander Pharmacy Scholarship Scheme and the Aboriginal and Torres Strait Islander Pharmacy Assistant Traineeship Scheme, which aim to increase the involvement of Indigenous people in the pharmacy workforce by encouraging them to study and achieve registration as pharmacists.
4.52 The review of the 4CPA Aboriginal and Torres Strait Islander workforce programs discussed above found that the programs have been generally successful in increasing the number of Indigenous people who complete a Bachelor of Pharmacy and go on to work as a pharmacist. A large proportion of participants agreed that they may not have undertaken traineeships in pharmacy without the programs. However, the review noted that better targeting of promotional activities to increase uptake and more support for scholars and trainees would improve the programs.
Research and Development Program ($10.6 million)
4.53 The Research and Development Program is intended to contribute to ‘maintaining and improving the health outcomes of Australians through evidence based best practice on issues related to community pharmacy and the provision of quality services to patients’.255
4.54 Health entered into a contract (a ‘project agreement’) with the Pharmacy Guild, for the Guild to manage the Research and Development Program.256 The objectives of the project are:
To identify and fund research and development projects against topic areas which have the greatest potential to deliver services with positive health outcomes for consumers and economic impacts for the health system and the PBS. To:
- Enhance the capacity of community pharmacy and community pharmacists to contribute to maintaining and improving the health of Australians.
- Develop and inform best practice professional and management standards and processes for delivery of cost effective services.
- Enhance and develop the role of community pharmacists as a member of the primary health care team.
- Deliver the R&D Program in accordance with government standards of accountability and transparency.257
4.55 Health’s contract with the Pharmacy Guild specifies six topic areas endorsed by the Minister for Health: Consumer Needs, Professional Integration, Mental Health, Rural Pharmacy Workforce, Chronic Illness, and Health Literacy. Six research projects were selected from a Pharmacy Guild tender process in 2011 (one project has since been cancelled). Another project was commissioned in 2013 by the Pharmacy Guild to analyse HMR patient eligibility.258 The research projects are outlined in Table 4.2.
Table 4.2: 5CPA Research and Development Program projects
|
Priority Area |
Project Aim |
Funding ($m) |
|
Consumer needs |
To collect qualitative and quantitative data on consumer expectations and consumer needs in relation to managing their health with support from the network of community pharmacies in the context of healthcare reform. |
1.66 |
|
Mental Health |
To develop a comprehensive training package for pharmacists to assist mental health consumers in the area of medication compliance. |
2.33 |
|
Rural Pharmacy Workforce |
To undertake research to examine the rural and remote pharmacy workforce and its impact on access to pharmacy services at a local level. |
0.57a |
|
Health Literacy |
To undertake research on health literacy and on the role community pharmacy can play in improving consumer outcomes through tailored communication of health information. |
1.08 |
|
Professional Integration |
To gather data from a variety of sources on the best practice model for the professional integration of community pharmacists in the primary health care setting. |
1.52 |
|
Chronic Illness |
To investigate the consumer perspective on the burden of chronic disease, and the role that community pharmacy can play in assisting them to better manage their condition/s. |
1.56 |
|
HMR: Refining Patient Eligibility Criteria |
To collate and assess evidence of the benefit of Home Medicines Reviews to different cohorts of patients in order to draft a set of recommendations detailing appropriately targeted criteria for HMR patient eligibility. |
0.29 |
Source: Department of Health and Pharmacy Guild of Australia, Research and Development: Current Projects, available from <http://5cpa.com.au/programs/research-and-development/current-projects/> [accessed 5 March 2014].
Note:
- This project was terminated after $70 000 was spent.
4.56 In representations to the ANAO, several stakeholders expressed a view that the Research and Development program would benefit from revised governance arrangements. For instance, one Faculty of Pharmacy advised the ANAO that:
The Faculty of Pharmacy believes that the research funding provided to the Guild under the Fifth Community Pharmacy Agreement should be provided instead to the NHMRC to ensure appropriate and independent administration of the funds. Should Government policy be such that research and development funding continues to be provided to the Guild under a Community Pharmacy Agreement, the Faculty would recommend that an independent panel of health and medical researchers be constituted by the Guild for the purpose of selection of applications for research grants and commissioned research.
4.57 While funding arrangements under the next community pharmacy agreement are a matter for government and the department, a relevant consideration in the administration of research funding is the promotion of confidence in the selection of research projects, and in project outcomes. In this respect Health advised the ANAO that:
…the Consumer Needs project panel consisted of representatives from the PSA, CHF, a George Institute for Global Health representative and Guild and Departmental members. This indicates that the tender selection process for R&D was not carried out exclusively by the Guild and the Department.
4.58 In addition, the Pharmacy Guild advised that:
An Advisory Panel was established for each project that assessed funding applications against the agreed selection criteria, selected the preferred applicant, considered and approved project deliverables and provided advice to the R&D Program Manager on the progress and outcomes of each project.
5CPA special projects
Supply and PBS claiming from a medication chart in Residential Aged Care Facilities ($3 million)
4.59 The purpose of this project, which was funded under the 5CPA, was to address issues faced in residential aged care facilities regarding the supply and PBS claiming of medicines from an available prescription. It was expected that introducing supply from a medication chart would streamline supply, claiming and governance issues for approved pharmacists; and ensure that medicines are supplied in accordance with the prescribers’ most recent intentions.
4.60 Health advised the ANAO that in 2013–14, a standardised National Residential Medication Chart was tested in over 20 residential care services by the Australian Commission of Safety and Quality in Health Care, and that an evaluation of this pilot259 found measurable improvements, including a reduction in the number of prescriptions per resident from 13.8 to 5.7, and a marked decrease in the number of medication-related adverse events from 9.2 errors per 1,000 prescriptions to 3.5 errors per 1,000 prescriptions.
Electronic Recording and Reporting of Controlled Drugs (ERRCD $5 million)
4.61 The purpose of this project was to support the development of a system to collect and report data relating to controlled drugs, to address the problems of forgery, abuse and doctor shopping.
4.62 Health advised the ANAO that in February 2012 a licensing agreement was executed to make a nationalised ERRCD system available to all jurisdictions for their use.
Medication Continuance
4.63 During the 5CPA the name of this project was changed to ‘Continued Dispensing’. The purpose of this project was described in the 5CPA as follows:
This program will support the development of protocols set out in an IT enabled auditable standard, in the agreed Standards, in relation to medication continuance.
4.64 Health advised the ANAO that as of December 2014, Continued Dispensing is permitted in all jurisdictions with the exception of Queensland260, and a report on the initiative was tabled in Parliament on 3 December 2014.261
Conclusion
4.65 Under the 5CPA, the Australian Government funds a range of pharmaceutical services, and programs that support the pharmacy network and develop the pharmacy workforce. The main pharmacy service funded under the agreement is the dispensing of PBS and RPBS medicines.
4.66 In addition, the 5CPA provides $663 million in government funding for over 30 programs and activities. Objective 2 of the agreement relates to ensuring that programs are patient-focused and target areas of need in the community:
- Some 26 per cent of 5CPA program funding ($163.9 million) is provided to fund medication management programs, which are patient-focussed services that clearly target an area of community need.
- Approximately 52 percent of program funding ($344 million) relates to pharmacy accreditation, which focuses on effective business operations and staff management in addition to the delivery of patient-focused professional services.
4.67 While some professional programs funded under the 5CPA can generally demonstrate alignment with Objective 2, in the case of the $344 million Pharmacy Practice Incentives Program (PPI)—which accounts for 52 per cent of all funding for 5CPA professional programs—the department is not well placed to assess program effectiveness and alignment with Objective 2. Health’s key performance indicator (KPI) for the PPI program is a proxy measure262 focusing on the percentage of community pharmacies participating in the program—that is, claiming payments.
4.68 The ANAO examined the detailed PPI program requirements for each of the six PPI priority areas, to assess how the overarching requirements for making PPI payments, as documented in the program guidelines—demonstrated delivery of agreed services to patients and demonstrated achievement of defined outcomes—are implemented. In respect of two PPI priority areas, payments are made quarterly on the basis of the number of patient services self-reported by the pharmacy and the pharmacy’s prescription volume. However, in respect to the four remaining PPI priority areas, the program guidelines indicate that payments are not made on the basis of actual patient services delivered, but as a flat annual amount depending on whether participating pharmacies have been accredited under a quality management system—currently the Quality Care Pharmacy Program (QCPP)—relating to the business and professional operations of a retail pharmacy.
4.69 One component of the PPI, the $97 million Clinical Interventions initiative, has attracted attention from the Department of Human Services and stakeholders on the grounds that the relevant guidelines may be open to interpretation, resulting in instances of ‘companion selling’ of complementary medicines or devices being claimed as PPI Clinical Interventions. Stakeholder observations regarding the operation of PPI Clinical Interventions suggest that these issues may warrant attention in the context of developing the next community pharmacy agreement.
5. Administration of Professional Programs
This chapter examines the governance and administrative arrangements for the 5CPA professional programs and services, and Health’s contractual arrangements with the Pharmacy Guild.
Introduction
5.1 The Fifth Community Pharmacy Agreement (5CPA) is the head agreement in a complex scheme of legal, financial and administrative arrangements involving both government entities and third parties in its implementation. The Department of Health (Health) provides policy advice to government on the 5CPA, and has primary responsibility for the overall administration of the agreement.
5.2 The 5CPA provides for other parties to assist Health administer payments for the various professional programs. These arrangements are set out in the Schedule to the 5CPA.263 Until 1 March 2014, the larger professional programs were administered by the Department of Human Services (Human Services) and the smaller programs were administered by the Pharmacy Guild, on behalf of Health. On 1 March 2014, Health transferred responsibility for the professional programs previously administered by Human Services to the Pharmacy Guild264, which now administers all 5CPA professional programs. Human Services continues to administer the Australian Government’s major pharmaceutical benefit schemes (the PBS and RPBS), and the pharmacy approvals system.
5.3 The 5CPA establishes the Agreement Consultative Committee (ACC) as the consultative mechanism for all aspects of the agreement’s implementation.265 The ACC has four Health members and four members from the Pharmacy Guild.266 The ACC is required to seek advice from an advisory committee, the Programs Reference Group (PRG), on the policy dimensions of professional programs funded by the 5CPA and their evaluation. The PRG is a broadly based committee which includes representatives from professional pharmacists’ associations, as well as the wider primary health care sector and individuals with particular expertise in program evaluation.
5.4 This chapter examines the governance and administrative arrangements for 5CPA professional programs, including the:
- roles of the Agreement Consultative Committee and Programs Reference Group;
- role of other parties assisting Health administer the professional programs—Human Services and the Pharmacy Guild; and
- Health’s contractual arrangements with the Pharmacy Guild.
5CPA governance and consultative arrangements
Agreement Consultative Committee (ACC)
5.5 Clause 5 of the 5CPA establishes the ACC and defines its role.267 The ACC is required to:
- be the mechanism for consultation between the parties on implementation of all aspects of the agreement, including issues relating to Approved Pharmacists’ payments (payments to pharmacy owners for PBS/RPBS dispensing), the CSO, Location Rules, Electronic Prescriptions, and professional programs;
- oversee professional programs, including, but not limited to, their design, business rules, timelines, outcomes and expenditure; and
- seek advice from the PRG on the policy dimensions of professional programs and their evaluation.
5.6 The terms of reference for the ACC, including meeting arrangements, reporting requirements and operating rules, are decided by Health and the Pharmacy Guild. Since the 5CPA commenced, the ACC has met between three and six times per year, with the frequency of meetings decreasing over time. Health provides secretariat services to the ACC, including the preparation of meeting records. The ACC does not publish records of its meetings.
Programs Reference Group (PRG)
5.7 Clause 6 of the 5CPA establishes an advisory committee, the Programs Reference Group (PRG). The functions of the PRG are:
- to provide advice to the Minister and the ACC, when such advice is requested, on the policy dimensions of new and continuing programs including, but not limited to, the scope, objectives, target groups and evaluation requirements, taking into account:
- the findings of any evaluations of programs under the 4CPA and the findings of any relevant research, particularly research conducted under the 4CPA Research and Development program; and
- the allocation of funds to the 5CPA professional programs; and
- any other function that may be agreed between the Minister for Health and the Pharmacy Guild.268
5.8 A review of the advisory committee established under the 4CPA269 had indicated that there was no overarching plan in place for the rollout of professional programs or consideration of how projects and programs interrelate. The review found that a lack of clarity around the previous committee’s responsibilities and a perceived lack of independence from the Pharmacy Guild prevented the committee from fulfilling its role to monitor program performance, accountability and transparency of program funding. To address these issues, the PRG was established with broader membership and the number of Pharmacy Guild representatives was reduced from five to one.
5.9 The PRG currently has 12 members, including representatives from professional pharmacists’ associations, other health practitioners, health consumers, an economist, an evaluator, a Pharmacy Guild representative, and a departmental representative. The PRG has met three times per year, and publishes summary statements of its meetings on Health’s website. The PRG’s terms of reference were developed by Health and the Pharmacy Guild.270
Role of the ACC and PRG in professional programs
5.10 The PRG is required to provide advice on professional programs funded under the 5CPA taking into account, among other things, the allocation of funds to the various professional programs. For its part, the ACC is required to have: ‘regard to the Commonwealth’s need to ensure transparent, contestable, merit based allocation of Funds within an accountability framework’271, including in respect of the professional programs.
5.11 In submissions to the ANAO, professional pharmacists’ bodies and health organisations represented on the PRG expressed a variety of concerns over aspects of the PRG’s and ACC’s operations relating to 5CPA professional programs.
5.12 In particular, the peak bodies represented on the PRG indicated that at times the PRG was not provided with key information relating to funding for professional programs under the 5CPA:
A complete lack of transparency surrounds the funding allocated to individual professional programs (total 5CPA funding $663 million). Repeated requests for information about the rationale for funding allocations and projections by members of the 5CPA Programs Reference Group have gone unanswered. This lack of transparency makes it difficult to determine exactly which factors have been employed to arrive at the funding allocated to individual programs … If any modelling exists, it has not been made available.
5.13 As discussed, the ACC is required to seek advice from the PRG on the policy dimensions of professional programs and their evaluation. Peak bodies represented on the PRG considered that, despite the efforts of the PRG in this regard, there were shortcomings in the development of professional programs, relating to:
- program design—the intent, objectives and content of programs were not always clearly articulated and did not always demonstrate how the consumer sits at the centre of each program;
- program benefits—the health or community-wide benefits of each program were not always demonstrated, and it was not clear how the program eligibility rules and payment systems supported these objectives; and
- program evaluation—the 5CPA evaluation framework was released in December 2011, some 18 months after the agreement commenced. Further, the lack of connection between program design, implementation and evaluation limited the extent to which program effectiveness could be rigorously evaluated; and there appeared to be limited capacity to assess the cost-effectiveness of any or all programs.272
5.14 In respect to the evaluation framework, Health advised the ANAO that:
The PRG was consulted on the development of the Evaluation Framework on multiple occasions … and had an ongoing role in advice on the evaluations including feedback on scope, requirements, questions, methodology, draft reports etc. Furthermore, the Evaluation Framework was developed predominately by two members of the PRG.
5.15 In submissions to the ANAO, peak bodies represented on the PRG also expressed concerns over changes made to medication management programs by the ACC:
Major changes to the home medicines review (HMR), residential medication management review (RMMR) and MedsCheck programmes were made without input from the PRG. Yet again the ACC made decisions in isolation and allocated budgets within the 5CPA without regard to the financial impact across the whole health system and the health of consumers …A lack of good clinical and corporate governance has led to:
- an inadequate budget allocation for this program [HMR];
- inappropriate decisions regarding program eligibility;
- HMR services being seen and delivered as a discrete service rather than as part of a ‘comprehensive medication review service’; and
- a perceived conflict of interest in the day-to-day management of the program …
5.16 On the issue of consultation between the ACC and PRG, Health advised the ANAO that:
Changes to programmes were not made based on policy dimensions, these were made on the basis of financial sustainability of the 5CPA, in accordance with terms of reference for the ACC. Dates where PRG were consulted include: 30 April 2013 PRG meeting [and] 21 August 2013 PRG meeting. The main issue or implication raised appears to be that the PRG did not have the ability to make financial decisions with respect to allocations under the 5CPA. However, the Fifth Agreement, signed by the Minister on 3 May 2010 clearly establishes the role of PRG as being to provide advice, where requested on policy dimensions relating to 5CPA professional programmes.
5.17 Health provided the ANAO with examples of the ACC considering PRG advice. However, the department also noted in its internal communications that due to the difficulties it experienced in obtaining the Pharmacy Guild’s agreement (through the ACC) to raise issues with the PRG, it had been necessary for Health to engage stakeholders through other means:
The terms of reference for the committee only allow for PRG to provide advice on the request of either the Agreement Consultative Committee or the Minister. The Agreement Consultative Committee has equal representation from both the Department and the Guild. The need for the Guild to agree on items for advice from the PRG has at times been difficult. The PRG is the only formal mechanism for stakeholder consultation in regard to Fifth Agreement programs. Due to the difficulties the Department has faced in obtaining the Guild’s consent to raise issues with the PRG, it has been necessary for the Department to engage stakeholders through a variety of other forums.
5.18 The Australian Government establishes, and often funds273, formal consultative and advisory arrangements to facilitate the effective implementation of its policies and programs. Where there is a risk of consultative or governance arrangements not fully achieving their intended purpose, or potentially operating in a manner not fully consistent with formal agreements or expectations, there is a senior management responsibility to: work with participants to re-focus effort on intended outcomes; and maintain the effective operation of formal advisory and consultative bodies, consistent with their terms of reference.
5CPA administrative arrangements
5.19 The Schedule to the 5CPA contains indicative budgets for the professional programs funded under the agreement. Most professional programs involve payments to third parties, such as: pharmacy owners; accredited pharmacists; pharmacy students and researchers. The Schedule specifies who will make the program payments for each program, which in most cases was either Human Services274 or the Pharmacy Guild.275
5.20 Prior to 1 March 2014:
- Human Services was allocated a departmental budget of $16.4 million to administer program payments of $583 million276 over five years; and
- Health entered into 62 contracts277 with the Pharmacy Guild, which provided the Guild with Commonwealth funding of $29 million to provide advisory services and administer program payments of $67 million over five years.278
5.21 From 1 March 2014:
- all contracts with the Pharmacy Guild that were active as at February 2014 were consolidated and incorporated into a single $259 million contract between Health and the Guild. Under the new contract the Pharmacy Guild would administer all programs formerly administered by itself and Human Services. The new contract provided additional administrative funding of $1.8 million to the Pharmacy Guild, and made provision for the Guild to make use of $7.2 million in unexpended funds for administrative purposes 279, 280; and
- Human Services retained its administrative responsibilities for other elements of the 5CPA including pharmacy remuneration for PBS/RPBS dispensing, and the administration of the pharmacy approval system on behalf of Health.
5.22 A summary of the five year budget and staffing for administering the 5CPA professional programs is shown in Table 5.1.
Table 5.1: Five year budget for the administration of 5CPA professional programs
|
Entity |
2010–15 budget before 1 March 2014 |
2010–15 budget after 1 March 2014 |
||
|
|
Staffing ASL |
Admin budget ($m) |
Staffing ASL |
Admin budget ($m) |
|
Health |
165.4 |
20.2 |
167.3 |
20.6 |
|
Human Services |
123.5 |
16.4 |
0 |
14.3a |
|
Pharmacy Guild |
- |
29.3 |
- |
31.2 |
Source: Health, Human Services and Pharmacy Guild information.
Notes: An entity’s administrative budget may cover a range of administrative costs in addition to staffing, such as ICT, property, legal and miscellaneous costs.
ASL is average staffing level. The Pharmacy Guild was unable to provide ASL figures.
- Human Services’ ongoing budget was reduced by the equivalent of $16.4 million over five years following the transfer of all professional programs to the Pharmacy Guild. Approximately $14.3 million had been expended by 1 March 2014 and Human Services’ actual budget was reduced by $2.1 million for the remaining term of the 5CPA.
5.23 Administrative arrangements for 5CPA professional programs before and after 1 March 2014 are shown in Figure 5.1 and Figure 5.2 respectively.
Figure 5.1: Administration of 5CPA professional programs before March 2014
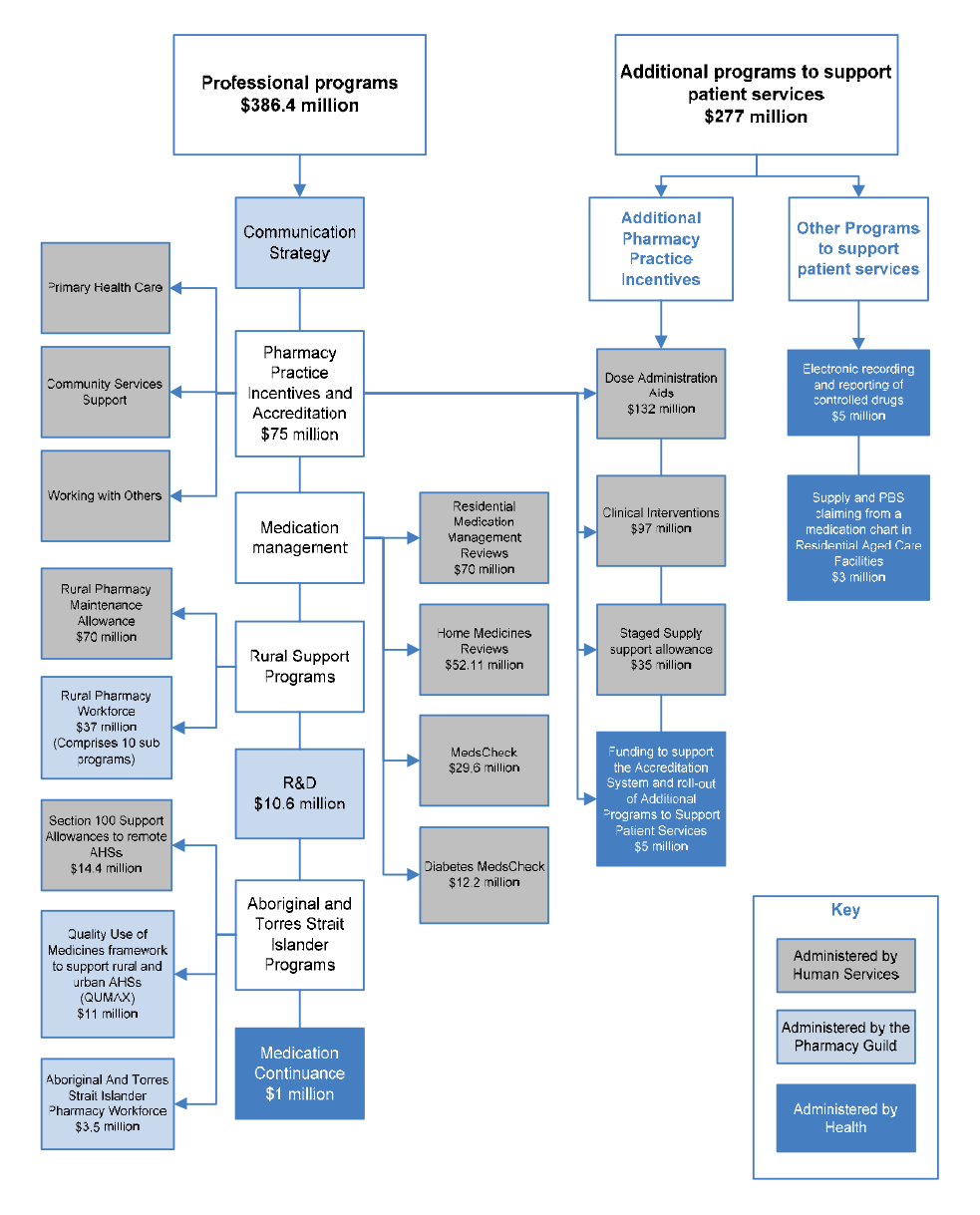
Source: Fifth Community Pharmacy Agreement and Health information.
Notes:
- The Communication Strategy was funded by re-allocating $5.8 million from funding for professional programs and activities.
- Funding allocations are the original budgeted amounts for the five year term of the 5CPA. Health and the Pharmacy Guild piloted MedsCheck and Diabetes MedsCheck early in the 5CPA.
