Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Australia’s COVID-19 Vaccine Rollout
Please direct enquiries through our contact page.
Audit snapshot
Why did we do this audit?
- The distribution and delivery of COVID-19 vaccines has been one of the largest exercises in health logistics in Australian history. COVID-19 and the vaccine rollout have impacted upon every person in Australia.
- This performance audit was conducted under phase two of the ANAO’s multi-year strategy that focuses on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic.
Key facts
- As at 1 July 2022, there had been 8.2 million reported cases of COVID-19 in Australia and 9,930 deaths.
- The Australian Government spent more than $8 billion on COVID-19 vaccines.
- During 2021, 42.6 million COVID-19 vaccine doses were administered.
What did we find?
- The Department of Health and Aged Care’s (Health’s) planning and implementation of the COVID-19 vaccine rollout has been partly effective.
- Health’s approach to planning became more effective as the rollout progressed.
- The final governance arrangements established to manage the COVID-19 vaccine rollout have been largely effective.
- Implementation of the COVID-19 vaccine rollout has been partly effective, with Health’s administration of vaccines to priority populations and the general population not meeting targets.
What did we recommend?
- The Auditor-General made two recommendations relating to: improving data quality and IT controls; and conducting a comprehensive review of the rollout to identify opportunities for improvement in the event of a future vaccine rollout.
- Health agreed to both recommendations.
90.2%
Percentage of people aged 12 or over in Australia double vaccinated by 31 December 2021.
87.2%
Percentage of residential aged care facility residents double vaccinated by 10 January 2022.
82.0%
Percentage of residential disability facility residents double vaccinated by 31 December 2021.
63.5%
Percentage of Aboriginal and Torres Strait Islander people aged 12 or over double vaccinated by 31 December 2021
Summary and recommendations
Background
1. Since its emergence in late 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic that is impacting on human health and national economies. On 21 January 2020, the Australian Government declared COVID-19 as a listed human disease under the Biosecurity Act 2015 (Biosecurity Act). The World Health Organization (WHO) declared COVID-19 to be a ‘public health emergency of international concern’ on 30 January 2020.
2. Early Australian Government responses to COVID-19 included:
- travel restrictions, international border controls and quarantine arrangements;
- delivery of substantial economic stimulus, including financial support for affected individuals, businesses and communities; and
- support for essential services and procurement and deployment of critical medical supplies (including the national vaccine rollout).
3. The Australian Government’s August 2020 Australia’s COVID-19 vaccine and treatment strategy committed the government to building a ‘diverse global portfolio of investments to seek to secure early access to promising vaccines and treatments’, using local manufacturing wherever possible.1 Between September 2020 and May 2021, the Australian Government entered into agreements with five vaccine manufacturers to purchase a total of 315.3 million vaccines of different types, with some vaccines being manufactured overseas and some produced locally.
4. The Department of Health and Aged Care (Health)2 has been the lead entity for the COVID-19 vaccine rollout. The COVID-19 vaccine rollout commenced on 22 February 2021. As at 30 December 2021, Health reported that 42,598,706 doses had been administered nationally with 18,845,485 people having been fully vaccinated.
Rationale for undertaking the audit
5. The COVID-19 pandemic and the pace and scale of the Australian Government’s response impacts on the risk environment faced by the Australian public sector. This performance audit was conducted under phase two of the ANAO’s multi-year strategy that focuses on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic.3
6. The distribution and delivery of COVID-19 vaccines has been one of the largest exercises in health logistics in Australian history. The COVID-19 vaccine rollout has required rapid and flexible planning, decision-making and implementation, to respond to the changing health, social and economic impacts of COVID-19, as well as the effective and timely acquisition and distribution of vaccines once they were created. The audit was conducted to provide independent assurance to the Parliament that Australia’s COVID-19 vaccine rollout was planned and implemented effectively.
Audit objective and criteria
7. The objective of the audit was to assess the effectiveness of the planning and implementation of the COVID-19 vaccine rollout.
8. To form a conclusion against this objective, the following high-level criteria were adopted.
- Has the approach to planning Australia’s COVID-19 vaccine rollout been effective? (Chapter 2)
- Have effective governance arrangements been established to manage the COVID-19 vaccine rollout? (Chapter 3)
- Has the COVID-19 vaccine rollout been effectively implemented? (Chapter 4)
Conclusion
9. Health’s planning and implementation of the COVID-19 vaccine rollout has been partly effective. While 90 per cent of the eligible Australian population was vaccinated by the end of 2021, the planning and implementation of the vaccine rollout to priority groups was not as effective.