Figure 5.2: Administration of 5CPA professional programs since March 2014
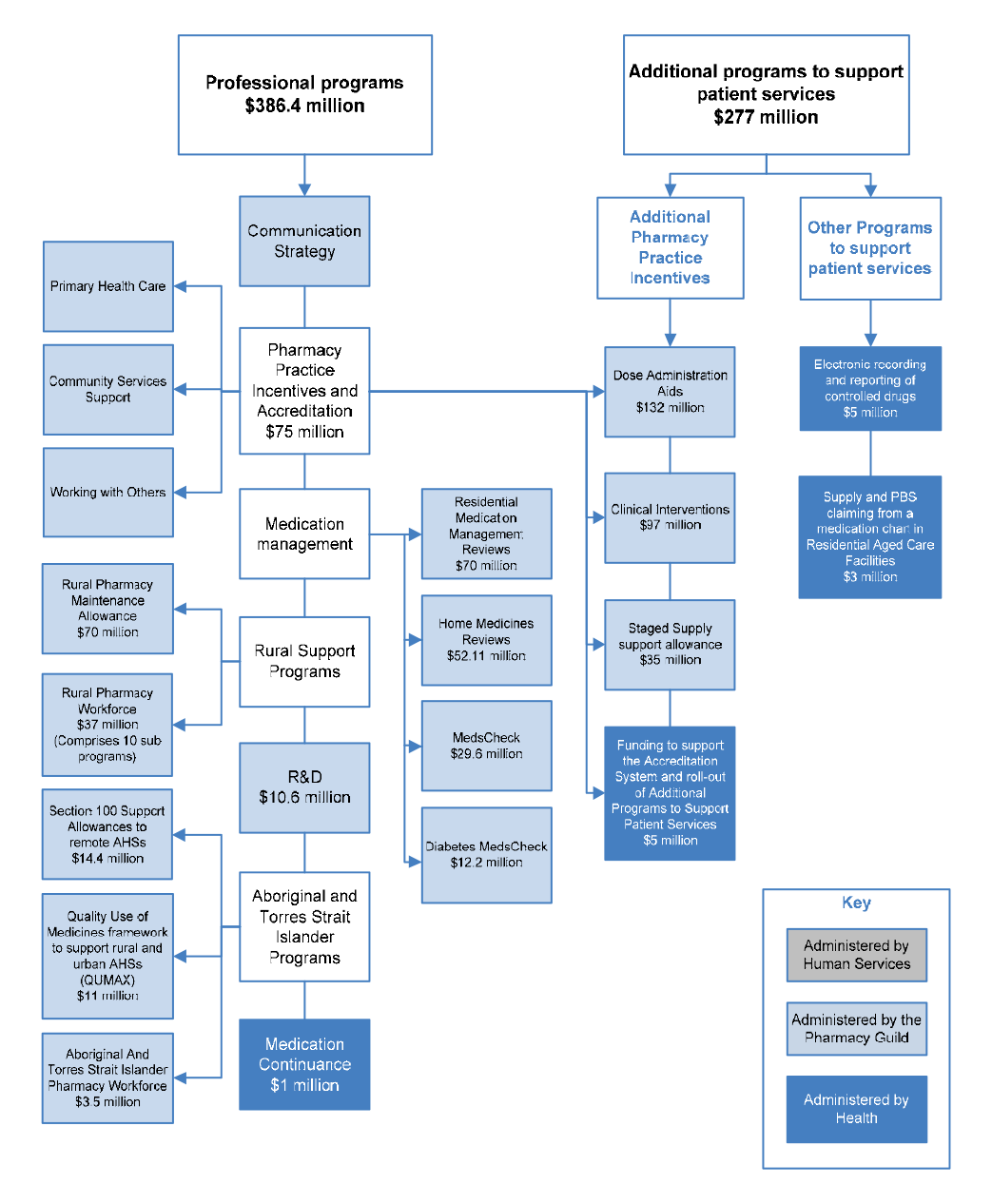
Source: Fifth Community Pharmacy Agreement and Health information.
Note: Funding allocations are the original budgeted amounts for the five year term of the 5CPA.
Administration of 5CPA programs prior to March 2014
Health’s role
5.24 Health has overall responsibility for the implementation and administration of the 5CPA, including the professional programs and services.281 The complexity of the 5CPA has at times created challenges for Health’s contract management and general administration.
Contract management
5.25 Effective management of contracts necessitates that government entities have ready access to the contract agreements and associated documentation. At the commencement of the audit, the ANAO sought Health’s assistance in identifying all agreements and contracts relevant to the 5CPA. Health subsequently provided the ANAO with a list of 310 contracts; and a list of 3551 departmental files, which Health indicated should contain the requested contracts.
5.26 Health’s 5CPA contract list did not contain any indication of which departmental files contained contracts relating to 5CPA administration. In addition, 86 contracts (28 per cent) on Health’s list had no corresponding description or contract manager.282
5.27 Two of the most significant 5CPA contracts were the Multi-Schedule Funding Agreements between Health and the Pharmacy Guild. These overarching agreements made provision for the Pharmacy Guild to receive government funding for performing projects related to 5CPA professional programs.283 However, these agreements were not included in Health’s contract list.284
5.28 The large number of contracts, and shortcomings in Health’s record keeping, meant that it took over two months from the ANAO’s first inquiry regarding 5CPA contracts for the department to identify the location of its 5CPA contracts and produce a complete list of contracts matched to departmental file records, where signed copies of the contracts were thought to be located. In this regard, Health advised the ANAO that:
Health does not disagree that its record-keeping could be improved, but TRIM has progressed this.285
5.29 There would be merit, given the large number of 5CPA records managed by Health and the department’s experience in locating key documents in the course of this audit, in reviewing record-keeping arrangements for the 5CPA and the next agreement in order to provide assurance that the department can effectively discharge its advisory, accountability and contract management functions in a timely manner.286
Recommendation No.5
5.30 In order to effectively discharge its advisory, accountability and contract management obligations in a timely manner, the ANAO recommends that the Department of Health reviews its record keeping arrangements for the Fifth Community Pharmacy Agreement and the next community pharmacy agreement.
Health response: Agreed.
Program management
5.31 Health is responsible for administering over 30 5CPA programs and activities, most with separate program guidelines and budgets. To assess Health’s approach to managing the large number of 5CPA programs, the ANAO asked Health to advise on the deliverables for 5CPA programs for the first three years of the 5CPA (from 2010–11 to 2012–13). Deliverables are the goods and/or services produced by a program in meeting its objective.287
5.32 Health responded with deliverables for several programs288, but for others, Health referred the ANAO to a large range of departmental files and spreadsheets but provided no clear statement identifying the deliverables for these programs. In response to further follow-up by the ANAO, Health subsequently finalised program deliverables.
5.33 An awareness of a program’s deliverables—the goods, services or outputs that a program is intended to deliver—is a key requirement for effective program administration. As discussed, the 5CPA funds a complex array of programs and activities, and it would be appropriate for Health to develop a more systematic approach to identifying and reporting on the deliverables and performance information for each of its 5CPA programs and any programs funded under a future community pharmacy agreement.289
5.34 The Pharmacy Guild also advised the ANAO of issues it had encountered with Health’s management of deliverables reporting:
The Guild is required to submit reporting deliverables for various Fifth Agreement programs as set out under the contracts between the Guild and Department. There are payments attached to the submission and acceptance of reporting deliverables. There have been numerous occasions where it has taken in excess of six months to receive feedback on reporting deliverables. The time lag means that any recommendations are unable to be implemented resulting in unnecessary delays and potentially impacting on program outcomes. In addition the time lag between report submission and feedback and eventual acceptance from the Department results in the Guild carrying program deficits for long periods of time…
5.35 During the course of this audit Health requested that the Pharmacy Guild provide copies of previously submitted and accepted progress reports, as the department had been unable to locate copies to respond to ANAO information requests. The department’s need to approach the Pharmacy Guild for previously submitted reports highlights the benefit, discussed in paragraph 5.29, of reviewing departmental record-keeping arrangements for community pharmacy agreements.
Human Services’ role
5.36 Prior to 1 March 2014, Human Services processed and paid claims for the larger 5CPA professional programs, comprising the:
- Pharmacy Practice Incentives programs ($339 million)290;
- Medication Management programs ($159.5 million)291;
- Rural Pharmacy Maintenance Allowance ($70 million); and
- Section 100 Support Allowance to Remote Area Aboriginal Health Services ($14.4 million).292
5.37 Until 1 March 2014 Human Services processed an estimated 20 000 claims per month293 from over 10 000 service providers. Claims and registrations for most 5CPA programs were manually processed by Human Services. Claiming timeframes and processes for participants submitting claims varied between programs. Some programs, such as the Pharmacy Practice Incentives and Accreditation priority areas, required only registration and verification of accreditation status by the Pharmacy Guild in order to receive annual payments. Other programs, such as the Medication Management Programs and the Home Medicines Review (HMR) program, required documentation for every service claimed to be submitted to Human Services on a monthly basis via fax or mail, and manually entered into departmental systems for payment.
5.38 Human Services was allocated approximately $16.4 million through a departmental appropriation to administer 5CPA program payments of $583 million294 over five years.
Processing issues
5.39 In November 2013, Health advised the Health Minister that for the HMR program, Human Services had an estimated backlog of claims for over 6000 services from July 2013 to be processed through the fax-based paper claiming system. In the course of this audit the ANAO received over 50 submissions, through its citizen’s input facility, regarding Human Services’ manual processing of 5CPA program claims, particularly for HMRs (representing 91 per cent of all complaints). The most commonly cited concerns expressed by pharmacy service providers related to:
- delays in Human Services’ processing and payment of HMR claims and financial hardship experienced as a result;
- the lack of an online claiming facility for HMRs and other 5CPA programs;
- the paper based claiming process, which was considered onerous and time consuming;
- claims only being partially paid295;
- some claims not being paid because Human Services had advised a claimant that the original claim was not received and the re-submitted claim was received outside the claiming timeframe; and
- Human Services’ use of fax machines, which were busy or unavailable when service providers tried to submit claims.
5.40 Human Services advised the ANAO that Health had not established performance targets for the manual processing of 5CPA professional program payments. There was, however, a processing target to pay claims by the fourth day of the following month. The ANAO requested evidence of a log of incoming claims and management reports to assess whether the processing target had been met. However, the department was not able to provide this information. Human Services further advised the ANAO that:
The department appreciates that paper based claiming processes are onerous and time-consuming to customers. In 2010 the department raised the idea of developing on-line functionality for programmes administered under the new 5CPA with Health. This option was not supported by Health due to the costs of implementing online solutions for multiple programs.296
5.41 More generally, Human Services advised that:
The department committed unfunded resources to the 5CPA programs and implemented processing improvements e.g. fax to email in an attempt to deliver the best possible service within the parameters set by Health.
Program costs
5.42 Human Services further advised the ANAO that three of the 5CPA professional programs—HMR, MedsChecks and PPIs—had higher take-up than Health anticipated, and while Human Services had worked closely with Health to modify policy parameters and tighten administration for these programs, expenditure continued to increase:
The department received higher than expected claims for services for the HMR and MedsCheck programmes from implementation. Health’s original policy intent expected to see a decrease in HMR services claimed from 1 July 2012 and a subsequent increase in MedsCheck services claimed. This did not happen. Instead, MedsCheck claiming increased and HMR services continued to remain at higher levels than expected by Health. The department’s anecdotal evidence suggests that pharmacies adjusted their business model to claim both MedsCheck and HMR services for the same patient. While this was not the intent of the policy changes, the HMR programme policy did not restrict the pharmacist from doing this. As a result, the overall programme expenditure continued to exceed expectations. In March 2013, Health introduced timeframes for HMR claiming. As such, any claims for HMR which the department received outside of the agreed timeframes for HMR were rejected … Between March 2013 and July 2013, the department consulted with Health a number of times to recommend they consider tightening PPI Programme Specific Guidelines to strengthen programme integrity.
5.43 Health advised the ANAO that in response to higher than expected claims under the HMR, PPI and MedsCheck programs:
On 25 November 2012, the Department directed DHS to undertake a targeted audit of pharmacies that had submitted high value Clinical Intervention claims. On 18 March 2013, DHS provided formal advice to Health on the outcomes of the audit. This advice suggested that Health may wish to tighten up the PPI Guidelines, in particular around the definition of Clinical Intervention which may be open to interpretation. The Department subsequently tightened the PPI Program Specific Guidelines to include the following: Clause 13.4 - It is the responsibility of the owner/manager of each Eligible Community Pharmacy to ensure all pharmacists, performing and recording Clinical Interventions on behalf of an Eligible Community Pharmacy, abide by the definition of a Clinical Intervention as detailed in the PSA [Pharmaceutical Society of Australia] Standards and guidelines for pharmacists performing clinical interventions. The Department also raised the issue of the CI definition at the ACC [Agreement Consultative Committee] where it was subsequently referred to the PRG [Programs Reference Group] for advice out-of-session. PRG advised the definition be unchanged.297
5.44 Human Services’ internal audits of the HMR program identified two further factors contributing to actual expenditure exceeding Health’s budget estimate:
- a large number of HMRs were being conducted outside the home (1500 per month); and
- registered pharmacists, instead of accredited pharmacists, were conducting patient interviews, which was intended to occur only in ‘exceptional circumstances’.
5.45 To control expenditure for the HMR program, the Pharmacy Guild proposed a moratorium on HMRs in January 2013.298 The Pharmacy Guild and Health subsequently negotiated a number of changes to the HMR guidelines, and from 15 March 2013:
- all HMR claims were to be submitted by the end of the calendar month following the month when the HMR was conducted; and
- prior approvals to conduct the HMR outside the home were to be submitted at least 10 working days before the interview took place, on a case by case basis. Exceptions only applied for cultural reasons and pharmacist safety. Prior approvals were required for a registered pharmacist (as opposed to an accredited pharmacist) to undertake a medication review.
5.46 Notwithstanding these changes, in late 2013 expenditure on HMR was again expected to exceed its budget for 2013–14 and 2014–15.299 Budget versus actual expenditure for the HMR program is shown in Figure 5.3.
Figure 5.3: HMR expenditure 2010–15
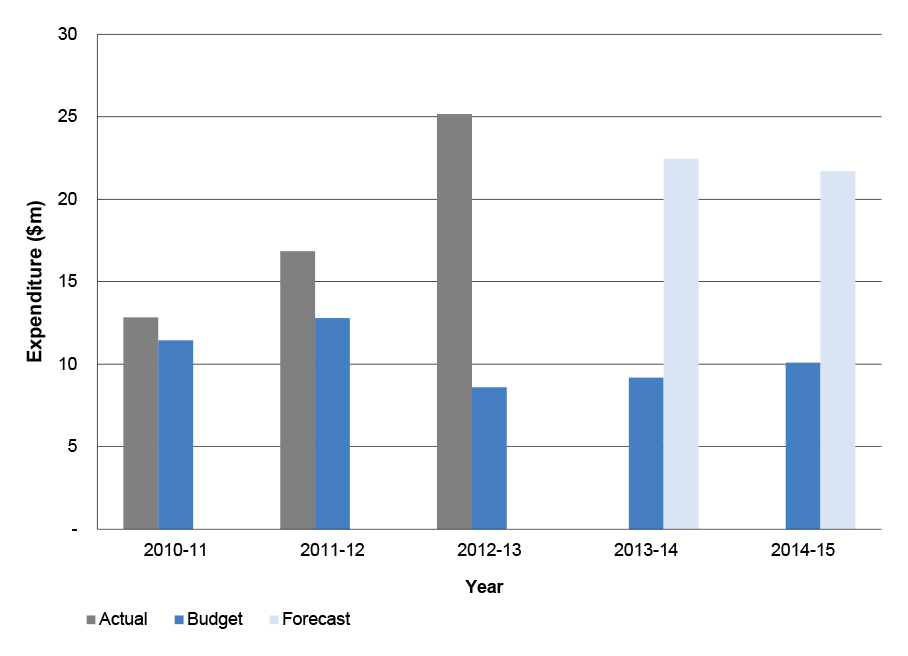
Source: ANAO analysis based on Health documentation.
5.47 In late 2013, an internal Health report indicated that 5CPA professional programs as a whole were projected to exceed their budgets by a cumulative $24 million in 2013-14 and $40 million in 2014–15. In a November 2013 minute, Health advised its Minister that the Pharmacy Guild had proposed a number of measures to manage the projected overspend, including:
- restricting eligibility criteria for HMRs and RMMRs;
- changing the timeframe of HMRs and RMMRs from one per patient per 12 months to one per 24 months300;
- introducing caps on the number of HMRs, MedsChecks and Diabetes MedsChecks that a pharmacy or accredited pharmacist can perform in each month; and
- cancelling a scheduled stakeholder forum on Aboriginal and Torres Strait Islander programs.
5.48 In respect to the proposed changes, the Pharmacy Guild advised the ANAO that:
The Guild and the Department worked together on a number of proposals to address the projected overspend. At a meeting of the ACC on 23 October 2013, the Department and the Guild discussed a number of potential strategies and the Guild undertook to provide the Department with a document of potential solutions. The Guild and the Department also liaised with other stakeholders regarding some of the measures including meetings on the 12 February (with AACP, SHPA, NPS & PSA)301 and a further consultation meeting with the PSA and the Department on 14 February 2014.
Revised administrative arrangements for programs
5.49 In November 2013, Human Services advised Health that it would cost an estimated $1.1 million and take 11 months to implement changes to Human Services’ processing systems to facilitate the proposed program changes. Human Services advised the ANAO that:
Health is aware the department [Human Services] has a minimum of a nine month lead time for all systems changes. It is also aware that Medicare systems are very old and difficult to make changes to and for the department to implement online functionality for all seven 5CPA programmes, would have meant significant costs and timeframes and that by comparison updating the Pharmacy Guild’s systems was likely a much quicker option.302
5.50 Health submitted a minute to the Minister for Health in late November 2013 with various options to implement the proposed program changes:
- agree to allow Human Services to make the necessary system changes, to be implemented in October 2014 at a cost of $1.1 million; or
- arrange for Health to assume responsibility for the processing of 5CPA professional program payments previously made by Human Services and introduce an electronic claiming system; or
- shift 5CPA professional programs processed by Human Services to the Pharmacy Guild through a new contract, and transfer associated administrative funding previously committed to Human Services to the Pharmacy Guild.
5.51 The Minister agreed to the third option on 29 November 2013, and Health commenced drafting a new contract with the Pharmacy Guild that consolidated all existing 5CPA contracts with the Guild, and added the professional programs processed by Human Services. Administrative funding for each program was combined into a single schedule to the new contract.
5.52 New arrangements for 5CPA program payments commenced on 1 March 2014 with the rollout of an electronic registration and claiming portal managed by the Pharmacy Guild. These new arrangements included limiting the number of HMR services claimed by an approved service provider to 20 per calendar month, in order to contain the higher than expected cost of the HMR program.
Procurement of the Pharmacy Guild’s services
5.53 The new arrangements introduced on 1 March 2014 resulted in the Pharmacy Guild administering most 5CPA professional programs.303 In seeking approval to enter into a new and expanded contract with the Pharmacy Guild, Health advised the financial delegate that its decision not to tender for the services relied on the relevant exemption in paragraph 10.3(b) of the 2012 Commonwealth Procurement Rules (CPRs), which provided that:
… where, for reasons of extreme urgency brought about by events unforeseen by the agency, the goods and services could not be obtained in time under open tender or prequalified tender …304
5.54 In its advice to the delegate dated 10 February 2014, Health indicated that it considered that the budgetary problems relating to 5CPA professional programs were unforeseeable by the department, and the Pharmacy Guild was the only entity able to take on the administrative functions and implement program changes by 1 March 2014, in order to mitigate the projected overspend within the 2013–14 financial year.
5CPA contracts with the Pharmacy Guild
5.55 The ANAO identified some 62 contracts between Health and the Pharmacy Guild relating to the 5CPA. The contracts have provided funding to the Pharmacy Guild for three purposes:
- payment for providing advisory services to the department (administrative funding);
- payment for providing administrative services to the department (administration funding); and
- funding for payments to recipients under 5CPA programs (program funding).
5.56 Until 1 March 2014, the following contracts made provision for payments to the Pharmacy Guild:
- one new contract and 11 variations to existing 4CPA contracts to extend arrangements for certain 4CPA programs the Pharmacy Guild administered into the first year of the 5CPA, valued at $12 million;
- an overarching Multi-Schedule Funding Agreement with 12 Schedules, valued at $3.4 million;
- an overarching Deed for Multi-Schedule Funding with 23 Schedules, valued at $78.8 million; and
- a contract for the provision of accreditation data for the Pharmacy Practice Incentives programs, valued at $2.7 million.
5.57 On 11 February 2014, Health entered into a $259 million ‘Contract for Services’ for the Pharmacy Guild to administer the professional programs formerly administered by Human Services, in addition to the programs previously administered by the Pharmacy Guild.305 The new contract, which took effect on 1 March 2014, replaced all contracts with the Pharmacy Guild that were active as at February 2014.306
5.58 The various 5CPA contracts with the Pharmacy Guild are summarised in Figure 5.4. Health’s management of Pharmacy Guild contracts is examined in the following paragraphs.
Figure 5.4: 5CPA contracts between Health and the Pharmacy Guild
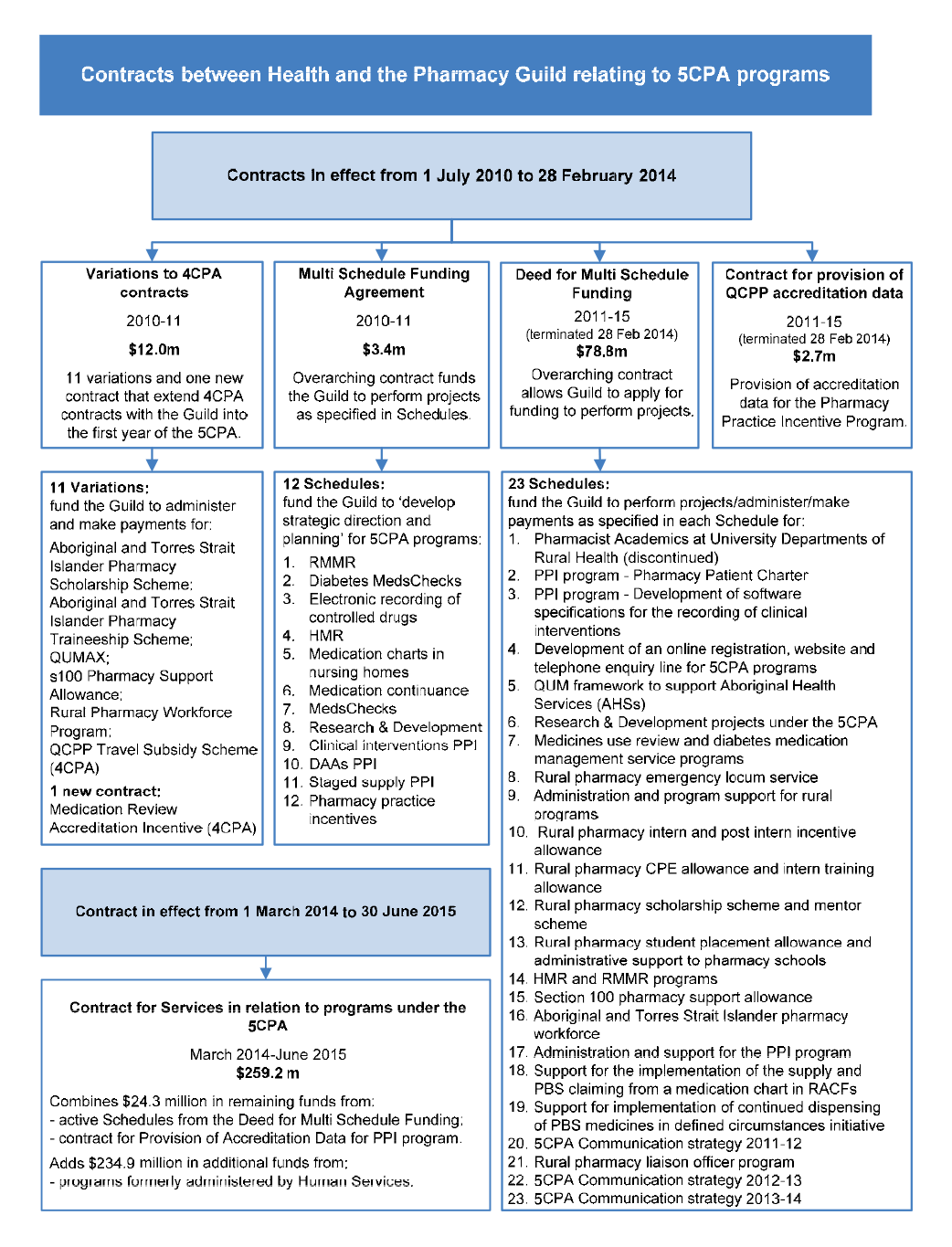
Source: Health internal documentation.
Note: Contract costs are GST inclusive, and include contract variations as at 31 December 2013.
Funding provided to the Pharmacy Guild
5.59 The ANAO examined each 5CPA contract to ascertain the value of administrative and program funding provided to the Pharmacy Guild. The ANAO’s estimate of funds provided to the Guild for administrative and program purposes is summarised in Table 5.2.307
Table 5.2: 5CPA contracts with the Pharmacy Guild—administrative and program funding
|
Contract |
Administrative (GST inclusive) |
Program (GST inclusive) |
Total (GST inclusive) |
|
4CPA programs transition funding 2010–11 |
$2 236 136 |
$9 710 525 |
$11 946 661 |
|
Multi Schedule Funding Agreement 2010–11 |
$3 391 605 |
$0 |
$3 391 605 |
|
Deed for Multi Schedule Funding 2011–15 |
$21 019 802 |
$57 770 484 |
$78 790 287 |
|
Provision of accreditation data for PPI program |
$2 696 738 |
$0 |
$2 696 738 |
|
Contracted amount for Pharmacy Guild before 1 March 2014 |
$29 344 281 |
$67 481 009 |
$96 825 290 |
|
Contract for Services 2014–15 |
$9 059 471 |
$250 149 427a |
$259 208 897a |
|
Contract for Services 2014–15 less funds from previous contracts |
-$7 244 471 |
-$17 029 430 |
-$24 273 901 |
|
Contracted amount for Pharmacy Guild from 1 March 2014 |
$31 159 281 |
$300 601 006 |
$331 760 287 |
Source: ANAO analysis of 5CPA contracts between the Pharmacy Guild and Health including variations, as at November 2014, and Health advice.
Notes:
- Health reported that program funds for the Contract for Services do not attract GST and therefore these figures are GST exclusive.
5.60 The ANAO estimated that over the life of the 5CPA, departmental contracts provided for the Pharmacy Guild to receive $31.2 million for administrative services, and to receive funding of $300.6 million for payments to recipients under 5CPA professional programs. In addition, Health entered into eight contracts with a Pharmacy Guild related entity, Fred IT Group, which provided up to $22.3 million for IT services.308 Many of the contracts with Fred IT Group are not directly related to the 5CPA.309
5.61 While Health has provided significant funding to the Pharmacy Guild for administering 5CPA professional programs, there is no mention of funding or the quantum of funding for the Pharmacy Guild in the 5CPA; a notable omission in an overarching agreement which establishes the framework for third-party administration of Commonwealth funded programs and services.
5.62 Further, in the context of seeking approval for the proposed 5CPA in April 2010, Health did not advise Ministers that administrative funding would be provided to the Pharmacy Guild from allocations for professional programs and services.
5.63 Health advised the ANAO that:
The Guild’s role in administration is a long standing part of Agreements … Based on the number and types of programs it is reasonable to expect the administration budget would not be insignificant. While the Fifth Agreement, signed on 3 May 2010 identified the Guild as program administrators for a number of programmes contained within the Agreement, Health can see value in being more explicit in future agreements in including for example percentages for such purposes.
5.64 Table 5.3 shows how indicative program funding for one professional program was recorded in the Schedule to the 5CPA, without explicit mention of Australian Government funding to the Pharmacy Guild for program administration.
Table 5.3: Indicative program funding as shown in the 5CPA
|
Program Name |
Program Description |
Indicative Funds |
Program governance |
|
Rural Support Programs |
|||
|
Rural Pharmacy Workforce |
The Program focuses on strengthening and supporting the rural pharmacy workforce, which in turn will provide increased access to quality pharmacy services for patients residing in rural and remote regions of Australia |
$37m |
Program Manager: Guild & Commonwealth Program payments made by: Guild |
Source: Fifth Community Pharmacy Agreement, p. 30.
5.65 To provide transparency and accountability for the funds they administer on the Government’s behalf, Government entities are required to clearly distinguish between expenditure used for administration and expenditure for programs. When Government entities contract with third parties to administer Commonwealth programs and services, the arrangements should similarly distinguish between payments for administrative services and funding provided to make program payments. Some of the 5CPA contracts in effect before 1 March 2014 did not clearly distinguish between payments to the Pharmacy Guild for its services to the department, and funds to be used to pay recipients under 5CPA programs. This lack of clarity was compounded by Health’s use of a single cost centre for each of four programs, representing $28.1 million of 5CPA funding, which combined administrative and program payments.
5.66 While acknowledging the potential need to revise estimates over the life of a five year agreement, Health should identify anticipated levels of Australian Government funding for third-party administration in the next community pharmacy agreement and in its related advice to government, as a means to improve transparency in agreement making, and to strengthen the quality of policy advice.
Recommendation No.6
5.67 To improve transparency in agreement-making, the ANAO recommends that the Department of Health documents anticipated levels of Australian Government funding for third party administration for the next community pharmacy agreement.
Health response: Agreed.
Funding for communication strategy ($5.8 million)
5.68 While 5CPA professional programs as a whole are cash limited, the agreement provides flexibility to re-allocate funding between professional programs, and Health adopted this approach to fund a $5.8 million communication strategy delivered by the Pharmacy Guild in the first four years of the 5CPA. The aim of the strategy was ‘to promote and increase uptake and understanding of the programs and initiatives’.
5.69 Four contracts between Health and the Guild, summarised in Table 5.4, made provision for the communication strategy.
Table 5.4: Contracts with the Pharmacy Guild for the 5CPA Communication Strategy
|
Contract |
Schedule |
Date signed |
Funding |
|
Development of an online registration system, website and telephone inquiry line for 5CPA programs |
4 |
22/2/2011 |
$ 267 806 |
|
5CPA Communication Strategy |
20 |
8/9/2011 |
$2 267 075 |
|
Variation 1 |
Variation 1 replaced Schedule 20 |
28/6/2012 |
$3 252 796 |
|
5CPA Communication Strategy |
22 |
16/7/2012 |
$1 733 197 |
|
5CPA Communication Strategy |
23 |
10/7/2013 |
$ 582 979 |
|
Total |
|
|
$5 836 779 |
Source: ANAO analysis of Health contracts.
5.70 The communication strategy included funding for a 5CPA website with Pharmacy Guild and Department of Health co-branding.310 However, the strategy was not submitted for approval as part of Health’s advice to Ministers on the proposed 5CPA in April 2010, and funding for the strategy was not included in the $663 million approved by Ministers for 5CPA professional programs and services. As a communication strategy is not a professional program, it would have been appropriate for Health to seek Ministerial approval to re-allocate $5.8 million from professional programs for this purpose.
5.71 Health advised the ANAO that the:
ACC [Agreement Consultative Committee] can agree to distribute dollars according to need, it was agreed there was a need to communicate information about programmes thus funding was diverted. ACC decisions record this … and there are funding approval minutes for communication strategy funding … The new schedule for the Communication Strategy was initiated by the Department to provide a more efficient and co-ordinated approach to the communication activities around all 5CPA programs. This also enhanced the monitoring and accountability for the communication elements of the Agreement.
5.72 The government funded 5CPA website includes links providing a direct link to both Health’s website and the Pharmacy Guild website, which is used as a vehicle for public advocacy and campaigns on behalf of Pharmacy Guild members. During November and December 2014, the Pharmacy Guild’s website home page featured information on the Pharmacy Guild’s national advertising campaign launched in October 2014, ‘Discover More. Ask Your Pharmacist’, and invited people to ‘Visit the campaign website.’ A further link was provided to the Guild’s campaign website.
5.73 Where government funding for communication purposes is provided to a third party that also has an advocacy role on behalf of its members, it is prudent for the administering department to consider how to avoid the risk of perceived Commonwealth endorsement for non-government campaigns. The minutes of 5CPA Agreement Consultative Committee (ACC) meetings, at which the Pharmacy Guild and Health had agreed and developed the communications strategy and source of funding, do not refer to managing the risk of linking a government sponsored website to a non-government website carrying advocacy and campaign materials311, and Health could not make available other documentation indicating whether these issues had been considered when developing the communications strategy and 5CPA website.
5.74 While the Minister was advised by Health on 30 May 2011 that the ‘ACC has agreed on an overarching communications strategy for all Fifth Agreement Programs’, she was not advised that $5.8 million would be re-allocated from 5CPA professional program funds to the Pharmacy Guild for this purpose, or that the website would be co-branded.
5.75 The unanticipated consequences of Health’s decision to co-brand the 5CPA website highlights the importance of government entities actively assessing risks through an agreement’s life cycle, and being alert to specific risks which may arise at the interface between the government and non-government sectors. Such risks can emerge regardless of the maturity or duration of the relationship between parties.
Reporting requirements for the Pharmacy Guild
5.76 In accordance with the Multi-Schedule Funding Deed and related Schedules, the Pharmacy Guild was required to submit progress reports to Health on its activities as set out in each Schedule. Payments to the Pharmacy Guild were generally made upon acceptance of the reports. In effect, the Pharmacy Guild self-reported its performance against the program aims, activities and timeframes.312 The Pharmacy Guild advised the ANAO that the self-reporting arrangements were developed by the department.
5.77 Until early 2014, Health had not implemented additional assurance measures for its contracts, such as periodic audit or other review activity. However, in seeking approval for new contract arrangements with the Pharmacy Guild in early 2014, Health advised the financial delegate that all expenditure of public money under the Contract for Services would be subject to an audit/compliance regime specifically established and managed by Health. In October 2014, Health advised the ANAO that:
From 1 March 2014, the Department assumed responsibility for audit and compliance activities for 5CPA Programmes which are administered by the Guild. The Department has contracted the development of a Compliance Assurance Strategy, developed in consultation with the [department’s] Audit and Fraud Branch. This strategy was deployed on 22 August 2014, with the Department issuing a “Request for Information” letter to 26 service providers identified through the Compliance Assurance Strategy, to request certain information to assist in gaining assurance of compliance. This related to Home Medicine Review, MedsCheck, Diabetes MedsCheck and Residential Medication Management Review service providers, for claims conducted between 1 January 2013 and 28 February 2014. Future steps and audits will be informed by the Compliance Assurance Strategy.
Application of financial framework
5.78 The purpose of the Australian Government’s financial management framework is to promote the proper use of public resources.313 Where departments use third parties to undertake administrative tasks or make payments on their behalf, it is necessary to consider whether framework requirements may also apply to officials of the non-government entity.
Handling of public money by outsiders
5.79 Section 12 of the FMA Act established special requirements for agencies that entered into agreements or arrangements for the receipt, custody or payment of public money by ‘outsiders’ (third parties such as the Pharmacy Guild). Agencies either had to ensure that outsiders complied with all the requirements of the financial framework, or alternatively could make special arrangements through a ‘section 12 agreement’, which specified a set of requirements to be met by the outsider.314
5.80 The ANAO sought Health’s advice on whether the department had an FMA section 12 agreement in place to cover its arrangements with the Pharmacy Guild. It took Health some time to respond that it did not have any agreements in place under Section 12 of the FMA Act to support its arrangements with the Pharmacy Guild. Accordingly, the ANAO asked Health to provide advice on the Pharmacy Guild’s compliance with financial framework requirements.315
5.81 Health initially advised the ANAO that it ensured that the Pharmacy Guild complied with the financial framework through the clauses in its contracts with the Guild, and was ‘pulling together’ evidence of the Guild’s compliance with framework requirements. However, in December 2013 Health advised that:
In reference to [ANAO] requests for confirmation and evidence that the Pharmacy Guild of Australia has met the requirements of the Commonwealth financial framework in regard to the Guild’s administration of the 5CPA programs, [Health] confirm that the Department does not consider that 5CPA funds, when paid to the Guild under the Deed for Multi Schedule Funding, are ‘public money’ for the purpose of the Financial Management and Accountability Act 1997, nor does the Guild act as an agent for the Department in this regard. Accordingly no confirmation or evidence can be provided.
5.82 The ANAO subsequently examined each Schedule of the Deed to establish whether there was a risk that the Schedules might constitute an agreement or arrangement for the receipt, custody or payment of public money and might therefore involve the Pharmacy Guild acting as an outsider or allocated official under the FMA Act.
5.83 As a number of Schedules to the Deed appeared to involve the Pharmacy Guild making program payments, the ANAO also examined whether Health had sought legal advice on these matters. Departmental records indicated that in the course of seeking financial approvals (under FMA Regulation 9) relating to the Schedules, Health had advised the financial delegate that legal advice was not required. Further, Health had not sought legal advice on arrangements for program payments being made by the Pharmacy Guild during the negotiation and drafting of the 5CPA.
5.84 It was not until the ANAO requested departmental advice on the ‘outsider’ issues that Health sought internal legal advice from its Legal Services Branch (LSB), in November 2013. However, the request for advice was focussed narrowly on one 5CPA program, the Research and Development program, and the advice was not able to provide assurance in respect of all programs where payments were made by the Pharmacy Guild. The internal advice indicated that the funds, once paid to the Guild under the Research and Development program, were not public money and therefore no section 12 authorisation was required.
5.85 Given the complexity of the 5CPA arrangements and the absence of broader internal legal advice on the ‘outsider’ issues, the ANAO sought external legal advice from the Australian Government Solicitor (AGS) on the application of the FMA Act and Regulations to the Pharmacy Guild.316 In summary, the AGS advice indicated that:
- 11 of the 23 Schedules to the Deed were agreements or arrangements for the receipt, custody or payment of public money by the Pharmacy Guild; and
- in respect to the affected arrangements, the Pharmacy Guild’s officers were performing tasks or procedures relating to the commitment, spending, management or control of public money.317
5.86 In consequence, officers of the Pharmacy Guild were at times performing financial tasks for Health and were allocated officials for the purposes of the FMA Act. Broadly speaking, officers of the Guild were in some circumstances subject to financial framework requirements relating to: the banking of public money318; the payment of public money; commitments to spend public money; and the improper use and loss of public money.319
5.87 The legal advice highlighted the need to consider the full implications of highly complex arrangements such as the 5CPA at the design stage, so as to avoid potential risks, including legal and reputational risks, for the parties.320 For the Pharmacy Guild, the risk under the arrangements outlined above was that its officers may not have complied with a range of requirements of the FMA Act and Regulations that applied to officials and to public money from the start of the 5CPA until 30 June 2014, when the FMA Act ceased to operate. In the case of Health, there was the risk of non-compliance with financial framework requirements—which FMA agencies were required to report in their annual Certificate of Compliance321—in addition to reputational risk to itself and its agent.
5.88 In the course of the audit, and after the ANAO raised the ‘outsider’ issues discussed above322, the responsible Health delegate gave an approval under section 12 of the FMA Act for the new $259.2 million 5CPA Contract for Services with the Pharmacy Guild, which took effect on 1 March 2014.323
5.89 Health advised the ANAO that:
Health recognised the ANAO’s opinion and subsequently addressed the ANAO’s possible concerns in March 2014 when entering into the new contract arrangements with the Pharmacy Guild.
New contract with Pharmacy Guild
5.90 The new contract between Health and the Pharmacy Guild incorporated all 5CPA professional programs, apart from those administered by Health.324 The new contract also classified the programs as either ‘Category A’ or ‘Category B’, corresponding to programs previously administered by Human Services and the Guild respectively. As part of the FMA Regulation 9 approval process relating to the new contract, the delegate was advised that:
The programmes that the Guild will make payments for under the New Agreement include discretionary grant-like programmes…
- Category B programmes comprise a number of low value grant-like activities. These are supported by established guidelines and involve less frequent payments. In many cases, payments for these programmes (such as Rural Scholarships) are made either quarterly, six- monthly or annually…
For Category B programmes, the New Agreement provides for payments to be made in accordance with the approved annual work plan and requires the Guild to provide financial reconciliations on a six-monthly basis. This is consistent with how Category B programmes (such as the Rural Pharmacy Workforce Programme) currently operate under the existing funding arrangements within the Deed for Multi-Schedule funding.325
5.91 The financial delegate was further advised that the programs administered by the Pharmacy Guild prior to 1 March 2014 were also programs of a ‘grant-like’ nature, and that:
The new contract requires, to the extent that the Guild performs such duties in relation to grants administration … on the behalf of the Commonwealth, the Guild must comply with the Commonwealth Grant Guidelines to the greatest extent possible. This was a requirement of the section 12 delegation.
5.92 The ANAO examined the new Contract for Services, and found that while Category A and Category B programs were clearly identified, and specific acquittal requirements were established, the contract did not identify which programs were ‘grant-like’ and therefore subject to the Commonwealth grants framework. In addition, the contract did not specify what compliance with the grants framework ‘to the greatest extent possible’ meant, or the specific framework obligations applying to the Pharmacy Guild.
5.93 The Contract for Services is intended to operate until 30 June 2015, and the requirements of the Commonwealth Grants Rules and Guidelines326—issued in July 2014—apply to all grants. To resolve any ambiguity, the application of the grants framework in respect to 5CPA programs should be clarified with the Department of Finance (Finance), which has responsibility for its administration at the whole-of-government level.
5.94 As part of that process, there would also be benefit in Health clarifying, in consultation with Finance, the basis on which Health has treated the Pharmacy Guild as the sole recipient of grants of Commonwealth financial assistance intended to be disbursed by the Guild to pharmacy owners and other parties as payments under professional pharmacy programs.327 While the schedules to the 5CPA provide for program payments for certain professional programs to be made by the Pharmacy Guild, they do not indicate that the monies become funds of the Guild for its own purposes. Health was unable to provide evidence that the relevant funds were authorised by Ministers as grants where the sole recipient of assistance would be the Pharmacy Guild.
5.95 The Public Governance, Performance and Accountability Act 2013 (PGPA Act) took effect on 1 July 2014, replacing the financial management framework established by the FMA Act, including the ‘outsider’ provisions. However, the complexity of administrative arrangements established to implement the 5CPA, including ongoing third party administration, suggests that Health should assess arrangements against the PGPA Act and Rules as part of the transition to the new resource management framework.
5.96 Health advised the ANAO that:
Health notes the raising of this matter … Health is considering this issue as part of the broader context of existing programs, noting the William’s case as mentioned, the new PGPA Act, CGG matters and legal advice on all aspects of program operations.
Conclusion
5.97 Stakeholders advised the ANAO of concerns relating to the interaction of the two 5CPA consultative committees—the Agreement Consultative Committee and Programs Reference Group—and the department acknowledged that there had at times been difficulties in obtaining agreement on issues. The Australian Government establishes, and often funds, formal consultative and advisory arrangements to facilitate the effective implementation of policy and programs. Where there is a risk of consultative or governance arrangements not fully achieving their intended purpose, or potentially operating in a manner not fully consistent with formal agreements or expectations, there is a senior management responsibility to work with participants to re-focus effort on intended outcomes.
5.98 Health has managed over 310 contracts related to the 5CPA, that were documented across 3551 departmental files. The large number of contracts, and shortcomings in Health’s record keeping, meant that it took some time from the ANAO’s first inquiry regarding 5CPA contracts for the department to identify the location of its 5CPA contracts and produce a complete list of contracts matched to departmental file numbers, where signed copies of the contracts were thought to be located. There would be merit, given the large number of 5CPA records managed by Health and the department’s experience in locating key documents in the course of this audit, in reviewing record keeping arrangements for the 5CPA and the next agreement in order to provide assurance that the department can effectively discharge its advisory, accountability and contract management functions in a timely manner.
5.99 The 5CPA provides flexibility to re-allocate money between professional programs, and Health adopted this approach to fund a $5.8 million communication strategy from professional program allocations. The strategy included funding for a 5CPA website with Pharmacy Guild and Department of Health co-branding. However, the strategy was not submitted for approval as part of Health’s advice to Ministers on the proposed 5CPA in April 2010, and funding for the strategy was not included in the $663 million approved by Ministers for 5CPA professional programs and services. As a communication strategy is not a professional program, it would have been appropriate for Health to seek Ministerial approval to re-allocate $5.8 million from professional programs for this purpose.
5.100 Further, the government-funded 5CPA website included a direct link to the Pharmacy Guild website, which is used as a vehicle for public advocacy and campaigns on behalf of Pharmacy Guild members. The minutes of the 5CPA Agreement Consultative Committee (ACC) meetings, at which the Pharmacy Guild and Health had agreed and developed the communications strategy and source of funding, do not refer to managing the risk of linking a government sponsored website to a non-government website carrying advocacy and campaign material.
5.101 While the Minister was advised by Health on 30 May 2011 that the ‘ACC has agreed on an overarching communications strategy for all Fifth Agreement Programs’, she was not advised that $5.8 million would be re-allocated from funding for 5CPA professional program funds and the Electronic Prescription Fee to the Pharmacy Guild for this purpose, or that the 5CPA website would be co-branded.
5.102 Under some of the contracts between Health and the Pharmacy Guild, officials of the Guild were at times performing financial tasks for Health and were therefore deemed—under the Financial Management and Accountability Act 1997 (FMA Act)—to be ‘allocated officials’ of the department when undertaking such tasks. Section 12 of the FMA Act made provision for the management of such arrangements through specific agreements between departments and third parties (known as ‘outsiders’); an approach which was not adopted by Health. In the absence of a ‘section 12’ agreement, there was a risk that affected Pharmacy Guild officials were not aware of their obligations relating to the handling of public money; giving rise to compliance and reputational risks for Health and the Guild.
5.103 Some of the 5CPA contracts in effect prior to 1 March 2014 did not clearly distinguish between payments to the Pharmacy Guild for its administration of services to the department and the actual funds used to pay recipients for the 5CPA programs. In addition, government funding of some $31 million for Pharmacy Guild administration relating to the agreement is not documented in the 5CPA. While acknowledging the potential need to revise estimates over the life of a five year agreement, Health should identify anticipated levels of Australian Government funding for third-party administration in the next community pharmacy agreement and in its related advice to government, as a means to improve transparency in agreement making, and to strengthen the quality of policy advice.
5.104 A new (February 2014) contract between Health and the Pharmacy Guild incorporated all 5CPA professional programs, apart from those administered by Health. As part of the internal approval process for the new contract, the departmental delegate was advised that a number of the 5CPA professional programs were ‘grant-like’, and the Pharmacy Guild was expected to comply with the Australian Government’s grants administration framework ‘to the greatest extent possible’. The ANAO found that whilst specific acquittal requirements were established, the contract does not identify which programs are ‘grant-like’ and therefore subject to the grants framework. In addition, the contract did not specify what compliance with the grants framework ‘to the greatest extent possible’ meant, or the specific framework obligations applying to the Pharmacy Guild.
5.105 To provide assurance that Health’s arrangements for community pharmacy agreements comply with the Australian Government’s revised resource management framework, there would be merit in the department consulting with Finance to review the application of the Public Governance and Accountability Act 2013 and Rules. As part of that review process Health should also clarify, in consultation with Finance, the basis on which Health has treated the Pharmacy Guild as the sole recipient of grants of Commonwealth financial assistance intended to be disbursed by the Guild to pharmacy owners and other parties as payments under professional pharmacy programs. While the schedules to the 5CPA provide for program payments for certain professional programs to be made by the Pharmacy Guild, they do not indicate that the monies become funds of the Guild for its own purposes. Health was unable to provide evidence that the relevant funds were authorised by Ministers as grants where the sole recipient of assistance would be the Pharmacy Guild.
6. Reporting and Evaluation
This chapter examines Health’s reporting and evaluation arrangements for the 5CPA.
Introduction
6.1 An objective of the 5CPA is to:
ensure transparency and accountability in the expenditure of the Funds.
6.2 The Fifth Community Pharmacy Agreement (5CPA) commits the Commonwealth to delivering $15.4 billion over five years for: pharmacy remuneration; a CSO Funding Pool for pharmaceutical wholesalers; and over 30 professional programs and activities.328 The 5CPA also documents that the initiatives covered in the agreement will result in $1 billion in savings over the term of the agreement.
6.3 Agencies publicly report performance and financial information on the programs that they administer in their annual reports. This chapter examines Health’s reporting on the 5CPA, including actual expenditure under the agreement and performance reporting on 5CPA professional programs and activities. The chapter also examines the 5CPA evaluation framework.
Departmental reporting of 5CPA costs
6.4 In its annual report Health reports on the 5CPA, among other programs, under ‘Outcome 2 Access to Pharmaceutical Services’.329 In 2013-14, Outcome 2 included four programs, two of which related to the 5CPA:
- Program 2.1: Community pharmacy and pharmaceutical awareness—covering 5CPA professional programs, the Premium Free Dispensing Incentive, and the Electronic Prescription Fee; and
- Program 2.2: Pharmaceuticals and pharmaceutical services—covering the PBS and CSO Funding Pool.