10. Health’s approach to planning Australia’s vaccine rollout became more effective as the rollout progressed. Health undertook largely appropriate planning to establish administration channels and logistics for the rollout and developed a fit-for-purpose communication strategy. Initial planning was not timely, with detailed planning with states and territories not completed before the rollout commenced, and Health underestimated the complexity of administering in-reach services to the aged care and disability sectors. Further, it did not incorporate the government’s targets for the rollout into its planning until a later stage. Health identified and continually reassessed risks to the rollout, adapting its planning in response to realised risks.
11. The final governance arrangements established to manage the COVID-19 vaccine rollout have been largely effective. Following the commencement of Operation COVID Shield in June 2021, senior level oversight of the program substantially increased. Health regularly consulted with four sector-specific stakeholder advisory groups. Health put in place effective monitoring and reporting arrangements using the best available data. However, it did not undertake sufficient reporting against targets, and it does not have adequate assurance over the completeness and accuracy of the data and third-party systems.
12. Health’s implementation of the COVID-19 vaccine rollout has been partly effective. While vaccines were delivered with minimal wastage, Health’s administration of vaccines to priority populations and the general population has not met targets. The vaccine rollout to residential aged care and residential disability were both slower than planned, and the vaccination rate for Aboriginal and Torres Strait Islander people has remained lower than for the Australian population. Health implemented its communication strategy, but its advertising campaign has not yet been evaluated.
Supporting findings
Vaccine rollout planning
13. The commencement of planning for the rollout was not timely and early planning did not include target dates for the rollout. Detailed engagement with the states and territories on rollout planning did not begin until November 2020 and Jurisdictional Implementation Plans were not agreed until February 2021, by which time the rollout of COVID-19 vaccines had already commenced. Initial planning on the prioritisation of vaccinations to vulnerable and at-risk groups was based on advice from the Australian Technical Advisory Group on Immunisation, an expert committee. Planning continued throughout the rollout. In June 2021, the Prime Minister announced the replacement of Health’s rollout taskforce with new Operation COVID Shield and a new Op COVID Shield National COVID Vaccine Campaign Plan was released in August 2021. Health subsequently developed a series of sub-plans which contained lower-level target dates for vaccinating specific priority groups and sectors. (See paragraphs 2.3 to 2.37)
14. Risks to the rollout were identified from June 2020. In January 2021, Health established a comprehensive risks and issues register which was used throughout 2021 to identify risks to the rollout. Risks were kept under regular review and, where necessary, corrective action was determined. The most significant risks and issues were referred to the taskforce leadership for review. (See paragraphs 2.38 to 2.47)
15. Health worked with state and territory authorities and relevant stakeholders to consider and establish a variety of administration channels that were suitable for priority groups and the population as a whole. Two logistics providers were engaged to transport and deliver vaccines throughout Australia. Health underestimated the magnitude and complexity of rolling out in-reach services for the residential aged care and disability sectors and did not engage sufficient in-reach providers early in the rollout. (See paragraphs 2.48 to 2.57)
16. Health developed a fit-for-purpose strategy for communicating the vaccine rollout. The communication strategy and its supporting advertising strategy identified target audiences and was tailored to addressing the concerns of these groups as determined through market research. The advertising strategy complied with government guidelines for advertising campaigns and had a plan to monitor progress. (See paragraphs 2.58 to 2.73)
Governance
17. Fit-for-purpose governance arrangements were established for the vaccine rollout following the commencement of Operation COVID Shield in June 2021. Senior level oversight of the program also substantially increased. Responsibilities of the Australian, state and territory governments and other stakeholders were documented in the Australian COVID-19 vaccination policy, Jurisdictional Implementation Plans and sector specific implementation plans. (See paragraphs 3.3 to 3.19)
18. Health put in place systems to monitor the vaccine rollout but does not have adequate assurance over the completeness and accuracy of the data and third-party systems. Health provided decision makers and the public with regular and detailed updates on the vaccine rollout that were tailored and developed using the best available data. These reports did not include progress against targets until August 2021. (See paragraphs 3.20 to 3.38)
19. Health developed a stakeholder engagement plan for the rollout, which assessed stakeholders based on their needs, influence, and impact on the program. Health received advice on the rollout from four sector-specific advisory groups that were established for the COVID-19 response. Feedback on the vaccine rollout from key stakeholders contacted by the ANAO was mixed. (See paragraphs 3.39 to 3.54)
Implementation
20. Vaccines were delivered within agreed logistics timeframes and with minimal wastage, with 60 per cent of vaccines administered through Australian Government managed channels. Health had a principle of allocating vaccines to states and territories on a proportional basis, however issues such as logistical constraints and responses to COVID-19 hotspots meant that allocations were not proportional in the early stages of the rollout. Health did not meet the government’s original target to ‘have the country vaccinated’ before the end of October 2021. (See paragraphs 4.3 to 4.29)
21. Health’s administration of the vaccine rollout to the residential aged care and disability sectors was slower than planned, due to Health initially contracting insufficient vaccine administration providers and other planning and implementation issues. Health’s target for the vaccination rate for Aboriginal and Torres Strait Islander people to be equal to or greater than the national target [80 per cent] has not been met in 2021. (See paragraphs 4.30 to 4.65)
22. Health’s communication about the vaccine rollout has been largely consistent with its communication strategy, and its monitoring of public sentiment has shown attitudes towards COVID-19 vaccination improved over time. Health implemented communication activities targeted at Aboriginal and Torres Strait Islander people and culturally and linguistically diverse communities, but these activities were not as effective at reaching these groups as communication to the general population. Health has not yet conducted an evaluation of the effectiveness of the advertising campaign as the campaign is ongoing. (See paragraphs 4.66 to 4.76)
Recommendations
Recommendation no. 1
Paragraph 3.25
The Department of Health and Aged Care establish processes, including during public health emergencies, to ensure it regularly obtains and reviews assurance over the data quality and IT controls in place in externally managed systems on a risk basis, including IT security, change management and batch processing.