6.5 The 5CPA is a key element in the delivery of Program 2.1 and Program 2.2. The administered expenditure (program costs) for these programs in 2013–14 is shown in Table 6.1.
Table 6.1: Health Outcome 2—Financial Resource Summary 2013–14
|
Health Outcome 2: Access to Pharmaceutical services |
Actual 2013–14 |
|
|
Program 2.1: Community pharmacy and pharmaceutical awareness |
||
|
Ordinary Annual Services (Annual Appropriation Bill 1) |
$374 606 000 |
|
|
Program 2.2: Pharmaceuticals and pharmaceutical services |
||
|
Ordinary Annual Services (Annual Appropriation Bill 1) |
$194 942 000 |
|
|
Special appropriation National Health Act 1953—Pharmaceutical Benefits |
$9 119 655 000 |
|
Source: Department of Health, Annual Report 2013–14, Outcome 2—Financial Resource Summary, p. 63.
6.6 It is not possible to identify the costs of the key material elements of the 5CPA—comprising pharmacy remuneration ($2.49 billion in 2013–14), CSO wholesaler payments ($192 million in 2013–14) and professional programs ($155 million)—from the department’s 2013–14 annual report or previous annual reports.330
6.7 Health advised the ANAO that:
although costs of elements of the 5CPA cannot be identified, it is also not appropriate that all components be reported in the Annual Report and other sources of reporting, such as the Departmental website, may be utilised. It should also be noted that more detailed reporting would have administrative resourcing and regulatory burden for Health and any other associated payment agency.
6.8 The ANAO notes that Health already reports on the disaggregated costs of key elements of the 5CPA—CSO wholesaler payments, 5CPA professional programs, and two components of pharmacy remuneration331—in an internal management report. This internal report informs preparation of the department’s annual report and could be used as the basis for improved public reporting on the key elements of the 5CPA.
6.9 In order to identify actual expenditure on the key elements of the 5CPA, the ANAO compared the costs in Health’s annual report with the detail in an internal report containing disaggregated financial information. The results of the ANAO analysis are shown in Table 6.2.
Table 6.2: Estimated actual expenditure under the 5CPA 2013–14
|
Health Outcome 2: Access to Pharmaceutical services |
Actual 2013–14 |
|
|
Program 2.1: Community pharmacy and pharmaceutical awareness |
||
|
5CPA professional programs |
$155 236 090 |
|
|
Premium Free Dispensing Incentive (part of pharmacy remuneration) |
$210 469 083 |
|
|
Electronic Prescription Fee (part of pharmacy remuneration) |
$6 562 453 |
|
|
Total (reported under Annual Appropriation Bill 1) |
$372 267 626a |
|
|
Program 2.2: Pharmaceuticals and pharmaceutical servicesb |
||
|
Community Service Obligation wholesaler payments (included in Annual Appropriation Bill 1) |
$191 960 898c |
|
|
PBS pharmacy remuneration (reported under Special appropriation National Health Act 1953—Pharmaceutical Benefits) |
$2 493 076 993d |
|
|
PBS cost of medicines (reported under Special appropriation National Health Act 1953—Pharmaceutical Benefits) |
$6 626 578 007d |
|
Source: ANAO analysis of Health annual report 2013–14 and internal financial information.
Notes:
- Program 2.1 (Annual Appropriation Bill 1) includes two items that are not elements of the 5CPA (PBS Concessional Validations and Post-Market Monitoring activity). The total for Program 2.1 is therefore some $2 million lower than the amount reported by Health (see Table 6.1).
- Program 2.2 (Annual Appropriation Bill 1) includes three items that are not CSO payments (PBS litigation, PBS data administration and non-5CPA legal costs). The total for CSO wholesaler payments is therefore some $3 million lower than the amount reported by Health under Annual Appropriation Bill 1 (see Table 6.1).
- CSO wholesaler payments also include $1.28 million for outsourced administration costs.
- The ANAO estimated the PBS cost of medicines and PBS pharmacy remuneration using ratios calculated for the whole of the PBS and RPBS from prescription claims data.
- Grey shaded boxes show the components of 5CPA pharmacy remuneration. Blue shaded boxes show 5CPA professional programs and CSO payments.
6.10 In summary, the ANAO’s analysis indicates that the following components of the 5CPA were either merged with other items, or divided between Program 2.1 and Program 2.2, in the department’s 2013–14 annual report:
- Health aggregates expenditure on 5CPA professional pharmacy programs ($155 million) with two components of pharmacy remuneration—the Premium Free Dispensing Incentive ($210 million) and the Electronic Prescription Fee ($6.6 million)—which are reported under Program 2.1;
- the five remaining components of pharmacy remuneration are allocated to Program 2.2. Health’s reporting on the Program 2.2 ‘Special appropriation National Health Act 1953—Pharmaceutical Benefits’ shows the total costs of the PBS, which combines the cost of medicines (a product) with the cost of pharmacy remuneration (a service); and
- Health combines its reporting on expenditure for the CSO wholesaler payments with its reporting on the costs of: PBS litigation; PBS data administration; non-5CPA legal costs; and the outsourced administration of the CSO. While this expenditure appears as a separate line item under Program 2.2, there is no explanation in the annual report that this expenditure primarily relates to the CSO wholesaler payments.
6.11 In summary, it is not possible to establish from Health’s annual report the actual annual cost of pharmacy remuneration under the 5CPA, as the components of pharmacy remuneration are allocated between Programs 2.1 and 2.2, with five components merged with the cost of pharmaceutical benefits, and two components merged with the cost of 5CPA professional programs.332 The three material elements of the 5CPA—pharmacy remuneration ($13.8 billion), CSO wholesaler payments ($950 million) and professional programs ($663 million)—are not clearly or transparently reported for the benefit of Parliament and other stakeholders.
Recommendation No.7
6.12 To improve transparency and the quality of program performance reporting, the ANAO recommends that the Department of Health reports annually on the actual cost of each major component of the Fifth Community Pharmacy Agreement and the next community pharmacy agreement, including pharmacy remuneration, CSO wholesaler payments and professional programs.
Health response: Agreed.
Performance reporting on the 5CPA
6.13 A central requirement of the Australian Government’s performance framework for government entities is the development of clearly specified program objectives, deliverables and appropriate key performance indicators (KPIs) to enable users to assess progress made towards the stated program objectives. Government entities report on program performance against program objectives in their annual reports.333
Annual reporting
6.14 As discussed, the 5CPA is reported under Programs 2.1 and 2.2. The ANAO examined Health’s annual reports for the first four years of the 5CPA (2010–11 to 2013–14), to assess the reporting of program objectives, deliverables and KPIs for the elements of the 5CPA.
Program objectives 2010–11 to 2013–14
6.15 The program objectives for Program 2.1, which covers 5CPA professional programs and two components of pharmacy remuneration (the Premium Free Dispensing Incentive and the Electronic Prescription Fee), and Program 2.2, which covers the other components of pharmacy remuneration (paid under the Pharmaceutical Benefits Scheme) and the CSO Funding Pool, are shown in Table 6.3.
Table 6.3: Objectives of Health programs covering the 5CPA
|
Program objectives |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
|
Program 2.1: Community pharmacy and pharmaceutical awareness (5CPA professional programs, Premium Free Dispensing Incentive and Electronic Prescription Fee) |
||||
|
Support timely access to medicines and professional pharmacy services through the implementation of the Fifth Community Pharmacy Agreement. |
Y |
Y |
Y |
Y |
|
Support quality use of medicines for Aboriginal and Torres Strait Islander peoples, through education and targeted support for consumers and health professionals. |
Y |
– |
– |
– |
|
Program 2.2: Pharmaceuticals and pharmaceutical services (Pharmaceutical Benefit Scheme and CSO Funding Pool) |
||||
|
Manage the Pharmaceutical Benefits Scheme (PBS) including gaining better value from competition between brands of medicines listed on the PBS. |
Y |
– |
– |
– |
|
Improve health outcomes and sustainability of the PBS by improving the evidence base for prescribing decisions and the assessment of effectiveness of listed PBS medicines. |
Y |
– |
– |
– |
|
List cost-effective new, innovative, clinically effective medicines on the PBS. |
Y |
– |
– |
– |
|
Improve community access to a range of pharmaceutical benefits when prescribed by appropriately trained and certified nurse practitioners and midwives. |
Y |
– |
– |
– |
|
To list cost-effective, innovative, clinically effective medicines on the PBS as well as improve health outcomes and the sustainability of the PBS by improving the evidence-base for prescribing decisions and assessing the effectiveness of PBS listed medicines. |
– |
Y |
– |
– |
|
To provide access to cost-effective, innovative, clinically effective medicines to all Australians, and ensure the sustainability of the PBS. |
– |
– |
Y |
Y |
Source: ANAO analysis of Health information.
6.16 The objectives for Program 2.1 and Program 2.2 have changed significantly over the first four years of the 5CPA, with amendments to seven out of eight program objectives over this period. In 2010–11, for example, Program 2.1 had a program objective relating to quality use of medicines for Aboriginal and Torres Strait Islander people, which was not included in the objectives for later years.
Performance reporting on key 5CPA components
6.17 The ANAO examined Health’s 2013–14 annual report to assess the extent of reporting of the costs, deliverables, and KPIs for the key components of the 5CPA. The results are shown in Table 6.4. The ANAO also examined the department’s website to identify any further information on costs, deliverables and KPIs.
6.18 In summary, in 2013–14 Health’s reporting of the key components of the 5CPA was limited, both in its annual report and on its website. Health has not published the separate costs for the key components of the 5CPA, or any other component. Similarly, there were no deliverables or KPIs reported for any of the key components of the 5CPA.
6.19 Without basic information about costs, deliverables or KPIs for 5CPA programs and activities it is difficult for stakeholders, including the Parliament, to form an overall view of what the 5CPA has actually delivered. In recognition of the move to more concise annual reports, the publication of information on matters of significant interest to stakeholders on websites provides a viable alternative.
Table 6.4: 5CPA reporting in Health’s 2013–14 annual report and website
|
Separate cost |
Deliverables |
KPIs |
|
|
Pharmacy remuneration |
Nil |
Nil |
Nil |
|
Premium Free Dispensing Incentive |
Nil |
Nil |
Nil |
|
Electronic Prescription Fee |
Nil |
Nil |
Nil |
|
Community Service Obligation |
Nil |
Nil |
Nil |
|
Location Rules |
Nil |
Nil |
Nil |
|
Pharmacy practice incentives (PPIs) |
|
|
93% of pharmacies participating |
|
Primary health care Community services support Working with others |
Nil |
Nil |
Nil |
|
Support for the provision of Dose Administration Aids |
Nil |
10 659 546c |
Nil |
|
Clinical interventions by pharmacists |
Nil |
3 269 325c |
Nil |
|
Staged supply support allowance |
Nil |
Nil |
Nil |
|
Administration of PPIs, accreditation, and the patient charter |
Nil |
Nil |
Nil |
|
Medication management programs |
|
|
|
|
Residential Medication Management (RMMR) |
Nil |
Nil |
Nil |
|
Home Medicines Review (HMR) |
Nil |
108 246 HMR services provided |
Nil |
|
MedsCheck formerly: Medicines Use Review (MUR) |
Nil |
Nil |
Nil |
|
Diabetes MedsCheck formerly: Diabetes Medication Management Service |
Nil |
Nil |
Nil |
|
Rural support programs |
|
|
|
|
Rural Pharmacy Maintenance Allowance |
Nil |
Nil |
86% (755 of 877) of rural pharmacies accessed ‘targeted rural programs’a |
|
Rural pharmacy workforce |
Nil |
Nil |
Nil |
|
Aboriginal and Torres Strait Islander programs |
|
|
|
|
S100 Pharmacy Support Allowance |
Nil |
Nil |
Nil |
|
Quality Use of Medicines framework to support Aboriginal Health Services (QUMAX) |
Nil |
Nil |
Nil |
|
Aboriginal and Torres Strait Islander pharmacy workforce |
Nil |
Nil |
Nil |
|
Other Programs |
|
|
|
|
Research and Development program |
Nil |
Nil |
Nil |
|
Continued dispensing of PBS medicines in defined circumstancesb |
Nil |
‘Continue measure phase in.’ |
Nil |
|
Electronic recording and reporting of controlled drugs |
Nil |
‘Continue measure phase in.’ |
Nil |
|
Supply and PBS claiming from a medication chart in a RACF |
Nil |
‘Continue measure phase in.’ |
Nil |
Source: ANAO analysis of Health’s 2013–14 Annual Report and website as at November 2014.
Notes:
- Health advised that ‘targeted rural programs’ referred to in the 2013–14 Annual Report was the Rural Pharmacy Maintenance Allowance
- Formerly known as ‘Medication Continuance’.
- Deliverables reported on Health’s website only.
6.20 Health advised the ANAO that the department’s 2014–15 annual report will provide ongoing data that will reflect all services, and a statement of the reasons for not providing this information previously. While it is not necessary for a department to report on the detail of all the programs and activities that it administers in its annual report, a department’s website can be utilised to provide key information of interest to stakeholders.
Key Performance Indicators (KPIs) 2010–2014
6.21 The department’s key performance indicators (KPIs) for the first four years of the 5CPA are shown in Table 6.5.
Table 6.5: Program 2.1 Key Performance Indicators 2010–2014
|
KPIs |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
|
Percentage of rural community pharmacies accessing targeted programs to ensure accessibility of community pharmacy in rural and remote Australia. |
Y |
Y |
Y |
Y |
|
Successful implementation and uptake of the subsidising PBS medicine co-payments measure by community pharmacies.a |
Y |
– |
– |
– |
|
Improved management of medicines for Aboriginal and Torres Strait Islander patients. |
Y |
– |
– |
– |
|
Percentage of Aboriginal Community Controlled Health Services participating in the Subsidising PBS Medicine Co-payments Measure. |
Y |
Yb |
– |
– |
|
Percentage of community pharmacies participating in the Pharmacy Practice Incentives (PPI) Program. |
–c |
Y |
Y |
Y |
Source: ANAO analysis of Health annual reports 2010–14.
Note:
- Health advised the ANAO that: ‘This KPI related to the implementation of the Closing The Gap measure in 2010-11. As this measure was implemented, it was not reported in subsequent years.’
- Health reported this KPI under Program 8.1 (Aboriginal and Torres Strait Islander Health) in 2011–12.
- Pharmacies that registered for certain PPI priority areas prior to 30 June 2011 were eligible for ‘start-up payments’ that were paid in the following year.
6.22 Overall, the KPIs have limited alignment with: the six objectives specified in the 5CPA; the key professional programs funded under the 5CPA; or material components of the 5CPA, such as pharmacy remuneration. In particular, the KPIs developed for Program 2.1 and Program 2.2 do not permit an assessment of the 5CPA’s high level objectives, which relate to the accessibility, efficiency and viability of retail pharmacy and the need for 5CPA programs to achieve positive health outcomes for the community.
6.23 Further, four out of five KPIs have changed over the first four years of the 5CPA, as shown in Table 6.5. After 2010–11, three KPIs were removed and a new KPI was added. The three KPIs that were removed related to Indigenous access to pharmacy services. The fourth KPI that was introduced in the second year related to the Pharmacy Practice Incentives and Accreditation (PPI) Program. While some parts of the PPI program were not fully implemented until 2011–12, it would have been informative for Health to have reported on the number of pharmacies that registered for PPI participation in 2010–11.334
6.24 The sole KPI consistently reported in the first four years of the 5CPA was: ‘the percentage of rural community pharmacies accessing targeted programs to ensure accessibility of community pharmacy in rural and remote Australia’. This KPI is broadly expressed and, from a reader’s perspective, may relate to any one of several 5CPA rural programs. Health advised the ANAO that this KPI relates solely to the Rural Pharmacy Maintenance Allowance.
Table 6.6: Program 2.1 quantitative Key Performance Indicators
|
Annual report quantitative KPIs |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
||||
|
Target |
Actual |
Target |
Actual |
Target |
Actual |
Target |
Actual |
|
|
Percentage of Aboriginal Community Controlled Health Services participating in the Subsidising PBS Medicine Co-payments Measure. |
80% |
95.7% |
85% |
85% |
– |
– |
– |
– |
|
Percentage of rural community pharmacies accessing targeted programs to ensure accessibility of community pharmacy in rural and remote Australia. |
60% |
83% |
65% |
89% |
70% |
82% |
75% |
86% |
|
Percentage of community pharmacies participating in the Pharmacy Practice Incentives Program. |
– |
– |
40% |
75% |
45% |
82% |
90% |
93% |
Source: Health Annual Reports 2010–11 to 2013–14.
Overall assessment of KPIs for the 5CPA
6.25 The ANAO has developed criteria to evaluate the appropriateness of Australian Government entities’ KPIs, and the completeness and accuracy of their reporting. Individual KPIs may be assessed for their relevance and reliability; while a set of KPIs may be assessed for its completeness, namely, the extent to which it allows an overall assessment of a program to inform users’ decision making.335
6.26 Overall, the KPIs adopted over the period 2010–14 for the 5CPA provide a limited basis on which to assess performance against high level 5CPA objectives, specific professional program objectives or the material components of Commonwealth expenditure such as pharmacy remuneration. The KPIs:
- are not related to health outcomes;
- address only selected aspects of the individual program objectives; and
- have been amended substantially.
6.27 The 5CPA is a key element in the delivery of the department’s Program 2.1 and Program 2.2. There is scope to review the overall performance reporting framework, to improve alignment between the next community pharmacy agreement and public reporting against the program objectives, deliverables and KPIs relating to Programs 2.1 and 2.2. The development of an appropriate and well aligned suite of performance measures, focusing particularly on effectiveness, can help government entities assess: the impact of policies and programs; adjust management approaches as required; and provide advice to government on the successes, shortcomings and/or options for revision to current policies. In addition to facilitating informed decision-making on the allocation and use of public resources, effective performance measurement and reporting enables the Parliament and other stakeholders to assess performance.
Recommendation No.8
6.28 To inform decision-making and the assessment of outcomes by stakeholders, the ANAO recommends that the Department of Health reviews performance reporting to improve alignment between the next community pharmacy agreement and public reporting against the program objectives, deliverables and KPIs relating to the department’s Program 2.1 and Program 2.2.
Health response: Agreed.
Reporting on historical costs of pharmacy remuneration
6.29 While the cost of pharmacy remuneration has been the major cost component of successive community pharmacy agreements336, it has not been publicly reported337 since the 1CPA commenced. The ANAO used Human Services and Health data to estimate overall pharmacy remuneration and pharmacy remuneration per prescription from 1991–92 to 2013–14.
|
Key issues in analysing pharmacy remuneration—1991 to 2014 To measure historical trends in pharmacy remuneration on a consistent basis since 1991 (the first agreement), the analysis must be restricted to remuneration for over co-payment prescriptions because data collection for under co-payment prescriptions did not commence until 1 April 2012.a Including under co-payment prescriptions from that date could lead to a misleading spike in pharmacy remuneration reported from that time. In addition, there was a major change to the way in which pharmacy wholesalers were paid for supplying PBS medicines on 1 July 2006, when the CSO Funding Pool was established. Prior to July 2006, the wholesale mark-up was 11.11 per cent of the cost of PBS medicines (an amount paid to pharmacists, who then pay wholesalers). From 1 July 2006, the wholesale mark-up was reduced to 7.52 per centb, and $150 million per year was allocated to a CSO Funding Pool for direct payments to eligible wholesalers.c The 5CPA definition of ‘pharmacy remuneration’ includes the wholesale mark-up but not the CSO Funding Pool—despite both amounts relating to wholesaler remuneration (notional or actual respectively). To produce comparable year by year information on pharmacy remuneration, the CSO Funding Pool should be added to wholesale mark up. Excluding the CSO Funding Pool could otherwise lead to a misleading fall in pharmacy remuneration reported from 1 July 2006. Alternatively, excluding both the wholesale mark-up and the CSO Funding Pool from pharmacy remuneration will also produce comparable year by year information. In the following analysis the ANAO: excluded prescriptions priced below the patient co-paymentd, and included the CSO Funding Pool, for the purposes of producing comparable year by year information. In reporting remuneration by financial year, the ANAO has grouped records by the ‘date of processing’, which is the date a pharmacy’s claim is finalised.e |
Notes:
- Retail pharmacies are remunerated directly from patients for under co-payment prescriptions, but reporting of under co-payment prescriptions did not commence until 1 April 2012.
- For items priced over $930.06, the wholesale mark-up was capped at $69.94 per item.
- The original CSO Funding Pool of $150 million per year was indexed to WCI9, and has progressively increased to $191 million in 2013–14.
- Prescriptions attracting a payment under the Closing the Gap initiative (which would have otherwise been under co-payment) have also been excluded for consistency.
- Most pharmacies submit their prescription data online to Human Services for payment as each prescription is dispensed. Human Services generally pays pharmacies in advance of receiving the paperwork required to support a pharmacy’s claim, which may contain thousands of paper prescriptions. Human Services validates the advance payments by checking the paper prescriptions, and makes any required adjustments to future payments to the pharmacy. If the value of a prescription is $10 000 or more, Human Services holds over payment until the paper prescription is submitted by the pharmacy. Human Services advised the ANAO that pharmacies are generally paid within nine to 16 days of submitting their prescription data online. The ANAO observed that there was, on average, approximately 43 days between the supply of a PBS/RPBS medicine and the date a pharmacy’s claim is finalised, with approximately 88 per cent of pharmacy claims finalised within 90 days.
Overall pharmacy remuneration and CSO payments
6.30 The ANAO’s analysis indicates that the annual cost of pharmacy remuneration and CSO payments for government subsidised prescriptions (over co-payment prescriptions) has risen from $546 million in 1991–92 to $3 087 million in 2013–14, as shown in Figure 6.1. Pharmacy remuneration and CSO payments have increased by 7.9 per cent per year from the 1CPA to the 5CPA. In contrast, the growth in corresponding prescription volumes for this period was 3.6 per cent per year.
6.31 The last survey of pharmacies’ costs of dispensing, conducted in 1989, indicated that pharmacies with more prescriptions had lower dispensing costs per prescription through economies of scale; suggesting that there was scope to reduce the rate of growth in government funded pharmacy remuneration over time by achieving economies of scale. Similarly, the policy rationale for introducing the Location Rules, which tightly restrict the opening of new approved pharmacies, was that the closure or merger of smaller pharmacies would see remaining pharmacies become more efficient and financially sustainable.
6.32 Health advised the ANAO that the policy and business landscape has evolved since the last survey of pharmacies’ costs of dispensing in 1989. There have also been changes across successive community pharmacy agreements, such as the introduction of Price Disclosure in 2007, which aims to align the price paid by government for PBS medicines to the actual market price.
Figure 6.1: Annual pharmacy remuneration and CSO payments 1991 to 2014
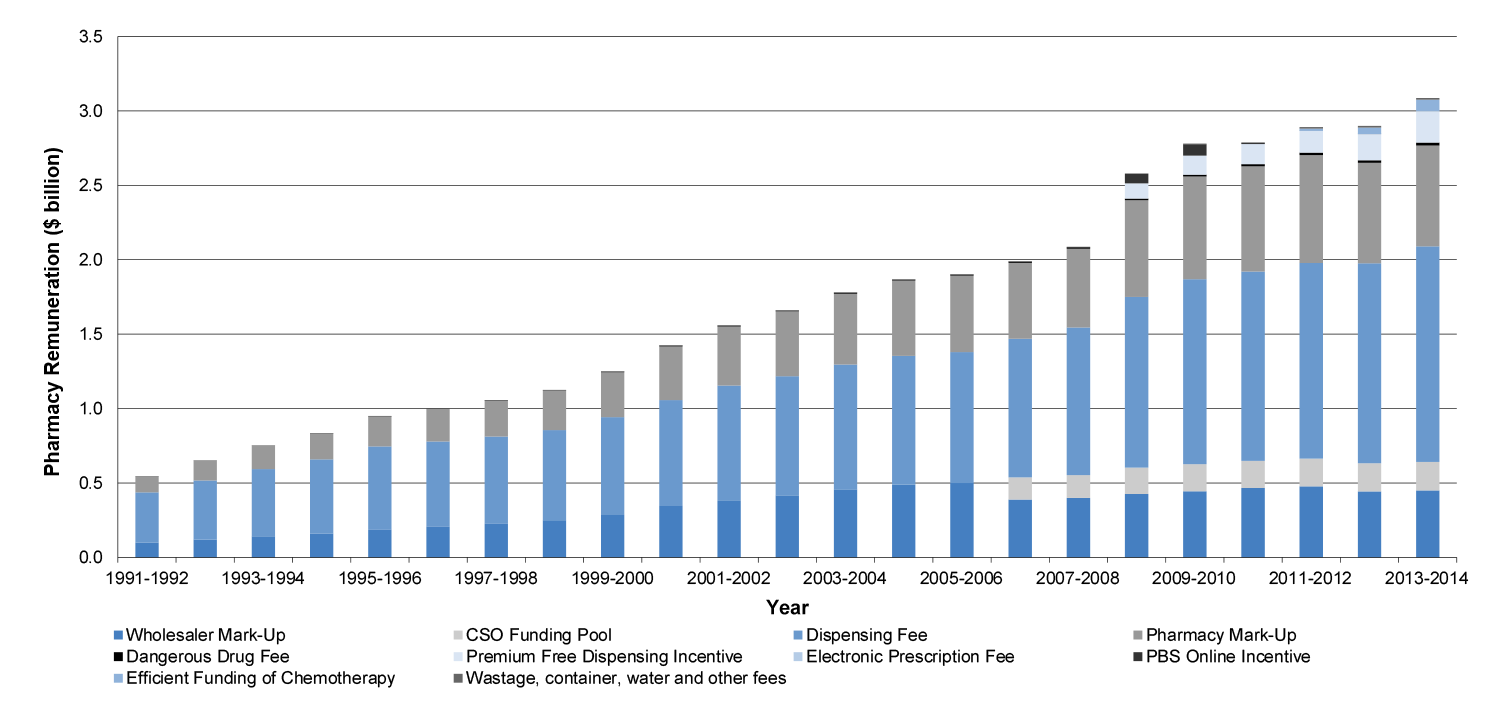
Source: ANAO analysis of Health information
Note: This graph shows annual pharmacy remuneration for over co-payment prescriptions, that is, prescriptions receiving a government subsidy under the PBS or RPBS.
Reporting on the retail pharmacy network
The number of approved retail pharmacies
6.33 Human Services reports on the number of approved PBS suppliers, including the number of approved retail pharmacies338, as at 30 June each year in its annual report. In 2013–14 Human Services reported that there were 5457 ‘approved pharmacists’—retail pharmacies approved under Section 90 of the National Health Act to supply pharmaceutical benefits to the public.
6.34 Health obtains information on the number and location of approved pharmacies from database extracts provided by Human Services, and uses this information, for example, to measure the rural/urban population to pharmacy ratios.339 The numbers of urban and rural pharmacies reported by Health as at 30 June each year during the 5CPA are shown in Table 6.7.
Table 6.7: Urban and rural pharmacies as at 30 June 2010–2014
|
Date |
Urban |
Rural |
Total pharmacies |
|
30-Jun-10 |
4,212 |
876 |
5,088 |
|
30-Jun-11 |
4,258 |
908 |
5,166 |
|
30-Jun-12 |
4,286 |
955 |
5,241 |
|
30-Jun-13 |
4,500 |
851 |
5,351 |
|
30-Jun-14 |
4,580 |
877 |
5,457 |
Source: Department of Health.
6.35 Health advised the ANAO that while there had been growth in the number of community pharmacies over the life of the 5CPA, changes to PhARIA (the geographical classification used by Health), which occurred in 2012 impacted on the count of rural and urban pharmacies. A number of pharmacies previously included as rural or remote in outer metropolitan and regional areas were reclassified as urban, resulting in an increase in those reported as urban (4500) as at 30 June 2013, and a reduction in those reported as rural (851).
6.36 The ANAO examined the Human Services database that records the details of approved pharmacies340, to assess the basis for public reporting.
6.37 The Human Services database records that, since records commenced, over 26 000 approval numbers have been issued for retail pharmacies. The ANAO calculated that 8809 unique approved pharmacies have existed since database records began. This information was then used to calculate the number of unique pharmacies that had submitted a claim for reimbursement in the course of each financial year from the 1CPA to the 5CPA. The number of unique approved pharmacies each year has been relatively stable, as presented in Appendix 11.
6.38 Some care is required when interpreting the Human Services database. While the overall number of pharmacies has remained relatively stable, this number in itself does not indicate the number of pharmacies that are closing (or opening) or the level of turnover in pharmacy ownership.
6.39 Currently Health does not require Human Services to record the registration numbers of pharmacy owners—approved under Section 90 of the National Health Act to dispense pharmaceutical benefits—in the pharmacy approvals database maintained by Human Services. Consequently, the number of pharmacists that own Section 90 approved pharmacies is not known. Human Services advised the ANAO that since the establishment of a national registration system for pharmacists341, such information would assist in conducting compliance activities and providing assurance that pharmacists meet their conditions of approval. There would also be merit in Health considering the option of recording pharmacists’ registration numbers as part of the approval process.
Reporting on the Location Rules
6.40 The Pharmacy Location Rules were introduced in 1990 to address the apparent oversupply of pharmacies in some urban areas, and the undersupply of pharmacies in rural and remote areas.342 Prior to 1CPA, it had been argued that some pharmacies (particularly those clustered in urban areas) would not be economically viable under the rationalised remuneration arrangements proposed by the Pharmaceutical Benefits Remuneration Tribunal. The then Government agreed to provide pharmacy restructuring packages for pharmacy owners to close or merge. The Location Rules were intended to discourage new pharmacies from opening in geographic areas that had a pharmacy, and encourage pharmacies to open in, or relocate to, less well serviced areas.
6.41 The ANAO examined Health’s reporting on the outcomes for the Location Rules. Health regularly reported on the number of people per pharmacy in rural areas compared to urban areas as a key performance indicator (KPI) in its annual reports from 1994 to 2010. However, Health has not reported the ratios of people per pharmacy in rural and urban areas for the first four years of the 5CPA (from 2010–11 to 2013–14).
6.42 Health advised the ANAO that the department supplies this data to the Productivity Commission every year, which reports the ratios in its annual Report on Government Services (ROGS). The ROGS provides information on the equity, effectiveness and efficiency of government services in Australia. For years where people per pharmacy ratios were not reported in Health’s annual report, the ANAO used the ROGS figures.
6.43 The ratios of population per pharmacy in urban and rural areas as at 30 June 1994 to 2014, as reported by Health or the Productivity Commission, are shown in Figure 6.2.
Figure 6.2: People per pharmacy in urban and rural areas 1994 to 2014—multiple remoteness classification systems
Source: ANAO analysis of Health and Productivity Commission information.
6.44 There is considerable year to year variation in the ratios Health has reported for the number of people per pharmacy. The ANAO examined the reasons for this variability.
6.45 Since the ratio was first reported in 1994, Health has used at least three different classification systems to measure the degree of ‘remoteness’:
- Rural, Remote and Metropolitan Areas (RRMA) classification;
- Accessibility/Remoteness Index of Australia (ARIA) classification; and
- Pharmacy Access/Remoteness Index of Australia (PhARIA) (a pharmacy specific measurement of remoteness).
6.46 In addition, the Human Services data extraction procedure did not accurately report compound addresses (where the pharmacy has a shop number or a unit number in addition to a street address). Consequently, the pharmacy classification defaulted to a postcode address, which places the pharmacy at the geographic centre of the postcode. The ANAO raised this issue with Human Services, which advised that it has since corrected the extraction routine.
6.47 The ANAO also calculated the people to pharmacy ratio using the Australian Statistical Geography Standard (ASGS), which was developed by the Australian Bureau of Statistics. The results are shown in Figure 6.3.
Figure 6.3: People per pharmacy in urban and rural areas 2001 to 2011—single remoteness classification system
Source: ANAO analysis.
6.48 When a single remoteness classification system is applied consistently across the years, a more consistent trend is observed. Using the ASGS to classify pharmacies as urban or rural, the ratio of people per pharmacy in urban areas shows an upward trend, rising from 3780 people per pharmacy in 2001 to 4270 in 2011. Over the same period, the number of people per pharmacy in rural areas rose from 4240 in 2001, to peak at 4430 in 2006, and has since declined to about the same level as in 2001.
6.49 In calculating the annual population to pharmacy ratios, the department uses a fixed measure of rural and urban populations from the last population census, and updates the number of pharmacies in rural and urban areas each year. While this approach captures annual changes in the number of pharmacies in rural and urban areas, it does not consider estimated annual changes to rural and urban populations during the inter-census years. Whenever there is a new census, the ABS re-bases its annual population estimates for the previous census period in order to provide accurate estimates of intercensal populations. Consequently, it would be useful for Health to review its population to pharmacy ratios when the ABS re-bases its population estimates following the most recent population census release.
6.50 While acknowledging that different measures of remoteness have been applied over time, a number of risks in the current approach were noted by a peak pharmacists’ association, which advised the ANAO that:
Under 5CPA there have been significant changes to the rural classification system, known as PhARIA, and significant changes to the rural program rules. These changes were not guided by evidence and were made without industry consultation. The changes have had negative financial implications for rural pharmacists, rural interns and rural pharmacy students … The PhARIA classification system provides an apposite example of the lack of integration and linkage between 5CPA and the broader health system. For example, it is unclear why DoHA [Health] maintains a classification system for 5CPA rural programs (PhARIA) that is quite distinct and separate from the rural classification system used by all other programs. This can lead to the quite ridiculous situation whereby a pharmacy is classified at a different degree of remoteness to the GP clinic located right next door. This is an important consideration in the light of a recent report from the Australian Institute of Health and Welfare which indicates that the proportion of the pharmacy workforce aged over 55 and the average age of employed pharmacists is higher in the Inner regional geographic classification compared with all other classifications … Ironically, pharmacists in many locations within this Inner regional classification would have lost their eligibility to access the 5CPA rural programs due to the changes to the PhARIA classification that were developed and implemented without any prior consultation with stakeholders and affected groups. Although the changes to the rural programs are likely to increase the difficulty for future rural workforce recruitment and retention, no studies or data on the rural pharmacy workforce is being collected under 5CPA. It is understood the changes will result in a sizable underspend for the program. Feedback indicates that communication of changes to the rural program to affected stakeholders has been extremely poor.
6.51 An examination of the different remoteness classification systems is outside the scope of this audit. However, the ANAO’s analysis and stakeholder observations indicate that anomalies may have arisen from the use, over time, of different remoteness classification systems to measure the population to pharmacy ratios in rural and urban areas.
Reporting on the CSO Funding Pool
6.52 The CSO Funding Pool arrangements for pharmaceutical wholesalers were established on 1 July 2006 under the 4CPA and have continued under the 5CPA. Under CSO arrangements, eligible pharmaceutical wholesalers (known as CSO Distributors) receive payments for meeting the CSO service standards, which include being able to supply PBS items to retail pharmacies usually within 24 hours.
6.53 The 5CPA states that the purpose of the CSO is to ensure that:
- all Approved Pharmacists [retail pharmacy owners] are able to obtain timely supply of the full range of PBS medicines, irrespective of the size or location of the pharmacy, the breadth of the PBS product range, the cost of the PBS medicines, or the cost of their distribution and supply to pharmacy; and
- all Australians have timely access to the PBS medicines they require, regardless of the cost of the medicine, or where they live.343
6.54 In practice, the CSO does not include the full range of PBS medicines, as it does not cover PBS medicines provided under Section 100 arrangements (Section 100 items). On its face, this arrangement is not strictly consistent with clause 14.1(a) of the 5CPA (as outlined in paragraph 6.53), which specifies that: ‘… all Approved Pharmacists are able to obtain timely supply of the full range of PBS medicines …’.344 In addition, the CSO does not cover items that are listed only on the RPBS, or PBS medicines supplied to public and private hospitals that are approved to dispense pharmaceutical benefits.
6.55 To access the CSO Funding Pool, pharmaceutical wholesalers must tender for registration as CSO Distributors345, and enter into a Deed of Agreement with Health. The CSO Service standards are to:
- supply to any Community Pharmacy and meet the minimum threshold for sales to rural and remote Community Pharmacies;
- supply any brand of PBS medicine;
- hold stock of at least one brand of every PBS medicine;
- supply any low volume PBS medicine and meet the threshold for sales of low volume PBS medicines;
- supply any PBS medicine at or below the Approved Price to Pharmacist, Claimed Price, or Price Per Unit; and
- supply any brand of PBS Medicine within 24 hours of the regular order cut off time and make available a daily delivery service to Community Pharmacies.346
6.56 There are currently four pharmaceutical wholesalers registered as CSO Distributors, who share a funding pool valued at approximately $190 million per year.347
6.57 Health has largely outsourced the administration of the CSO Funding Pool to a non-government entity, Australian Healthcare Associates (AHA). However, Health continues to make payments to eligible pharmaceutical wholesalers. The costs of the CSO Funding Pool for the first four years of the 5CPA are shown in Table 6.8.
Table 6.8: Costs of the CSO Funding Pool
|
Component |
2010–11 |
2011–12 |
2012–13 |
2013–14 |
|
CSO Funding Pool |
$181 319 306 |
$184 898 274 |
$187 862 437 |
$190 680 539 |
|
CSO Administration Agency |
$1 217 227 |
$1 150 663 |
$1 261 437 |
$1 280 359 |
|
Total actual payments |
$182 536 533 |
$186 048 937 |
$189 123 874 |
$191 960 898 |
|
CSO original budget |
$182 300 000 |
$186 100 000 |
$189 900 000 |
$197 700 000 |
Source: Department of Health information.
6.58 The payment system relies on self-reporting by pharmaceutical wholesalers of their monthly sales volumes to retail pharmacies of PBS items. Pharmaceutical wholesalers are required to deduct sales data relating to the sale of PBS items to hospitals, or over the counter medicines348 that are supplied to retail pharmacies, supermarkets or non-pharmacy suppliers. Although Health receives a range of reports from the AHA on its administration of the CSO, Health does not currently report any performance information relating to the CSO Funding Pool. Health advised the ANAO that:
The CSO is a supply driven remuneration arrangement with each wholesaler and information such as payments and market share percentages are treated as commercially confidential, and are therefore not made publicly available.
6.59 In April 2009, in the context of seeking approval to commence 5CPA negotiations, Health advised Ministers that pharmacies operate in a different market to, and have advantages not enjoyed by, other retail businesses, such as the CSO. The CSO generally ensures delivery of PBS medicines to retail pharmacies within 24 hours, and alleviates the burden of maintaining significant levels of PBS stock.349 While the 5CPA provides $949.5 million for the CSO Funding Pool, the department has not assessed—and as discussed below, does not plan to assess—whether the CSO has made any significant difference to the timeliness of PBS deliveries to pharmacies, or whether it has led to any significant reduction in pharmacies’ stock holding levels as originally advised to Ministers.
Evaluation framework for the 5CPA
6.60 Clause 34.1 of the 5CPA specifies that the parties to the agreement will participate in a review of the agreement prior to its expiry in June 2015, to inform the negotiations for any subsequent agreement.350
6.61 The 5CPA Evaluation Framework was released publicly by Health in December 2011, and is summarised in Figure 6.4. The Framework states that:
This Framework provides a structure for the review of the Fifth Agreement, incorporating independent evaluations of its component parts. The Framework will be used by the Department, the Guild and the ACC to provide guidance on reviewing, monitoring and evaluating the Fifth Agreement.
Figure 6.4: Evaluation framework for 5CPA and its components

Source: Evaluation Framework for the Fifth Community Pharmacy Agreement December 2011.
6.62 The Framework indicates that the review will be at a high whole-of-agreement level, with preliminary findings to be available at the commencement of negotiations for the next agreement, in approximately July 2014.351 While the Framework scheduled the initial surveys (six in total) to be conducted at the mid-point of the agreement (December 2013), in November 2013 Health advised the ANAO that a survey would not get underway until early 2014. In October 2014, Health advised the ANAO that all evaluations scheduled to be undertaken were either completed or in progress.
Stakeholder views on the evaluation framework
6.63 Stakeholders, including those represented on the PRG, have expressed some dissatisfaction with the 5CPA evaluation framework. The release of the framework some 18 months after the 5CPA was signed was considered to be late, as evaluation should have been part of the development of 5CPA professional programs. Other concerns expressed by stakeholders related to:
- (in effect) self-evaluation by the Agreement Consultative Committee (ACC)352—an approach considered to introduce potential for conflicts of interest and which may inhibit objective and independent evaluation;
- the role of members of the Programs Reference Group (PRG)353—a number of whom considered that their advice on the evaluation framework had not been appropriately considered, as the framework was finalised before their input was received;
- data collection—a number of stakeholders considered that very little data was being collected and analysed to inform evaluation of 5CPA programs;
- the focus of evaluation activity—the evaluation framework was considered to have a minimal focus on patient and consumer outcomes; and
- the rigour of evaluation activity—some of the 4CPA evaluations lacked the rigor or coverage that was considered necessary, and their recommendations were not implemented in the design of 5CPA programs.
Review of professional programs
6.64 The evaluation of the 5CPA professional programs, as detailed in the 5CPA Evaluation Framework, is divided into two types of activity: program specific reviews; and thematic reviews relating to consumer experience, access to PBS medicines and the Quality Use of Medicines. The program specific reviews will take the form of either implementation reviews or progress/outcome reviews. Candidates for the implementation reviews are the new programs introduced under the 5CPA and 5CPA projects, whilst progress/outcome reviews will be completed on some of the continuing programs from the 4CPA.
6.65 As at October 2014, two independent 5CPA evaluations had been commissioned by Health: the baseline data collection and assessment of Continued Dispensing and Medication Charts, which was completed by Urbis in November 2012; and the Deloitte evaluation of the MedsCheck and Diabetes MedsCheck pilot program, also completed in 2012.354 A review of Medication Management programs commenced in July 2013 and was ongoing as at 1 October 2014. A scoping paper for the review of 5CPA governance was also produced for provision to the ACC in October 2013. In October 2014, Health advised the ANAO that:
a review of Governance commenced in August 2014, and a combined thematic review of Access, Quality Use of Medicines and Consumer Experience commended in August 2014. These reviews are currently ongoing and due to complete prior to conclusion of the 5CPA in June 2014.
No review of pharmacy remuneration or CSO Funding Pool
6.66 The Evaluation Framework states that no reviews will be conducted of: pharmacy remuneration ($13.8 billion); medicine supply via wholesalers (CSO Funding Pool—$950 million); and the Pharmacy Location Rules.355
6.67 The Location Rules were considered by the 2014 Commission of Audit and the National Competition Review.356
6.68 While pharmacy remuneration for dispensing pharmaceutical benefits accounts for 90 per cent of 5CPA funding—some $13.8 billion—and there has been no review of pharmacy remuneration since 1989, Health has no plan to review the largest material component of the current agreement. Similarly, there is no plan to review the second largest material component of the 5CPA, medicine supply via wholesalers (CSO Funding Pool) valued at $950 million.
6.69 The substantial levels of Commonwealth funding provided under the 5CPA, the complexity of the administrative and program arrangements, and the issues raised by stakeholders, discussed above, suggest that the 5CPA required a commensurate level of evaluation and review, to provide: a sound basis for assessing performance and value for money; and for the purposes of planning and negotiating the next pharmacy agreement.
Conclusion
6.70 Shortcomings in Health’s performance reporting and 5CPA evaluation framework mean that the department is not well positioned to assess whether the Commonwealth is receiving value for money from the agreement overall, or performance against its six principles and objectives.
6.71 In its annual report, the department aggregates the cost to government of pharmacy remuneration (comprising expenditure on services) with the cost to government of PBS medicines (comprising expenditure on products), without differentiating between the two types of expenditure. A consequence of this approach is that there is no ready basis for the Parliament or other stakeholders to determine the actual cost of pharmacy remuneration delivered under the 5CPA. To improve transparency and the quality of program performance reporting, the department should report annually on the actual cost of each major component of the 5CPA and the next community pharmacy agreement, including pharmacy remuneration, CSO wholesaler payments and professional programs.
6.72 The 5CPA is a key element in the delivery of the department’s Program 2.1 and Program 2.2. Health’s KPIs have limited alignment with the six objectives specified in the 5CPA. In particular, the KPIs developed for Programs 2.1 and 2.2 do not permit an assessment of the 5CPA’s high level objectives, which relate to the accessibility, efficiency and viability of retail pharmacy and the need for 5CPA programs to achieve positive health outcomes for the community. There is scope to review the overall performance reporting framework, to improve alignment between the next community pharmacy agreement and public reporting against the costs, program objectives, deliverables and KPIs relating to Programs 2.1 and 2.2. The development of an appropriate and well aligned suite of performance measures, focusing particularly on effectiveness, can help government entities assess: the impact of policies and programs; adjust management approaches as required; and provide advice to government on the successes, shortcomings and/or options for revision to current policies. In addition to facilitating informed decision-making on the allocation and use of public resources, effective performance measurement and reporting enables the Parliament and other stakeholders to assess performance.
6.73 While some aspects of the 5CPA will be evaluated, Health’s 5CPA evaluation framework does not make provision for reviews of the agreement’s two major financial components—pharmacy remuneration ($13.8 billion) and Community Service Obligation (CSO) payments to pharmaceutical wholesalers ($950 million). Pharmacy remuneration, which lies at the heart of the 5CPA and previous community pharmacy agreements—accounting for some 90 per cent of funding delivered under the current agreement—has not been fully reviewed since 1989.
Appendices
Appendices
Please refer to the attached PDF for the Appendices:
- Appendix 1: Entity Responses
- Appendix 2: Example of PBS Pricing
- Appendix 3: Authorised Prescribers and Approved Suppliers under the PBS
- Appendix 4: 5CPA Savings and Spending Measures
- Appendix 5: Distribution of Under and Over Co-payment Pharmacy Remuneration 201213 and 2013–14
- Appendix 6: Subsidised PBS and RPBS Items Per Person Per Year from 2002 to 2014
- Appendix 7: 5CPA Professional Programs Budget and Actual Expenditure
- Appendix 8: 5CPA Professional Programs Deliverables
- Appendix 9: QCPP Elements
- Appendix 10: Pharmacy Remuneration Data Tables 1991 to 2014
- Appendix 11: Number of unique pharmacies per year
Abbreviations
|
4CPA |
Fourth Community Pharmacy Agreement |
|
5CPA |
Fifth Community Pharmacy Agreement |
|
ACC |
Agreement Consultative Committee |
|
ACPA |
Australian Community Pharmacy Authority |
|
AHA |
Australian Healthcare Associates |
|
ANAO |
Australian National Audit Office |
|
CSO |
Community Service Obligation |
|
DVA |
Department of Veterans’ Affairs |
|
EFC |
Efficient Funding of Chemotherapy |
|
ERRCD |
Electronic Recording and Reporting of Controlled Drugs |
|
EPF |
Electronic Prescription Fee |
|
ERC |
Expenditure Review Committee |
|
Finance |
Department of Finance |
|
Health |
Department of Health |
|
HMR |
Home Medicines Review |
|
HSD |
Highly Specialised Drugs |
|
Human Services |
Department of Human Services |
|
PBRT |
Pharmaceutical Benefits Remuneration Tribunal |
|
PBS |
Pharmaceutical Benefits Scheme |
|
PES |
Prescription Exchange Service |
|
PFDI |
Premium Free Dispensing Incentive |
|
Pharmacy Guild |
The Pharmacy Guild of Australia |
|
PhRANCIS |
Pharmacy Remuneration and Negotiation Cost Information System |
|
PPIs |
Pharmacy Practice Incentives |
|
PRG |
Programs Reference Group |
|
PSA |
Pharmaceutical Society of Australia |
|
QCPP |
Quality Care Pharmacy Program |
|
RMMR |
Residential Medication Management Reviews |
|
RPBS |
Repatriation Pharmaceutical Benefits Scheme |
|
S100 |
Section 100 of the National Health Act |
|
SHPA |
The Society of Hospital Pharmacists of Australia |
|
SPBC |
Strategic Priorities and Budget Committee of Cabinet |
|
WCI9 |
Wage Cost Index 9 |
Glossary
|
5CPA |
The Fifth Community Pharmacy Agreement—the fifth in a series of five year Agreements (beginning in 1990) between the Australian Government and the Pharmacy Guild that sets out remuneration arrangements for PBS dispensing (among other things). |
|
ACC |
The Agreement Consultative Committee is the principal governance committee for all elements of the 5CPA. Membership comprises four members from the Pharmacy Guild and four members from the Department of Health. |
|
CSO |
The Community Service Obligation was established under the Fourth Agreement and provides funding for participating pharmaceutical wholesalers to supply PBS medicines to retail pharmacies within 24 hours. |
|
EFC |
Efficient Funding for Chemotherapy arrangements set out remuneration for injectable or infusible chemotherapy drugs under Section 100 of the National Health Act. |
|
FMA Act |
Financial Management and Accountability Act 1997, which was replaced by the Public Governance, Performance and Accountability Act 2013. |
|
The Pharmacy Guild |
The Pharmacy Guild of Australia is a registered employers’ organisation under the Fair Work (Registered Organisations) Act 2009, which represents about 77 per cent of retail pharmacy owners. |
|
HSDs |
Highly Specialised Drugs are a number of expensive drugs funded under the PBS for a number of chronic conditions. They may only be prescribed through public or private hospitals that have access to appropriate specialist facilities. |
|
Location Rules |
Rules governed by legislation which restrict the opening of new pharmacies and the relocation of existing pharmacies. |
|
National Health Act |
The National Health Act 1953. |
|
PGPA Act PBRT |
Public Governance, Performance and Accountability Act 2013 The Pharmaceutical Benefits Remuneration Tribunal is an independent tribunal under the National Health Act that is empowered to determine remuneration for PBS dispensing and gives effect to the 5CPA. |
|
Pharmaceutical Benefit or PBS Item |
A pharmaceutical supplied under the Pharmaceutical Benefits Scheme. |
|
PhARIA |
The Pharmacy Access/Remoteness Index of Australia is a remoteness index (based on ARIA) used by Health to classify pharmacies for the purposes of 5CPA and other pharmacy programs. |
|
PRG |
The Programs Reference Group is an advisory body established under the 5CPA comprised of pharmacy stakeholders that provides advice on 5CPA programs upon request. |
|
Price Disclosure |
Arrangements introduced in 2007 to align the government price of a PBS item more closely with the market price. |
|
Retail pharmacy |
A pharmacy approved under Section 90 of the National Health Act to dispense PBS and RPBS subsidised medicines to the public. |
|
S100 |
Section 100 of the National Health Act allows the Minister to make special arrangements for the supply of pharmaceutical benefits, including funding for HSDs, EFC and allowances to remote area Aboriginal Health Services. |
Footnotes
1 Actual expenditure under the agreements has not been reported publicly. Health advised the ANAO that the actual total costs of community pharmacy agreements (CPAs) were: 1CPA ($3.286 billion); 2CPA ($5.497 billion); 3CPA ($8.804 billion); 4CPA ($12.158 billion). The original cost of the 5CPA as recorded in the agreement was $15.384 billion, which Health has since revised to $15.610 billion.