Department of Health and Aged Care response: Agreed
Recommendation no. 2
Paragraph 3.55
Before 31 December 2022, the Department of Health and Aged Care conduct a comprehensive review of the COVID-19 vaccine rollout which:
- invites contribution from all key government and non-government stakeholders;
- examines all aspects of the COVID-19 vaccine rollout;
- identifies what worked well and what did not; and
- makes recommendations to the Australian Government about opportunities for improvement in the event of a future vaccination rollout.
Department of Health and Aged Care response: Agreed
Summary of entity response
23. Health’s summary response is provided below and its full response is included at Appendix 1.
The Department of Health and Aged Care (the Department) welcomes the findings in the report and the recommendations directed to the Department. The Department is committed to effective implementation of recommendations and has already commenced steps to address the issues identified in this audit.
It was pleasing to note the audit recognises the significant planning, consultation and engagement that underpinned the vaccine rollout. I also note the acknowledgement of appropriate governance and risk management processes developed through the rollout, and the fit-for-purpose communication strategy that informed Australians and key stakeholders on the vaccine program. The audit also found that vaccines were delivered within agreed logistics timeframes and with minimal wastage.
The audit found some shortcomings on assurance received over the completeness and accuracy of the data and third-party systems that underpinned the vaccine rollout. To address this finding the Department plans to undertake a review of IT controls for data received from externally managed systems. The audit also recommended the Department undertake a comprehensive review of the vaccine rollout. The Department notes that such a review would logically form part of an expected broader review into the COVID-19 pandemic with the timing still to be agreed by Government.
Key messages from this audit for all Australian Government entities
Below is a summary of key messages, including instances of good practice, which have been identified in this audit and may be relevant for the operations of other Australian Government entities.
Policy/program implementation
Governance and risk management
Performance and impact measurement
1. Background
Introduction
1.1 Since its emergence in late 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic that is impacting on human health and national economies. On 21 January 2020, the Australian Government declared COVID-19 as a listed human disease under the Biosecurity Act 2015 (Biosecurity Act).4 The World Health Organization (WHO) declared COVID-19 to be a ‘public health emergency of international concern’ on 30 January 2020.
1.2 From January 2020, the Australian Government commenced the introduction of a range of policies and measures in response to the emergence of COVID-19. On 18 March 2020, in response to the pandemic in Australia, the Governor-General of the Commonwealth of Australia declared that a human biosecurity emergency exists.5
1.3 The Australian Government’s health and economic response has included:
- travel restrictions, international border controls and quarantine arrangements;
- delivery of substantial economic stimulus, including financial support for affected individuals, businesses and communities; and
- support for essential services and procurement and deployment of critical medical supplies (including the national vaccine rollout).
1.4 With the release of the 2022–23 Budget on 29 March 2022, the Australian Government reported that it had committed $314 billion to economic response measures.6 In addition, as at April 2022, the Australian Government had committed more than $45 billion to the COVID-19 health response, including more than $8 billion related to COVID-19 vaccines and booster doses.
Development, approval and acquisition of COVID-19 vaccines
1.5 The Australian Government’s initial response to COVID-19 was contained in the Australian Health Sector Emergency Response Plan for Novel Coronavirus (COVID-19), which was published on 18 February 2020.7 The plan focussed on measures to reduce transmission, minimise the burden on health systems and ‘inform, engage and empower the public’. It also noted that ‘availability of a customised novel coronavirus vaccine would be the greatest tool in reducing the impact [of COVID-19]’ and committed the Australian Government to:
fast-track assessment and approval of a customised vaccine, should this become available; procure vaccines; develop a national novel coronavirus vaccination policy and a national novel coronavirus immunisation program; and communicate immunisation information on the program to the general public and health professionals.8
1.6 Australia has had mass vaccination campaigns since 1924. In 1997, the Australian, state and territory governments jointly established the National Immunisation Program (NIP)9, which currently provides free vaccination against 17 diseases for babies, young children, teenagers and older Australians.10 The NIP has achieved very high immunisation rates, particularly for children.