2 Also known as ‘pharmaceutical benefits’ or ‘PBS items’.
3 The Pharmacy Guild is a registered employers’ organisation, which advised the ANAO that it represents the owners of approximately 77 per cent of the 5457 retail pharmacies currently approved to supply PBS items.
4 Figure 1.3 of this audit report reproduces the terms of the 5CPA relating to funding.
5 Pharmacy remuneration relates only to payment for supplying the medicines, and excludes the ex-manufacturer cost of the medicines.
6 Pharmacy remuneration comprises $11.6 billion provided by government and $2.2 billion provided by patients through co-payments for PBS and RPBS medicines.
7 The 5CPA professional programs range from patient services to pharmacy practice incentives, workforce development, and financial support.
8 Price Disclosure is a system for adjusting the official government price of PBS medicines to reflect the actual market price charged by manufacturers, which is often heavily discounted following patent expiry.
9 Clause 1.2(e) of the 5CPA provides that: ‘The initiatives covered by this Agreement result in $1 billion in savings over the Term of the Agreement against the Commonwealth forward estimates.’
10 On 1 March 2014 a single contract for services took effect between Health and the Pharmacy Guild, replacing some 62 contracts previously in operation.
11 Health continues to manage three projects with a total value of $9 million.
12 For instance, under the $10.6 million Research and Development program, the Pharmacy Guild is funded to identify and fund research and development projects relating to six topic areas.
13 Known as the Agreement Consultative Committee (ACC).
14 The National Commission of Audit recommended opening up the retail pharmacy sector to competition, including through the deregulation of ownership and the Location Rules, in order to achieve more efficient service delivery and the development of alternative retail models—such as pharmacists available to dispense medicines at supermarkets. See Commonwealth of Australia, Towards Responsible Government: The Report of the National Commission of Audit Phase One, February 2014, pp. 112-113.
15 The National Competition Policy Review’s draft report recommended that the pharmacy ownership and the Location Rules be removed in the long term interests of consumers. The draft report noted: ‘The current regulations impose costs on consumers; yet it is not clear how restricting the location of pharmacies or requiring that only pharmacists can own a pharmacy ensures the quality of advice provided to consumers.’ Australian Government, Competition Policy Review Draft Report, September 2014, p. 69, p. 110.
16 There is no formal mechanism in place to reconcile actual expenditures on pharmacy remuneration against funding specified in the 5CPA.
17 Budget Paper No.2, Budget Measures 2010-11, pp. 212-13.
18 The difference of $0.4 billion results from additional funding of $285.5 million for professional programs and $82.6 million to encourage the electronic processing of prescriptions by pharmacies, as agreed by Ministers in the context of settling the 5CPA.
19 Developed for 5CPA planning and negotiating purposes and for the provision of advice to Ministers.
20 On 23 December 2009, Health advised Finance that it had used ‘WCI9 [Wage Cost Index 9] (2%)’ in its costings. However, the official forecast WCI9 indexation factors released by Finance ranged from 1.5 to 1.6 per cent per year. In advice to the ANAO, Finance confirmed that while it had agreed to Health costings that included the use of a 2 per cent indexation factor, it was unclear from Finance records why this value was used. Finance suggested that one likely explanation was that the relevant Finance officer accepted the information received from Health on face value.
21 The ANAO has estimated that as at 30 June 2014, actual pharmacy remuneration delivered under the 5CPA was $10.9 billion, compared to forecast remuneration of $10.8 billion. Actual and original forecast remuneration align closely, with an overall difference of approximately 0.7 percent.
22 The payment was intended to encourage pharmacists to dispense premium free brands in place of brands with a price premium. However, Health implemented the incentive more broadly, and paid the incentive on all premium free brands whether or not they had been substituted for a brand with a price premium.
23 The first estimates variation of $226 million, which was agreed with Finance in January 2011 (some six months into the 5CPA), was due to data adjustments being made for National Health Act Section 100 prescriptions, DVA scripts and PBS growth factors. A further increase of $66 million, which was agreed with Finance in January 2013, was due to the medicine atorvastatin coming off patent.
24 The 5CPA principles and objectives are listed in paragraph 8 of this audit report.
25 Health advised the Senate Community Affairs References Committee in 2013 that its understanding of the cost structures and business models of retail pharmacies remains limited.
26 The last survey into pharmacists’ costs of dispensing was conducted by the Pharmaceutical Benefits Remuneration Tribunal in 1989. A limited review of supply and remuneration arrangements for drugs provided under Section 100 of the National Health Act was finalised in February 2010. Prescriptions for Section 100 items are less than one per cent of PBS dispensing.
27 The $2.2 billion in patient co-payments is only delivered by the Government in the sense that legislation requires patients to make such payments in defined circumstances.
28 The two service providers, known as Prescription Exchange Services, provide the systems for downloading electronic prescriptions. There are two PES providers: eRx (a wholly owned subsidiary of FRED IT, a related entity of the Pharmacy Guild) and MediSecure Limited (supported by the Royal Australian College of General Practitioners).
29 Australian National Audit Office and Department of the Prime Minister and Cabinet, Successful Implementation of Policy Initiatives—Better Practice Guide, Canberra, October 2014, p. 31.
30 Department of Health and Ageing, Annual Report 2013–14, Canberra, 2014, p. 55 (note: at page 63 of the annual report the actual cost is reported as $9.12 billion). Reported PBS expenditures relate to the cost to government of medicines subsidised under the PBS and the cost to government of most components of ‘pharmacy remuneration’ funded by the Australian Government under the 5CPA. Pharmacy remuneration includes: a range of flat-rate and percentage mark-ups on the base cost of medicines; and specified fees for dispensing certain types of prescriptions.
31 Department of Veterans’ Affairs, Annual Report 2013-14, Canberra, 2014, p 91.
32 The Pharmacy Guild is a registered employers’ organisation, which represents the owners of about 77 per cent of the 5457 retail pharmacies currently approved to supply PBS items. Pharmacy Guild of Australia, Summary of Guild Membership and Associates at June 2013, available at: http://www.guild.org.au/docs/default-source/act-branch-public/publications/annual-report/appendices-(5).pdf?sfvrsn=0 [accessed 28 May 2014].
33 Australian Commission on Safety and Quality in Health Care, Windows into Safety and Quality in Health Care 2011 [Internet], available from: <http://www.safetyandquality.gov.au/wp-content/uploads/2011/11/Windows-into-Safety-and-Quality-in-Health-Care-2011.pdf> [accessed 31 January 2014]
34 The Pharmacy Guild of Australia, Guild Digest 2013, Canberra, 2013, p. 4.
35 The ANAO calculated the total number of subsidised and non-subsidised PBS and RPBS prescriptions dispensed from retail pharmacies from Health’s 2012–13 prescription data.
36 A co-payment is a fixed payment that the patient contributes to the cost of a PBS/RPBS medicine.
37 Pharmacy Board of Australia, Guidelines for Dispensing of Medicines, available from: <http://www.pharmacyboard.gov.au/Codes-Guidelines.aspx> [accessed 31 January 2014].
38 In 2011, Australia had the highest per capita consumption of anticholesterols in the OECD and the second highest consumption of antidepressants. Health at a Glance 2013: OECD Indicators, OECD 2013, available at: <http://www.oecd.org/health/health-systems/health-at-a-glance.htm> [accessed 14 July 2014].
39 National Prescribing Service, Older, Wiser, Safer, MedicineWise News, September 2013, available from: <http://www.nps.org.au/publications/health-professional/medicinewise-news> [accessed 17 January 2015].
40 LM Kalisch et. al., The Prescribing Cascade, Australian Prescriber 2011; 34, pp. 162–66.
41 Australian Commission on Safety and Quality in Health Care, Windows into Safety and Quality in Health Care, ACSQHC, 2011, p. 34.
42 E Roughead, S Semple and E Rosenfeld, Literature Review: Medication Safety in Australia, Australian Commission on Safety and Quality in Health Care, August 2013, p. 8.
43 The 5CPA references the Location Rules, which set out location based criteria that must be met in order for the Australian Community Pharmacy Authority to recommend approval of a new pharmacy or relocation of an existing pharmacy that dispenses pharmaceutical benefits.
44 Community pharmacy agreements and the location rules have attracted criticism over time, including on economic and regulatory grounds. See: D Gadiel, Harmacy: The Political Economy of Community Pharmacy in Australia, The Centre for Independent Studies, CIS Policy Monograph 89, 2008; T Barnes, ‘A Prescription for Pharmacy Reform’, Policy, Vol. 27 No. 4, Summer 2011–12; J Albrechtson, ‘Pharmacists’ Cosy Cartel Needs Dose of Reality’, The Australian, 16 April 2014; and Senator D Leyonhjelm, ‘Want a pill that will make you rich?’, The Australian Financial Review, 19 September 2014. For a rebuttal to Senator Leyonhjelm, on behalf of the Pharmacy Guild, see: D Quilty, ‘Leyonhjelm needs a reality check from his fairytale’, The Australian Financial Review, 22 September 2014. The economic and policy issues relating to pharmacy regulation have been the subject of a number of reviews, including the 1999 National Competition Policy Review of Pharmacy (the Wilkinson review), the 2014 National Commission of Audit and the 2014 National Competition Policy Review. See paragraph 1.27.
45 Pharmacy Board of Australia, Pharmacy Registrant Data: March 2014, available from: <http://www.pharmacyboard.gov.au/About/Statistics.aspx> [accessed 30 May 2014].
46 Department of Human Services Annual Report 2013–14.
47 Unpublished Human Services’ data. Friendly Society pharmacies are not-for-profit entities that have owned and operated pharmacies continuously since the 1840s, prior to the introduction of prohibitions on non-pharmacists owning pharmacies. They are managed by registered pharmacists.
48 The term ‘pharmaceutical benefit’ refers to a medicine supplied under the PBS or the RPBS—also known as a ‘PBS item’ or a ‘RPBS item’. A pharmaceutical benefit is not a benefit payment, but a medicine that is provided under the PBS or the RPBS.
49 The Pharmacy Board’s guidelines may be used as evidence of what constitutes appropriate professional conduct or practice for pharmacy in proceedings under the National Law or a law of a coregulatory jurisdiction against a health practitioner.
50 The practice standards of the professional associations are not mandatory unless referenced in the Pharmacy Board of Australia’s professional codes and guidelines.
51 The Pharmacy Guild is a registered employers’ organisation, which represents the owners of about 77 per cent of the 5457 retail pharmacies currently approved to supply PBS items.
52 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia, Canberra, 2010.
53 ANAO estimate. The $13.8 billion does not include pharmacy remuneration from under co-payment (unsubsidised) PBS prescriptions, which are paid entirely by patients, nor does it include private prescriptions or remuneration from other sources.
54 ‘Pharmacy remuneration’ as set out in the 5CPA includes ‘wholesale mark-up’, although this is a notional amount. Health advised the ANAO that the amount actually paid by pharmacies to wholesalers is a matter between those parties.
55 Pharmacies may be paid other fees under the PBS—such as wastage factors applying to broken packs, container fees and diluent fees. A diluent is a diluting agent. These fees are not directly mentioned in the 5CPA.
56 A list of PBS authorised prescribers and suppliers is at Appendix 3.
57 Not all drugs listed on the PBS are ‘prescription only’. Some are classified as ‘over the counter’ and may be sold in pharmacies or supermarkets without a prescription. However, the PBS rules require that to qualify for a subsidy, the item must generally be prescribed by an authorised prescriber. In limited circumstances (such as an emergency), a pharmacist may dispense a PBS item without a prescription.
58 Department of Health, Browse the PBS, available at: http://www.pbs.gov.au/browse/body-system [accessed 28 May 2014].
59 The patient co-payment is set by the Australian Government. A patient may also be charged a price premium or a special patient contribution for certain PBS items, see Department of Human Services, Pricing of PBS Medicine, available at: <http://www.medicareaustralia.gov.au/provider/pbs/pharmacists/pricing.js…> [accessed 7 February 2014]
60 Department of Human Services, Pricing of PBS Medicine, [Internet].
61 A drug is the active component of a pharmaceutical.
62 Health advised the ANAO that these figures were as at 1 September 2014.
63 The approval is made under Section 90 of the National Health Act. Pharmacy premises that have been approved for the purposes of dispensing pharmaceutical benefits may be referred to as ‘Section 90 pharmacies’ or, more generally, as ‘approved pharmacies’. The owners of Section 90 approved pharmacies are termed ‘approved pharmacists’ under the National Health Act.
64 The Commonwealth does not require pharmacy owners to work in any pharmacy that they own. The Commonwealth approval number is unique to the pharmacy and to the owner/s.
65 Urbis, Review of the Pharmacy Location Rules under the Fourth Community Pharmacy Agreement June 2010 Final Report, Urbis, Canberra, 2010, p. 1.
66 ibid.
67 Department of Human Services Annual Report 2013–14, available at: <http://www.humanservices.gov.au/corporate/publications-and-resources/annual-report/resources/1314/chapter-04/pharmaceutical-benefits-scheme> [accessed 13 November 2014].
68 Department of Health, Pharmacy Location Rules Applicants Handbook, p. 4, available at: <http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pbs-general-pharmacy-acpa-acpaintro.htm> [accessed 3 June 2014].
69 Human Services receives pharmacists’ applications to supply pharmaceutical benefits from particular premises, issues registration numbers, refers applications to ACPA, makes approvals on behalf of the Health Secretary on the recommendation of ACPA, and maintains the pharmacy approvals database.
70 The National Commission of Audit recommended opening up the retail pharmacy sector to competition, including through the deregulation of ownership and location rules, in order to achieve more efficient service delivery and the development of alternative retail models—such as pharmacists available to dispense medicines at supermarkets. See Commonwealth of Australia, Towards Responsible Government: The Report of the National Commission of Audit Phase One, February 2014, pp. 112-113, available at: http://www.ncoa.gov.au/report/index.html [accessed 13 November 2014].
71 The National Competition Policy Review’s draft report recommended that the pharmacy ownership and location rules be removed in the long term interests of consumers. The draft report noted: ‘The current regulations impose costs on consumers; yet it is not clear how restricting the location of pharmacies or requiring that only pharmacists can own a pharmacy ensures the quality of advice provided to consumers.’ Australian Government, Competition Policy Review Draft Report, September 2014, p. 69, p. 110, available at:<http://competitionpolicyreview.gov.au/draft-report/> [accessed 13 November 2014].
72 Department of Health and Ageing, Community Service Obligation Funding Pool–Operational Guidelines, July 2012, available from <https://www.health.gov.au/internet/main/publishing.nsf/ Content/BE2CB73EF2EE20D4CA257BF0001DC53C/$File/CSO%20Operational%20Guidelines%20July%202012.pdf> [Accessed 29 August 2014].
73 Health advised the ANAO that ‘Medication Continuance’ is now formally known under legislation as ‘Continued Dispensing’.
74 While the 5CPA refers to this program as ‘Electronic recording of controlled drugs’, the 5CPA website refers to it as the ‘Electronic Recording and Reporting of Controlled Drugs (ERRCD) initiative’.
75 For Section 100 items, the term ‘dispensed price’ is used.
76 The Financial Framework Legislation Amendment Act (No. 3) 2012 (the Act), which commenced on 28 June 2012, was enacted in response to the High Court decision of 20 June 2012 in Williams v Commonwealth of Australia (Williams No.1), which related to the validity of Commonwealth spending programs not supported by legislation other than an appropriation Act. The Act amended the Financial Management and Accountability Act 1997 (the FMA Act) with the purpose of establishing legislative authority for the Commonwealth to make payments in relation to particular programs, grants and arrangements; and transitional provisions were included in the Act with the purpose of protecting programs, grants and arrangements in place before the Act commenced. The Act also amended the Financial Management and Accountability Regulations 1997 (FMA Regulations) to include a new schedule, which specified relevant grants and programs drawing legislative authority from the FMA Act for payments, where such authority did not otherwise exist. Schedule 1AA, Part 4 (item 415.007) of the FMA Regulations specified ‘Community pharmacy and pharmaceutical awareness’ intended to ‘provide funding to improve access to medicines and pharmacy services’. From 1 July 2014 the Financial Framework (Supplementary Powers) Act 1997 replaced the FMA Act for this purpose.
77 Part 5 of the Repatriation Pharmaceutical Benefit Scheme Instrument 2013 No.R43.
78 The Health Secretary has delegated this function to the Secretary of Human Services.
79 From 1 July 2014 the Financial Framework (Supplementary Powers) Act 1997 replaced the FMA Act for this purpose—see footnote 81.
80 The Pharmacy Guild of Australia and the Department of Health, Standards and Guidelines [Internet], available from: <http://5cpa.com.au/resources/standards-and-guidelines/> [accessed 26 March 2014].
81 From 1 July 2014 the Financial Framework (Supplementary Powers) Act 1997 replaced the FMA Act for this purpose—see footnote 81.
82 Health has entered into a number of further contracts and agreements with other entities. As discussed in paragraph 5.25, this audit identified some 310 contracts relating to the 5CPA.
83 Health continues to manage three projects with a total value of $9 million.
84 For instance, the Guild made representations, supported by research it had commissioned, to the Competition Policy Review. See ‘Media release: New analysis confirms benefits of community pharmacy model’, 18 November 2014, available at: <http://www.guild.org.au/docs/default-source/public-documents/news-and-events/media-releases/2014/mr_competition_response_ 18november2014.pdf?sfvrsn=2> [accessed 18 November 2014].
85 A recent national advertising campaign, launched in October 2014, is ‘Discover More. Ask Your Pharmacist’. See http://www.guild.org.au/news-events/consumer-campaign. [accessed 11 November 2014].
86 A Commonwealth grant is defined in the Commonwealth Grant Rules and Guidelines as an arrangement for the provision of financial assistance by the Commonwealth or on behalf of the Commonwealth (paragraph 2.3, p. 6).
87 For instance, under the $10.6 million Research and Development program, the Pharmacy Guild is funded to identify and fund research and development projects relating to six topic areas.
88 For instance, the Pharmacy Guild owned 50 per cent of FRED IT, a provider of pharmacy software, until 2013.
89 Known as the Agreement Consultative Committee (ACC).
91 The ANAO provided Health with detailed discussion papers and findings for comment in April 2014, September 2014 and December 2014, including potential recommendations. Extracts of ANAO discussion papers were also provided to affected entities in the course of the audit. The proposed audit report was provided to Health in January 2015 and extracts were provided to Human Services, DVA, the Department of Finance, the Pharmacy Guild and MediSecure Limited in January 2015.
92 The department advised the ANAO that negotiations commenced in July 2009, while the Pharmacy Guild advised the ANAO that negotiations commenced in September 2009.
93 Health’s consultation with stakeholders is discussed later in this chapter.
94 Health subsequently advised the ANAO that in addition to the 4CPA evaluations, a number of reviews were undertaken during the 4CPA. Of the eleven reviews planned under the 4CPA, Health reported that seven were completed, but only six reviews had final reports. Of these six reviews, five were completed before the 5CPA was signed and one was completed afterwards. Health advised the ANAO that the 4CPA Research and Development program also informed the development of the 5CPA.
95 The 4CPA had two levels of wholesaler mark-up; six levels of pharmacy mark-up; and several other fees and incentive payments.
96 Health advised the ANAO that the lack of technical capability related to the limitations of available software at the time.
97 Fifth Community Pharmacy Agreement Departmental Workshop —Session 1 (PBD) Minutes of meeting dated 1 October 2008, Agenda Item 5.4.
98 Health was considering a range of reforms to Price Disclosure for PBS medicines. The changes that were eventually adopted were called Expanded and Accelerated Price Disclosure (EAPD). This audit report refers to the options under consideration at the time as ‘expanded Price Disclosure’.
99 Price Disclosure is not part of the 5CPA, but affects pharmacy remuneration as it reduces the Government agreed price for PBS medicines, which in turn, reduces the wholesale and pharmacy mark-ups paid on PBS medicines. Health advised the ANAO that it was considering options to ‘improve the sustainability of the PBS, including enhancements to Price Disclosure and other statutory pricing arrangements’.
100 The ANAO estimates that in 2010–11, approximately 1.67 per cent of all PBS and RPBS subsidised (over co-payment) prescriptions were dispensed by suppliers other than retail pharmacies. ‘Section 85 items’ are PBS items listed in the General Pharmaceutical Benefits section of the Pharmaceutical Benefits Schedule, and account for approximately 98.7 per cent of all PBS and RPBS prescriptions. Section 85 of the National Health Act refers to the declaration of medicines listed on the PBS.
101 ‘Section 100 items’ are PBS items that are supplied through ‘special arrangements’ under Section 100 of the National Health Act, and account for approximately 0.3 per cent of all PBS and RPBS prescriptions. Section 100 items generally have a different pricing structure to Section 85 items.
102 Following price disclosure reductions in 2012, some chemotherapy providers signalled that they would be unable to continue providing their services under the new pricing arrangements, and subsequently, chemotherapy remuneration was significantly increased. This issue, which was the subject of a parliamentary inquiry, is discussed further in paragraphs 3.7 to 3.9.
103 In August 2009.
104 In the course of the audit Health advised the ANAO that the actual cost of the 4CPA was estimated to be $12.158 billion, comprising: $11.041 billion for pharmacy remuneration (including wholesaler mark-up); $663 million for CSO wholesaler payments; and $453 for 4CPA professional programs.
105 In addition to pharmacy and wholesaler remuneration, the 5CPA $16.0 billion baseline figure included the costs of the CSO funding pool and professional programs.
106 As noted in paragraph 2.30, Health advised the ANAO that the PBS forward estimates for the 5CPA were only amended to reflect the agreed changes from 4CPA arrangements, and did not include the cost of patient co-payments.
107 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement, Canberra, 2010, pp. 2–3, [Internet]. Figure 1.3 of this audit report reproduces the terms of the 5CPA relating to funding.
108 The $2.2 billion in patient co-payments is only delivered by the Government in the sense that legislation requires patients to make such payments in defined circumstances.
109 In 2014, the maximum amount paid by a General patient for a pharmaceutical benefit (in the form of a co-payment) was $36.90. Concessional patients paid a maximum of $6.00. Additional costs may be incurred by patients for certain pharmaceutical benefits.
110 [Emphasis added.] Australian Government, Budget Measures: Budget Paper No. 2: Budget 2010–11, Commonwealth of Australia, Canberra, p. 213, available from: <http://www.budget.gov.au/2010-11/content/bp2/html/bp2_expense-13.htm> [accessed 1 March 2014].
111 WCI9 is produced by the Department of Finance using data from the Australian Bureau of Statistics and the Treasury. WCI9 is weighted as: 75 per cent wage cost component and 25 per cent CPI through the year growth to the previous March.
112 WCI9 had been used as the indexation factor for the dispensing fee since the commencement of the 3CPA in 2000, and continued throughout the 4CPA and 5CPA. On 24 December 2009, the Health Minister exchanged a letter of intent with the Pharmacy Guild, which agreed that indexation for dispensing under the 5CA would continue with the same general structure as under the 4CPA. In April 2010, Health advised Ministers that the dispensing fee had been routinely indexed on 1 July each year using WCI9, and that to achieve specified savings, WCI9 indexation would not be applied to the first two years of the 5CPA.
113 Health’s analysis indicated that the structural adjustment package had more than compensated pharmacy for the flow-on costs of price reductions under Price Disclosure; with funding for pharmacies under the 4CPA estimated to be approximately 8 per cent higher than would have been the case had there been no pricing reform. The structural adjustment package was also intended to be time limited, and the 4CPA was amended to allow the elements of the package to continue until 30 June 2011 (the end of the first year of the 5CPA).
114 This issue is discussed further in paragraphs 2.85 to 2.101.
115 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, paragraph 1(e), p. 2.
116 Budget Paper No.2, Budget Measures 2010-11, pp. 212-13.
117 The difference of $0.4 billion results from additional funding of $285.5 million for professional programs and $82.6 million to encourage the electronic processing of prescriptions by pharmacies, as agreed by Ministers in the context of settling the 5CPA.
118 Other 5CPA savings measures reported in the 2010-11 Commonwealth Budget were: re-structuring private hospital pharmacy remuneration ($35.3 million); and cessation of the Western Australian Freight Allowance ($5.3 million). Estimated 5CPA pharmacy remuneration was not reduced by $35.3 million, although pharmacy remuneration for most PBS private hospital prescriptions (Section 85 items) was included in the $13 771.6 million estimate of pharmacy remuneration.
119 As reported in Commonwealth Budget Paper No. 2, Budget Measures 2010–11, p. 213.
120 The PBS Online system was introduced by Medicare in 2005. Prior to the implementation of the incentive, around 365 pharmacies out of about 5000 pharmacies were using PBS Online. Medicare was incorporated into the Department of Human Services in 2011.
121 Under the then Commonwealth Budget rules, Lapsing Program Reviews (LPR) were required for program measures which did not have a termination date and needed ERC or Cabinet reconsideration before they could be extended. There was no record of an LPR being conducted for this measure, or Ministers re-considering the extension of the measure.
122 Emphasis added.
123 Although not specifically mentioned in departmental advice to Ministers on the 5CPA, the PBS Online incentive was a lapsing measure. Under the then Commonwealth Budget rules, Lapsing Program Reviews (LPR) were required for program measures which did not have a termination date and needed ERC or Cabinet re-consideration before they could be extended.
124 Health also estimated that an additional $14.6 million in savings would be achieved due to patients taking longer to qualify for the Safety Net due to lower PBS prices.
125 Not including the overestimate of savings from the flow-on effect to the Safety Net.
126 See paragraph 2.29.
127 See paragraph 2.15.
128 Budget Paper No. 2, Budget Measures, 2010–11.
129 See paragraph 2.30.
130 See paragraph 2.40.
131 Office of Best Practice Regulation, Best Practice Regulation Report 2009-10, OBPR, Canberra, available from: <http://www.dpmc.gov.au/deregulation/obpr/reporting-publications/publications/report-09-10/index.cfm> [accessed 24 February 2014].
132 Office of Best Practice Regulation, OBPR Guidance Note: Post-implementation Reviews [Internet], OBPR, p. 8, available from: <http://ris.dpmc.gov.au/files/2012/03/pir_guidance_note.pdf> [accessed 24 February 2014].
133 Available at: <http://ris.dpmc.gov.au/> [accessed 2 December 2014].
134 A Letter of Intent was co-signed by the Guild on 23 December 2009 and the Health Minister on 24 December 2009.
135 Consumers Health Forum of Australia, Fifth Community Pharmacy Agreement National Consumer Consultation Workshop 18 February 2010 Report, March 2010.
136 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia, Canberra, 2010, Clause 1.2(c), available from [Internet].
137 While Section 98BAA(1) of the National Health Act provides for the Health Minister (acting on behalf of the Commonwealth) and the Pharmacy Guild to enter into an agreement about how the Commonwealth price for pharmaceutical benefits is to be worked out, the Act does not prevent the Commonwealth from consulting, negotiating and reaching agreement with other parties—including peak pharmacy bodies
138 Departmental records examined by the ANAO contain a one page minute relating to discussions on 28 January 2010.
139 Shortcomings in departmental record-keeping at times affected the conduct of this performance audit.
140 One dispute, discussed at paragraph 3.7 of this audit, related to whether agreement had been reached on remuneration arrangements for dispensing chemotherapy infusions.
141 Department of the Prime Minister and Cabinet and Australian National Audit Office, Implementation of Programme and Policy Initiatives: Making Implementation Matter, Better Practice Guide, October 2006, p. 19.
142 For long-term projects, strategic implementation plans are commonly prepared to provide structure to the implementation effort, prioritise activity and help maintain momentum. The ANAO similarly observed that Health did not develop an implementation plan for its recent implementation of diagnostic imaging reforms. See ANAO Performance Audit Report No.12 2014-15, Diagnostic Imaging Reforms.
143 As noted in paragraph 2.69, the consumer consultation did not commence until after the main elements of the 5CPA had been settled in a Letter of Intent, exchanged between the Health Minister and the Pharmacy Guild. Health received the CHF’s 5CPA Consumer Consultation Project report 24 days after the 5CPA was signed.
144 In establishing the original savings target, Ministers agreed that the savings from the 5CPA would be exclusive of the impact of further reform to Price Disclosure (that was under consideration at the time), and the implementation of more appropriate remuneration for chemotherapy infusions.
145 Shortfalls in achieving the savings target were discussed in paragraph 2.40.
146 The Pharmacy Guild wished to retain all elements of the 4CPA transitional structural adjustment package apart from the $0.40 PBS Online payment, which the Guild had identified as a $431 million savings measure.
147 During the audit, Health advised the ANAO that its forecast, at the time, of the impact of proposed further PBS reforms (revised Price Disclosure) on retail pharmacy would be savings of $1816.8 million comprising a reduction in: wholesale and pharmacy mark-ups of $299.7 million (of which the Pharmacy Guild agreed that retail pharmacy would accept a $68.7 million reduction in remuneration, and sought compensation of $231 million); and a reduction in ex-manufacturer prices of $1517.1 million (which the Pharmacy Guild claimed would reduce the discounts received by pharmacies by $396 million, and sought compensation of $46 million). The figure of $277 million was the aggregate sum of $231 million and $46 million. In its advice to the ANAO, the Pharmacy Guild indicated that the $396 million was an estimate provided to the Guild by Health, based on the department’s modelling.
148 Until October 2013, the Pharmacy Guild was a 50 per cent owner of Fred IT Group, which owns eRx Script Exchange, one of the two electronic Prescription Exchange Services in Australia. Fred IT Group states that it has: ‘grown over the past 21 years to be Australia’s largest dedicated provider of business and professional solutions to the retail and pharmacy industries’, available from: <https://www.fred.com.au/> [accessed 21 March 2014].
149 Health and the Pharmacy Guild advised the ANAO that in the course of negotiations, Health had advised the Guild that the forecast savings from revised Price Disclosure arrangements was approximately $1816.8 million. However, Health subsequently advised the ANAO that these figures were only the savings to government (or 83.5 per cent of the total forecast savings of revised Price Disclosure). The full savings from revised Price Disclosure was forecast to be approximately $2175.8 million, comprising a reduction in: wholesale and pharmacy mark-ups of $358.9 million; and a reduction in ex-manufacturer prices of $1816.9 million.
150 The risks of inadequate record keeping for accountability and other purposes were discussed at paragraph 2.76 of this audit.
151 See ANAO Audit Report No. 26 2013–14, Medicare Compliance Audits, p. 23, paragraph 30.
152 There are two PES providers: eRx (a wholly owned subsidiary of FRED IT, which was 50 per cent owned by the Pharmacy Guild) and MediSecure Limited (supported by the Royal Australian College of General Practitioners).
153 This issue is discussed further in Chapter 3.
154 See footnote 156.
155 The 5CPA definition of pharmacy remuneration is reproduced in Figure 1.3 of this audit report.
156 Pharmacies receive higher remuneration for more expensive medicines because the wholesale mark-up and pharmacy mark-up are generally based on a stepped percentage mark-up of the relevant base price, and are only capped at a flat fee for the highest price bracket.
157 The term ‘pharmacy remuneration’ is not defined in the National Health Act. The components of pharmacy remuneration are specified in the 5CPA.
158 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia, Canberra, 2010, [Internet].
159 An explanation of PBS pricing, including the PBS dispensed price, may be found at: Department of Human Service, Pricing of PBS Medicine, available at: <http://www.medicareaustralia.gov.au/provider/pbs/pharmacists/pricing.js…> [accessed 21 October 2014].
160 Health advised the ANAO that 5CPA ‘pharmacy remuneration’ does not include other PBS related remuneration to pharmacies, such as discretionary additional fees, safety card issue fees and container fees, which add to overall pharmacy remuneration.
161 Senate Community Affairs References Committee, Supply of chemotherapy drugs such as Docetaxel May 2013, available from: <http://www.health.gov.au/internet/main/publishing.nsf/Content/ chemotherapy-review/$File/appendix-b.pdf> [accessed 8 April 2014].
162 ibid. Health advised the Senate Committee that the Pharmacy Guild was informed of changes to Price Disclosure in the context of the 5CPA negotiations.
163 Health advised the ANAO that: ‘The Parliamentary inquiry mentioned supported Health’s view regarding Price Disclosure and Chemotherapy infusions were included in negotiations as part of the 5CPA.’
164 The Minister made her determination under Section 100 of the National Health Act.
165 The role of the Tribunal is discussed in Chapter 1, Table 1.1.
166 The Tribunal made its determination under Section 98B(1)(a) of the National Health Act.
167 Australian Government, ComLaw: Legislative Instruments, available at: <http://www.comlaw.gov.au/Browse/ByTitle/LegislativeInstruments/Current> [accessed 11 March 2014].
168 Item 415.0007 of Schedule 1AA, FMA Regulations. See Chapter 1, footnote 81.
169 The proposed fee was a 15 cent per script incentive payment to pharmacies to offset the 25 cent per script cost of downloading electronic prescriptions from a Prescription Exchange Service.
170 AGS considered that payments provided for under the National Health Act are clearly ‘pharmaceutical benefits’ under section 51 (xxiiiA) of the Constitution, and by including the EPF in the Commonwealth price, the EPF would become part of a permissible pharmaceutical benefit payment for constitutional purposes.
171 The constitutional validity of Commonwealth payments relying on the executive power of the Commonwealth was challenged in the two ‘Williams’ cases heard by the High Court in 2012 and 2014.
172 Sections 26 and 28 of the Repatriation Pharmaceutical Benefit Scheme Instrument 2013 No.R43.
173 A sponsor is a person or company who does one or more of the following: exports therapeutic goods from Australia; imports therapeutic goods into Australia; manufactures therapeutic goods for supply in Australia or elsewhere; arranges for another party to import, export or manufacture therapeutic goods. See: Department of Health, Therapeutics Goods Administration, Role of the sponsor, https://www.tga.gov.au/role-sponsor [accessed 14 November 2014].
174 The ex-manufacturer price and ‘price to pharmacist’ are based on the price for the maximum quantity of the item as listed in the PBS Schedule. In addition, where the maximum quantity does not correspond to the manufacturer’s pack size, Health provides the price for a single pack incorporating the wholesale mark-up and the pharmacy mark-up (refer Section 3 of the PBS Schedule for further detail). Health advised the ANAO that its data files contain ‘comprehensive listing and pricing information for each item on the PBS and RPBS Schedules’.
175 Department of Human Services, Service Delivery Reform: Transforming government service delivery, DHS, Canberra, p. 4, available from: <http://www.humanservices.gov.au/spw/corporate/about-us/resources/service-delivery-reform-overview.pdf> [accessed 11 March 2014].
176 Department of Health and Ageing and Department of Human Services, Business Agreement Between the Department of Health and Ageing and Department of Human Services relating to the Pharmaceutical Benefit Scheme, DHS and Health, Canberra, Schedule A, p. 3.
177 Also known as the ‘price to pharmacist’.
178 Health advised that prior to 1 October 2012, the legislative instrument that implemented 5CPA pharmacy remuneration (a section 98(B1) determination under the National Health Act) did not include the wholesaler mark-up. This was added when the National Health Act was amended to use ex manufacturer price rather than price to pharmacist as the basis for price agreements between the Minister and the Responsible Person (sponsor). Prior to 1 October 2012, the wholesale mark-up was only included in the 5CPA.
179 The maximum quantity as specified in the PBS and RPBS Schedules.
180 General pharmaceutical benefits are determined by the Minister for Health under Section 85 of the National Health Act 1953, on the advice of the Pharmaceutical Benefits Advisory Committee.
181 ANAO analysis of Human Services data for over co-payment and under co-payment prescriptions.
182 Section 100 Highly Specialised Drugs (HSDs) and Efficient Funding of Chemotherapy (EFC), have different pricing structures.
183 The PFDI was introduced in the 4CPA and the EPF was introduced in the 5CPA.
184 A substitutable PBS item has at least one alternative bioequivalent PBS brand. Department of Health, How Pharmacists Claim Reimbursement: Information Required [Internet], available from: <http://www.pbs.gov.au/info/healthpro/explanatory-notes/section1/Section_1_7_Explanatory_Notes> [accessed 11 March 2014].
185 Department of Human Services, PBS Reforms, available at: http://www.medicareaustralia.gov.au/ provider/pbs/pharmacists/reforms.jsp#dispensing [accessed 14 July 2014]
186 A related development was that some of the brands entitled to charge a price premium chose to forego their premium in order to match the price of the generic brands. Health advised that consumer demand for premium free medicines had resulted in a response from manufacturers to reduce the number and proportion of brands to which premiums applied. As a consequence, the PFDI was paid on all brands of certain drugs, as no brand of the drug had a price premium.
187 Health’s analysis undertaken in May 2009 had indicated that pharmacists’ substitution rates did not increase significantly following the introduction of the PFDI, and this information was not included in advice of October 2009 to Ministers on the 5CPA negotiations.
188 The Pharmacy Guild advised the ANAO that until September 2013, FRED IT was 50 per cent owned by the Guild, and Guild ownership subsequently dropped to 35 per cent.
189 MediSecure Pty Ltd was the original operating entity up to 1 July 2014.
190 Software vendors sell or lease proprietary software applications to pharmacies.
191 Including under co-payment PBS and RPBS prescriptions.
192 Health advised Ministers that the Pharmacy Guild had agreed that the EPF would only be paid in the following circumstances: for e-prescriptions processed and claimed for by approved suppliers, which were generated electronically by prescribers in accordance with the specifications of the National eHealth Transition Authority (NEHTA); and if NEHTA specifications changed or a Commonwealth approved individual electronic health record became available, the criteria may be reviewed.
193 In October 2014, Health advised the ANAO that the then Health Minister did not consider this proposal due to a change in Minister.
194 Health’s original proposal to the then Health Minister (not approved) was dated 12 December 2011. Health advised the ANAO that a new Health Minister commenced on 14 December 2011. Health re-submitted the proposal dated 15 December 2011 to the new Minister, which was also not approved. Health then submitted a revised proposal dated 30 January 2012 to the new Health Minister. The new Health Minister approved part of Health’s revised proposal on 29 February 2012.
195 Health advised the ANAO that although the study identified issues, these were then actively managed by the department.
196 In addition to 15 cents per prescription paid to pharmacies, bringing the total Electronic Prescription Fee to $1.00 per prescription.
197 In addition to 15 cents per prescription paid to pharmacies, bringing the total Electronic Prescription Fee to 50 cents per prescription.
198 In October 2014, Health advised the ANAO that: ‘EPF was included in a broader e-Health Minute as one facilitator for the viability of e-Health records and as a key part of the implementation of the personally Controlled Electronic Health Records. Due to the development of the e-Health landscape the EPF (incentive) on its own could not deliver end to end interoperability nor encourage prescribers to utilise electronic prescriptions. The Minister decided, under executive power, to utilise EPF funds to improve the update of electronic prescriptions.’
199 The $1.00 EPF was set from 1 July 2012 to 31 December 2012, with 85 cents paid to PESs and 15 cents paid to pharmacies. The EPF was further adjusted to 50 cents from 1 January 2013 to 30 June 2013, with 35 cents paid to PESs and 15 cents paid to pharmacies.
200 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement, Canberra, 2010, p. 2, available from: [Internet]
201 Department of Health, Annual Report 2013–14, Canberra, 2014, p. 63. Department of Veterans’ Affairs, Annual Report 2013–14, Canberra, 2014, p. 90–91.
202 As discussed in Chapter 2, the cost of pharmacy remuneration as specified in the 5CPA is approximately $2.8 billion per year. However, the actual cost to government is approximately $2.3 billion per year if patient co-payments are not included.
203 These are total costs, which include both government payments and patient co-payments.
204 The 5CPA definition of pharmacy remuneration is reproduced in Figure 1.3 of this audit report.
205 When the CSO Funding Pool of $949.5 million is added to 5CPA pharmacy remuneration, the notional wholesaler remuneration accounts for 22 per cent of total pharmacy remuneration under the 5CPA.
206 The ANAO obtained PBS/RPBS payment records from 2010–11 to 2012–13, and extracted payments to retail pharmacies. Payments to all other suppliers (public and private hospitals, dispensing doctors and Aboriginal Health Services) were removed. Retail pharmacy payments included payments for dispensing: Section 85 items; Section 100 items; RPBS Schedule items and nonlisted items.
207 There have always been a small number of prescriptions where Health could not derive the wholesale cost of the medicine from Human Services prescriptions data (such as RPBS prescriptions for unlisted medicines) but the total value of these prescriptions was relatively low.
208 Known formally in the PBS as Section 100 (Efficient Funding of Chemotherapy) items.
209 Health advised the ANAO that the department’s costing model developed for the 6CPA (PhRANCIS 3) will deliver the capacity to revise public financial reporting to include the cost of each major component of pharmacy remuneration in the future.
210 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement, Canberra, 2010, pp. 1– 2, [Internet].
211 Official Committee Hansard, Senate Community Affairs References Committee, Supply of chemotherapy drugs such as docetaxel, 28 March 2013, p. 31.
212 Pharmacies that did not have approval under Section 90 of the National Health Act for at least 364 days per year were excluded.
213 Health advised the ANAO that retail pharmacies dispensing less than 1000 prescriptions per year were not likely to be trading pharmacies.
214 In addition, the Community Service Obligation funding pool for pharmaceutical wholesalers assists retail pharmacies to access PBS medicines in a timely manner at an agreed price. Three 5CPA programs were development projects: ‘Medication Continuance’; ‘Supply and PBS Claiming from a Medication Chart in Residential Aged Care Facilities’; and ‘Electronic Recording of Controlled Drugs’.
215 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement, Canberra, 2010, p. 11, available from [Internet].
216 The conditions of approval for a person as an approved pharmacist are set out in Subsection 92A (1) (a) to (f) of the National Health Act, with paragraph (f) referring to ‘any other condition as determined by the Minister’.
217 Determination No. PB 42 of 2007, National Health (Pharmaceutical Benefits) (Conditions of approval for approved pharmacists) Determination 2007.
218 See <http://www.guild.org.au/docs/default-source/public-documents/news-and-events/media-releases/2014/mr_unapproved_measures_2december14.pdf?sfvrsn=0> [accessed 5 December 2014].
219 A subsidised dispensing service is the dispensing of a PBS or RPBS item priced above the patient co-payment. Health did not receive data on under co-payment PBS or RPBS items prior to April 2012.
220 Health advised the ANAO that patient data prior to 2002–03 was not reliable. The data supporting this analysis is detailed in Appendix 6.
221 Since April 2012, Health has also received data on non-subsidised (under co-payment) PBS/RPBS prescriptions. When under co-payment prescriptions for this cohort are taken into account, the ANAO estimates that in 2013-14 there were, on average, 27 items dispensed per person per year from retail pharmacies, with the highest users of dispensing services under 5CPA arrangements receiving several hundred medicines each. This group of people received at least one subsidised PBS or RPBS prescription during 2012–13.
222 Subsidised medicines are those priced over the patient co-payment. All concessional prescriptions are priced over the patient co-payment as the dispensing fee is higher than the concessional co-payment. The data includes PBS and RPBS scripts, based on date of processing by Human Services. The data supporting this analysis is detailed in Appendix 6.
223 Department of Health and Pharmacy Guild of Australia, Programme Specific Guidelines Pharmacy Practice Incentives (PPI), February 2014, available at: <http://5cpa.com.au/programs/pharmacy-practice-incentives/> [accessed 30 March 2014].
224 Health contracted the Pharmacy Guild to produce the Community Pharmacy Service Charter and Customer Service Statement template.
225 To become accredited, and maintain accreditation, pharmacies are required to pay the Pharmacy Guild: an annual QCPP membership fee; a QCPP assessment fee each time that they are assessed; and the cost of purchasing a QCPP Requirements Manual. Health advised the ANAO that: ‘All matters relating to the operation and fees relating to the QCPP are a matter for the Guild, independent of Government or the Department. The level of the annual QCPP fees were determined and announced by the Guild subsequent to the finalisation of the Fifth Agreement negotiations.’
226 The 18 QCPP elements are set out in Appendix 9 of this audit report.
227 Department of Health and Ageing and the Pharmacy Guild of Australia, Pharmacy Practice Incentives Program Specific Guidelines, Health and the Guild, Canberra, February 2014, available from: <http://5cpa.com.au/programs/pharmacy-practice-incentives/> [accessed 2 April 2014].
228 To be accredited, pharmacies are currently required to provide proof to the QCPP assessor, among other things, that the pharmacy has a system for delivering a program or service, and proof of compliance with any checklist of requirements that applies to the program or service. In the case of the Community Services Support priority area, for instance, a pharmacy must register to provide at least three of the eight Community Support elements. As at 2014, the QCPP manual contained checklists for two of the eight Community Support elements, and procedures for three of the eight Community Support elements.
229 Proxy measures are output-level performance indicators which indirectly measure program effectiveness.
230 Objective 2 of the agreement relates to ensuring that programs are patient-focused and target areas of need in the community.
231 Until 1 March 2014, Human Services administered the PPI Program, prior to administration being transferred to the Pharmacy Guild.
232 Pharmacy dispensing is funded by the Australian Government under the $13.8 billion pharmacy remuneration component of the 5CPA
233 Australian Government and the Pharmacy Guild of Australia, A Stafford et al., IIG-021-VALMER The Economic Value of Home Medicines Reviews, 2009, p. 1, available at: <http://5cpa.com.au/programs/ research-and-development/previous-projects/> [accessed 5 November 2014].
234 Australian Commission on Safety and Quality in Health Care, Windows into Safety and Quality in Health Care, ACSQHC, 2011, p. 34, available from: <http://www.safetyandquality.gov.au/wp-content/uploads/2011/11/Windows-into-Safety-and-Quality-in-Health-Care-2011.pdf> [accessed 31 January 2014].
235 E. Roughead, S. Semple and E. Rosenfeld, Literature Review: Medication Safety in Australia, Australian Commission on Safety and Quality in Health Care, August 2013, p. 8, available from: <http://www.safetyandquality.gov.au/publications/literature-review-medication-safety-in-australia/> [accessed 3 March 2014].
236 Accredited pharmacists have specialised postgraduate training in conducting medication management reviews.
237 The ANAO identified independent studies published in peer-reviewed scientific journals reporting the effectiveness of collaborative, in-home medicines reviews. For example see: R. Castelino et al., Are interventions recommended by pharmacists during Home Medicines Review evidence-based?, Journal of Evaluation in Clinical Practice 2011; 17: 104–110; E. Roughead et al., Collaborative Home Medicines Review delays time to next hospitalization for warfarin associated bleeding in Australian war veterans, Journal of Clinical Pharmacy and Therapeutics 2011; 36: 27–32; E. Roughead et al., The effectiveness of collaborative medicine reviews in delaying time to next hospitalization for patients with heart failure in the practice setting: results of a cohort study, Circulation: Heart Failure 2009; 2: 424–428.
238 Savings occur through a reduction in health resource utilisation, such as fewer hospital admissions and reduced medicine costs. For example: University of South Australia, Veterans’ Medicines Advice and Therapeutics Education Services Final Report: Volume 2 Cost-consequences analysis, August 2010, reported average savings of $631 for each HMR conducted in the veteran cohort. A. Stafford et al., IIG-021-VALMER The Economic Value of Home Medicines Reviews, 2009, available at: [Internet], reported that 16 per cent of HMRs potentially resulted in cost savings greater than $323.80, which completely offset the cost of the HMR service (including GP costs). The latter analysis did not include savings in out-of-pocket costs to consumers.
239 Health’s evidence to support the effectiveness of in-pharmacy medicines reviews was a 2010 report by two UK pharmacy organisations, which examined medicine use reviews by accredited pharmacists (unlike the MedsChecks/Diabetes MedsChecks programs, where pharmacists are not required to be accredited) available at: https://www.npa.co.uk/Documents/Docstore/PCO_LPCs/MUR_support_ evaluation.pdf [accessed 9 June 2014]; and a 2008 UK doctoral thesis, available at: http://gala.gre.ac.uk/6499/ [accessed 9 June 2014].
240 Specifically: Deloitte Access Economics, Evaluation of the MedsCheck and Diabetes MedsCheck Pilot Program, Department of Health and Ageing, July 2012; G Harding G&M Wilcock, Community pharmacists’ perceptions of medicines use reviews and quality assurance by peer review. Pharm World Sci 2010, DOI l0.1007/s11096-010-9381-1; and A Blenkinsopp et al, Medicines Use Review: adoption and spread of a service innovation, Int J Pharm Pract 2008:16.
241 It is not evident from departmental records why the budget allocation for HMRs declined over time.
242 Further details of the Medication Management programs are at Appendix 7 and Appendix 8.
243 5CPA website, Rural Pharmacy Initiatives, available at: <http://5cpa.com.au/programs/rural-pharmacy-initiatives/> [accessed 12 July 2014].
244 The Rural Pharmacy Workforce Program (RPWP) had eleven components prior to 1 March 2014. The eleventh program component ‘Rural Pharmacy Workforce Programs Administration’ was rolled into the administrative funding provided to the Pharmacy Guild under new arrangements that commenced on 1 March 2014.
245 Health’s 2013-14 Annual Report reported that 755 out of 877 rural community pharmacies accessed one or more elements of targeted rural programs.
246 KPMG, Evaluation of the Rural Pharmacy Programs: Final Report, KPMG, 2010, available at: <http://www.health.gov.au/internet/main/publishing.nsf/Content/F520A0D5EDEA0172CA257BF0001D7B4D/$File/RPWP%20Report.pdf> [accessed 5 June 2014]
247 There has been no departmental analysis of the adequacy of the Rural Pharmacy Maintenance Allowance, taking into account the impacts of Price Disclosure on remote and rural pharmacies.
248 For example, Health was unable to provide management reports that used or assessed the information provided in progress reports submitted by the Pharmacy Guild to the department. The ANAO compiled the annual deliverables for each component of the Rural Pharmacy Workforce Program (as presented in Appendix 8) from information in the Pharmacy Guild’s reports. The Pharmacy Guild advised the ANAO that: ‘The Department has contacted the Guild to provide copies of previously submitted and accepted progress reports as they have been unable to locate their copies for the purpose of the ANAO Audit’.
249 Health advised the ANAO that: ‘AHSs must be participating in supply arrangements under S100 (that is, the Remote Area Aboriginal Health Services Program)’.
250 Quality Use of Medicines Maximised for Aboriginal and Torres Strait Islander People.
251 Senate Community Affairs References Committee, The effectiveness of special arrangements for the supply of Pharmaceutical Benefits Scheme (PBS) medicines to remote area Aboriginal Health Services, (2011) available at <http://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/ Completed_inquiries/201013/pbsmedicines/report/~/media/wopapub/senate/committee/clac_ctte/completed_inquiries/2010-13/pbs_medicines/report/report.ashx> [accessed 5 June 2014].
252 Some of the other recommendations included: that the Commonwealth Government provide specific funding for remote area AHSs to be able to provide dose administration aids (DAAs) to their patients; and that the Commonwealth Government urgently support the development and introduction of efficient standardised systems for accurate labelling of medicines in remote area AHSs, and that these systems are developed to ensure accurate collection of medicine data and use.
253 Evaluation of Indigenous Pharmacy Programs Final Report 28 June 2010, available at: <https://www.health.gov.au/internet/main/publishing.nsf/Content/F520A0D5EDEA0172CA257BF0001D7B4D/$File/Indigenous%20Programs%20Report.pdf> [accessed 12 July 2014].
254 There had not been a response as at 1 October 2014.
255 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement website, Research and Development, available at: <http://5cpa.com.au/programs/research-and-development/> [accessed 14 July 2014].
256 The contract is Schedule Six to the Deed for Multi Schedule Funding between Health and the Pharmacy Guild, dated 25 January 2011.
257 The Pharmacy Guild must also establish and maintain an Advisory Panel for each body of research, which includes a representative from the Guild, the department, the Pharmaceutical Society of Australia and consumer representatives where appropriate. Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement website, Research and Development, Advisory Panel—Terms of Reference [Internet].
258 The Pharmacy Guild advised the ANAO that project topics were a result of a stakeholder consultation process, learnings from previous projects, consideration of other current research, and national health priority areas.
259 Refer: <http://www.safetyandquality.gov.au/wp-content/uploads/2014/12/NRMC-phased-implementation-evaluation-report.pdf>.
260 South Australia, Tasmania, Victoria and Western Australia had made enabling amendments to permit continued dispensing by 1 September 2013. New South Wales introduced amendments on 20 September 2013, and the Australian Capital Territory in November 2013. Northern Territory amendments took effect in May 2014.
261 Refer: http://www.pbs.gov.au/publication/reports/continued-dispensing/report-to-parliament-operation-of-s-89a.pdf
262 Proxy measures are output-level performance indicators which indirectly measure program effectiveness.
263 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, 3 May 2010, pp. 29–33, [Internet].
264 Programs transferred from Human Services to the Guild comprised: HMR, RMMR, MedsCheck, Diabetes MedsCheck, PPIs, RPMA and the s100 Support Allowance.
265 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, 3 May 2010 [Internet].
266 Department of Health, Fifth Community Pharmacy Agreement—Governance Arrangements, [Internet], Health, available from <http://www.health.gov.au/internet/main/publishing.nsf/Content/fifth-community-pharmacy-agreement-consultative-committee> [accessed 19 November 2013].
267 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, p. 8, [Internet].
268 Department of Health, Fifth Community Pharmacy Agreement—Governance Arrangements, [Internet], Health, available from <http://www.health.gov.au/internet/main/publishing.nsf/Content/fifth-community-pharmacy-agreement-consultative-committee> [accessed 19 November 2013].
269 The 4CPA advisory committee, known as the Professional Programs and Services Advisory Committee (PPSAC), comprised five members appointed by the Pharmacy Guild and five members appointed by the Minister for Health. The PRG was established following a KPMG review of the PPSAC. See: KMPG, Review of the Professional Programs and Services Advisory Committee Case Study Report, Research and Development Steering Committee, KPMG, May 2010, available at: <http://www.health.gov.au/internet/main/publishing.nsf/Content/fourth-community-pharmacy-agreement-evaluation-reports> [accessed 15 February 2014].
270 Health advised the ANAO that the PRG terms of reference were approved by the then Minister for Health and Ageing.
271 Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, 3 May 2010, Clause 5, [Internet].
272 During the 4CPA, members of the 4CPA PPSAC Evaluation Steering Committee provided advice to Health that program evaluations needed to be considered and designed in parallel with the design and implementation of the programs.
273 For example, the Pharmacy Guild received public funding to provide advice to the ACC. While other peak bodies are not funded for their advisory role on the PRG, Health advised the ANAO that: ‘Funding provided under contractual arrangements to the Guild reflects the joint role in implementing identified 5CPA programmes, administering the ACC and preparing papers for the ACC. Independent PRG members receive sitting fees, and other members representing waged roles under the advocacy bodies receive travel, accommodation and incidental expenses in accordance with Remuneration Tribunal Determinations.’
274 Referred to in the 5CPA as ‘Medicare Australia’, because the 5CPA was signed before the incorporation of Medicare Australia into Human Services in 2011.
275 The 5CPA Schedule does not identify who will make the program payments for several programs. These programs were generally developmental projects, and their payments were made by Health.
276 This figure includes budgeted amounts for 5CPA programs excluding budgeted amounts for the Pharmacy Guild to make MedsChecks and Diabetes payments in the pilot phase of those programs.
277 This number includes all separately executed agreements, schedules and variations. A contract is defined by the Australian Government Solicitor as being a ‘legally binding agreement containing the rights and obligations of each party’. Australian Government Solicitor, Avoiding common legal pitfalls in contract management’, Fact sheet for contract managers No. 14 October 2011, available at: http://www.ags.gov.au/publications/fact-sheets/ [accessed 14 July 2014].
278 Further details of the contracted services provided by the Pharmacy Guild are at Figure 5.4.
279 The $259 million comprised $250 million (GST exclusive) for program payments and $9 million for the Pharmacy Guild’s administrative services. The $9 million (GST inclusive) for administrative services included unexpended funds of $7.2 million from previous contracts with the Guild, and an additional $1.8 million for administering programs transferred from Human Services. The new contract was signed on 11 February 2014, with the revised arrangements coming into effect on 1 March 2014.
280 In the 2014-15 Budget Measures, the Government announced that an additional $2.1 million over two years would be provided to the Pharmacy Guild to administer the payment functions for 5CPA professional programs. However, on 6 February 2014 Health proposed to the Department of Finance that it would provide $1.7 million to the Pharmacy Guild and re-allocate $0.4 million to Health to undertake audit and compliance activities.
281 Health advised the ANAO that: ‘while Health has overall responsibility, a number of administrative roles are shared with other agencies’.
282 When the ANAO sought Health’s assistance in identifying which files held the 5CPA contracts, the department advised that: ‘[Health] cannot provide the advice you are seeking as [we] do not have a separate list which identifies which contracts are in which specific files. We are providing access to the Branch/5CPA files for examination by your team, and this will ensure that we do not end up in a position of inadvertently not providing you with access to requested documents. There are a large number [of] contracts, documentation for which may cover multiple files’.
283 Whenever Health agreed to fund the Pharmacy Guild to perform a project, Health and the Guild executed a Schedule under the Multi-Schedule Funding Agreements.
284 The department advised the ANAO that: ‘the multi schedule agreements in themselves do not have a contract registration number in SAP [Health’s IT system], but where contracts are entered into relating to the individual schedule items a contract registration number is registered in SAP and it is these records that are in the list that [Health] had provided. There can be more than one SAP record per schedule item where there are multiple contracts for that item.’
285 ANAO Comment: TRIM is the department’s electronic record management system.
286 Shortcomings in departmental record keeping for the 5CPA negotiations and contracting with the Pharmacy Guild were examined in Chapter 2 of this audit report.
287 Department of Finance, Performance Information and Indicators, October 2010, available at: http://www.finance.gov.au/financial-framework/financial-management-policy-guidance/performance-information-and-indicators.html [accessed 14 July 2014].
288 These were primarily the programs administered by Human Services.
289 Health’s reporting arrangements and performance information for 5CPA programs are discussed further in Chapter 6 of this audit.
290 The Pharmacy Practice Incentives programs included an additional $5 million administered by Health to ‘support the Accreditation System and roll-out of Additional Programs to Support Patient Services’. Australian Government and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, 3 May 2010, p. 32 [Internet].
291 Medication Management programs included an additional $4.4 million administered by the Pharmacy Guild for the pilot phase of the MedsChecks and Diabetes MedsChecks programs.
292 The costs listed in paragraph 5.36 are the budgeted costs for the five year 5CPA. Health advised the ANAO that the actual amounts paid by Human Services prior to 1 March 2014 were: PPI programs—$169.74 million; Medication Management programs—$150.34 million; Rural Pharmacy Maintenance Allowance—$49.59 million; s100 Support Allowance—$6.10 million.
293 Health advised the ANAO that one claim may cover several services.
294 The ANAO’s estimate excludes funds to ‘support the Accreditation System and roll-out of Additional Programs to support Patient Services’ and MedsChecks and Diabetes pilot phase payments.
295 Human Services advised the ANAO that in some instances this was the result of claims being received incomplete, in duplicate, or with incorrect claiming data provided.
296 Health advised the ANAO that: ‘Like all government agencies, DHS may approach government for appropriations to support activities as needed per the budget process.’
297 ANAO comment: Health records indicate that PRG members were not provided with the relevant compliance report from Human Services. Instead, Health provided PRG members with one example from of a list of over 30 cases in Human Services’ report.
298 Many HMRs are delivered by accredited pharmacists in addition to retail pharmacies. The Pharmacy Guild’s proposal was not accepted by the then Government.
299 Health advised the ANAO on 1 October 2014 that: ‘while the changes did not fully ameliorate 2013-14 and 2014-15 expenditure, it did reduce the incidence of HMRs being conducted outside the home’.
300 Whilst HMRs and RMMRs are intended to be performed on an asneeds basis, not as an annual review, a patient’s GP is able to request more frequent reviews if considered clinically necessary.
301 The AACP is the Australian Association of Consultant Pharmacists; SHPA is the Society of Hospital Pharmacists of Australia; NPS is the National Prescribing Service; and PSA is the Pharmaceutical Society of Australia.
302 Health advised the ANAO that: ‘There are examples, such as implementation of arrangements for prior approval in 2013 where DHS have implemented changes in a more timely manner … Given the main parameter changes proposed to DHS were the introduction of a service cap, there was no expectation from Health that a change to introduce such a cap would take 11 months or require significant resources to implement.’
303 The Pharmacy Guild now administers all 5CPA professional programs apart from special projects: Medication Continuance; Electronic recording of controlled drugs; and Supply and PBS Claiming from a Medication Chart in a Residential Aged Care facility.
304 Under the CPRs agencies could only conduct a procurement valued at or above $80 000 through a limited (that is, sole source) tender in the various circumstances set out in paragraph 10. Department of Finance, Commonwealth Procurement Rules July 2012, available at: <http://www.finance.gov.au/ procurement/docs/cpr_commonwealth_procurement_rules_july_2012.pdf> [accessed 14 June 2014].
305 A total of $24.3 million under this contract related to unspent funding from previous contracts, including program and administrative funds.
306 The previous 5CPA contracts between Health and the Pharmacy Guild included GST for both program and administrative payments. In contrast, the new Contract for Services with the Pharmacy Guild treated funding for program payments as GST exempt. Health advised the ANAO that it sought guidance on whether payments of program funds to the Pharmacy Guild attracted GST as follows: ‘In terms of the Project Funds made available to the Guild for payments to third parties (under new arrangements from 1 March), advice was sought from OCFO [Office of the Chief Financial Officer] relating to this issue. Formal written advice was then sought confirming verbal discussions, and on 17 March 2014 confirmation in writing was provided … In effect given the new split of project funds under new arrangements, and that the Guild was merely holding funds on behalf of the Commonwealth, advice was provided that GST should not apply to this arrangement.’ Health records indicate that verbal discussions with Health’s OCFO regarding the GST occurred on 11 March 2014, followed by a written request on 17 March 2014. The OCFO sought legal advice, which confirmed its view that the project funds remained public money, and were not a payment for the supply of services from the Pharmacy Guild.
307 While some contracts clearly distinguished between funding for administration and program purposes, others did not. For these contracts, the ANAO sought Health’s advice on the allocation of administrative and program funding. This issue is discussed further in paragraph 5.65.
308 As at 25 November 2014, Health was unable to confirm that Health had not entered into additional contracts with Fred IT up to 1 October 2014.
309 Three contracts with Fred IT group related to the 5CPA. They provided up to $9.5 million in Commonwealth payments, much of which related to Fred IT’s eRx Prescription Exchange Service. The two largest non-5CPA contracts relate to the development and testing of the Personally Controlled eHealth Record, and the hosting, support and operation of the National Prescription Dispense Repository.
310 http://5cpa.com.au/ [accessed 11 November 2014]. The website indicates that it is ‘Funded by the Australian Government Department of Health as part of the Fifth Community Pharmacy Agreement’, and features the Commonwealth coat of arms and Pharmacy Guild symbol.
311 The minutes of the 8 December 2010 ACC meeting record that four members of the Pharmacy Guild and four departmental members attended, and that the ACC ‘AGREED to the development of a communications strategy’. The minutes of the 16 February 2011 ACC meeting record that the committee ‘NOTED funding will be negotiated between the Department and the Pharmacy Guild based on activities, with program related activities to be funded from program allocations …’
312 The ANAO has previously observed that there are inherent limitations in self-assessment processes, which are unlikely of themselves to consistently provide a high level of assurance. Well-targeted quality assurance activity, focussing on higher risk, more significant, or higher volume transactions, complements self-assessments. These considerations are particularly relevant where a third party may be handling public money, as discussed in Chapter 5. See: ANAO Report No.38 2010–11, Management of the Certificate of Compliance Process in FMA Act Agencies, paragraph 22.
313 Prior to 1 July 2014, the primary legislation establishing the requirements for Australian Government agencies was the Financial Management and Accountability Act 1997 (the FMA Act). From 1 July 2014, the FMA Act was replaced by the Public Governance, Performance and Accountability Act 2013 (the PGPA Act). Both the FMA Act and PGPA Act require public officials to make ‘proper use’ of public resources. Proper use means efficient, effective, economical and ethical use.
314 An outsider was defined as ‘any person other than the Commonwealth, an official or a Minister’. An outsider who performed financial tasks in relation to public money and who was not doing so under an authorised section 12 agreement was deemed to be an ‘allocated official’ of the relevant agency in relation to the tasks undertaken, and was therefore subject to all relevant provisions of the FMA Act and FMA Regulations. See Finance Circular No. 2011/01 Commitments to spend public money (FMA Regulations 7 to 12), pp. 40–42.
315 Under the FMA Act it was an offence for an outsider to receive, have custody of or make a payment of public money unless properly authorised.
316 The advice related to Schedules 1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16 and 21 of the Deed for Multi Schedule Funding (the Deed) executed in January 2011.
317 However, to the extent that any amounts payable were for the provision of administrative or management services by the Pharmacy Guild in relation to a program, those amounts, when received by the Pharmacy Guild, would not be public money.
318 In respect of banking, for example, Section 11 of the FMA Act provided that public money had to be deposited in an official account, and prohibited the depositing of public money in a non-official bank account. The FMA Act included penalties for non-compliance with Section 11.
319 AGS advice to the ANAO, March 2014.
320 Australian National Audit Office and Department of the Prime Minister and Cabinet, Successful Implementation of Policy Initiatives—Better Practice Guide, Canberra, October 2014, p. 31.
321 The Certificate of Compliance was an annual process requiring FMA Act agencies to report on their compliance with the financial framework to their Minister, copied to the Finance Minister.
322 The ANAO provided Health with a copy of the legal advice received from AGS.
323 The new Contract for Services was discussed in paragraph 5.57.
324 Programs transferred from Human Services to the Pharmacy Guild comprised: HMR, RMMR, QUM, MedsCheck, Diabetes MedsCheck, RPMA, s100 Support Allowance and the PPI program.
325 In seeking approval for the new arrangements with the Pharmacy Guild, Health also advised the delegate that all expenditure of public money under the Contract for Services will be subject to an audit/compliance regime specifically established and managed by Health. In October 2014, Health advised the ANAO that: ‘From 1 March 2014, the Department assumed responsibility for audit and compliance activities for 5CPA Programmes which are administered by the Guild. The Department has contracted the development of a Compliance Assurance Strategy, developed in consultation with the [department’s] Audit and Fraud Branch. This strategy was deployed on 22 August 2014, with the Department issuing a “Request for Information” letter to 26 service providers identified through the Compliance Assurance Strategy, to request certain information to assist in gaining assurance of compliance. This related to Home Medicine Review, MedsCheck, Diabetes MedsCheck and Residential Medication Management Review service providers, for claims conducted between 1 January 2013 and 28 February 2014. Future steps and audits will be informed by the Compliance Assurance Strategy.’
326 The Commonwealth Grants Rules and Guidelines replaced the Commonwealth Grant Guidelines following the introduction of the Public Governance, Performance and Accountability Act 2013 (PGPA Act).
327 Health advised the ANAO that the Pharmacy Guild was the sole recipient of grants relating to all 11 components of the Rural Pharmacy Workforce Program. One grant is provided to the Pharmacy Guild by Health for the purposes of its administration of the program, while 10 grants have the purpose of assisting rural pharmacies and/or developing the rural pharmacy workforce. The Guild uses these funds to make payments to owners and staff of rural pharmacies for initiatives such as: continuing professional education; financial incentives to employ interns; and scholarships. The Guild also uses the funds for a rural emergency locum service.
328 The 5CPA professional programs and activities are listed in Appendix 7 and Appendix 8.
329 Department of Health, Annual Report 2013–14, Volume 1, p. 52.
330 These program costs are not identified in the department’s financial resource summary, nor in any explanatory material.
331 However, the report does not contain all components of pharmacy remuneration, with the exception of PFDI and EPF.
332 The cost of pharmaceutical benefits includes the cost of medicines.
333 Key performance indicators have been examined in: ANAO Audit Report No.21 Pilot Project to Audit Key Performance Indicators, and ANAO Audit Report No.28 The Australian Government Performance Measurement and Reporting Framework - Pilot Project to Audit Key Performance Indicators.
334 Health’s KPI for the PPI program is discussed further in paragraph 4.27.
335 The KPIs were assessed against the criteria in ANAO Audit Report No.21 2013–14 Pilot Project to Audit Key Performance Indicators, p. 41.
336 Comprising 90 per cent of 5CPA costs.
337 Health has not reported pharmacy remuneration separately to the cost of PBS and RPBS medicines.
338 The number of retail pharmacies approved to supply pharmaceutical benefits under Section 90 of the National Health Act is termed “approved pharmacists”. It should be noted that this is not the number of pharmacists approved to own Section 90 pharmacies (which is not measured or reported) but the number of premises approved under Section 90 of the National Health Act.
339 This information is reported in the Productivity Commission’s annual Report On Government Services.
340 Over the last 24 years, the agency responsible for issuing pharmacy approval numbers was, in chronological order, the Health Insurance Commission, Medicare Australia, and Human Services.
341 All pharmacists must be registered by the Pharmacy Board of Australia, as supported by the Australian Health Practitioner Regulation Agency.
342 Urbis, Review of the Pharmacy Location Rules under the Fourth Community Pharmacy Agreement June 2010 Final Report, Urbis, Canberra, 2010, p. 1.
343 Australian Government and the Pharmacy Guild of Australia, The Fifth Community Pharmacy Agreement, 2010, p. 16, available from [Internet].
344 [Emphasis added.]
345 Health advised the ANAO that an open Request for Tender was undertaken to select CSO Distributors, which was advertised in February 2011 and completed in May 2011.
346 Department of Health and Ageing, Community Service Obligation Funding Pool–Operational Guidelines, July 2012, p. 9 available from <https://www.health.gov.au/internet/main/publishing.nsf/ Content/BE2CB73EF2EE20D4CA257BF0001DC53C/$File/CSO%20Operational%20Guidelines%20July%202012.pdf> [Accessed 29 August 2014].
347 The CSO Funding Pool is indexed annually to WCI9.
348 Over the counter medicines are medicines that are supplied without a doctor’s prescription.
349 In the course of this audit Health advised the ANAO that: ‘There is no evidence under existing arrangements outside the CSO that supply in greater than 24 hours occurs … The CSO is around ability to supply in a timely manner, and no pharmacy holds all PBS product stock (numbering in thousands of items). It is therefore difficult to genuinely assume this leads to real reduction in stock holding levels’.
350 Department of Health and Ageing and the Pharmacy Guild of Australia, Fifth Community Pharmacy Agreement, Health, Canberra, 3 May 2010, p. 26.
351 Department of Health and Ageing, Evaluation Framework for the Fifth Community Pharmacy Agreement, Health, Canberra, December 2011.
352 The ACC comprises four Health members and four Pharmacy Guild members.
353 As discussed in Chapter 5, the PRG provides advice on professional programs to the Minister and the ACC.
354 Deloitte Access Economics, Evaluation of the MedsCheck and Diabetes MedsCheck Pilot Program, Health, Canberra, July 2012, available from <http://www.health.gov.au/internet/main/publishing.nsf/ Content/fifth-community-pharmacy-agreement-medscheck> [accessed 15 November 2013].
355 The Framework notes that reviews of: Section 100 supply and remuneration arrangements; the administration of the CSO Funding Pool; and the Pharmacy Location Rules were finalised in 2010.
356 See paragraph 1.27 of this audit.