Development and approval of COVID-19 vaccines
1.7 From January 2020, research organisations and pharmaceutical companies worldwide began developing and testing COVID-19 vaccines at unprecedented speeds.11 The vaccines developed ranged from viral vector and protein vaccines, such as the Oxford AstraZeneca viral vector vaccine (AstraZeneca), to new genetic vaccines, such as the mRNA Pfizer vaccine (Pfizer).
1.8 The Therapeutic Goods Administration (TGA), which forms part of the Department of Health and Aged Care (Health)12, is Australia’s regulatory authority for therapeutic goods.13 The TGA assessed COVID-19 vaccines for safety, quality and effectiveness before they could be supplied in Australia.14 While achieving full registration for a therapeutic good such as a vaccine can take several years, where the TGA assesses that the benefit of early availability outweighs the risk inherent in the fact that additional data are still required, it can provisionally register the vaccine. All COVID-19 vaccines used in Australia have used the provisional registration process.15
1.9 Once the TGA approves use of a therapeutic good, it monitors the use of the product to identify potential safety issues. The TGA has monitored the COVID-19 vaccine to identify adverse effects since 22 February 2021 and published a weekly safety report on any issues identified.
Australia’s acquisition of COVID-19 vaccines
1.10 By mid-2020, many potential COVID-19 vaccine candidates had entered clinical trials and countries such as the United States began entering into advance purchase agreements with pharmaceutical companies.16
1.11 In August 2020, the Australian Government established the Science and Industry Technical Advisory Group (SITAG) to ‘support the Australian Government to make decisions about purchasing and manufacture of COVID-19 vaccines and treatments’. SITAG’s terms of reference state that its purpose is to provide advice on:
- the scientific validity of research into safety and efficacy of COVID-19 vaccine candidates;
- potential purchasing arrangements for COVID-19 vaccine candidates for Australia;
- the scientific validity of research into new therapeutics for COVID-19, including tests and treatments;
- potential purchasing arrangements for COVID-19 therapeutics for Australia;
- the viability of options for manufacturing and packaging COVID-19 vaccines and treatments in Australia;
- distribution and logistics associated with COVID-19 vaccine candidates; and
- other technical matters related to COVID-19 vaccines and treatments, as requested by the Chair.17
1.12 In August 2020, the Australian Government released Australia’s COVID-19 vaccine and treatment strategy, which committed the Australian Government to building a ‘diverse global portfolio of investments to seek to secure early access to promising vaccines and treatments’, using local manufacturing wherever possible.18 The criteria which were used to guide investment decisions were:
- availability of the vaccine candidate in 2021;
- experience in the technology platform;
- the track record of the company or partner company in vaccine development;
- complexities involved in the distribution and administration of the candidate (such as the temperature at which they must be stored and transported prior to administration19);
- the likely volume able to be secured in 2021;
- the clinical trial stage reached; and
- costs related to securing candidates.
1.13 Vaccine efficacy was not one of the factors taken into account in the initial selection of vaccines for purchase. This was because, at that time, none of the vaccines being considered had reached Phase 3 clinical trials where efficacy is tested.20
1.14 Commencing in August 2020, Health presented SITAG with information about a number of vaccine candidates and sought its endorsement to proceed with negotiations with the five companies which produced the vaccines that were ultimately purchased (see Table 1.1). Health then sought the government’s approval to proceed with advance purchase agreements with the selected companies.
Table 1.1: Australia’s COVID-19 vaccine purchase agreements
|
Vaccine |
Number of doses per agreementa (millions) |
Total number of doses per vaccine (millions) |
Date agreement announced |
Vaccine type |
Approved by TGA |
|
|
AstraZeneca (Vaxzevria) |
33.8 |
56.3 |
07/09/2020 |
Viral vector |
✔ |
15/02/2021 |
|
22.5 |
11/12/2020 |
|||||
|
University of Queensland |
51.0 |
51.0 |
08/10/2020 |
Protein |
✘ |
N/Ab |
|
Pfizer (Comirnaty) |
10.0 |
131.0 |
05/11/2020 |
mRNA |
✔ |
25/01/2021 |
|
10.0 |
04/02/2021 |
|||||
|
20.0 |
09/04/2021 |
|||||
|
0.5c |
13/5/2021 |
|||||
|
85.0 |
25/07/2021 |
|||||
|
1.0 |
15/08/2021 |
|||||
|
0.5d |
31/08/2021 |
|||||
|
4.0e |
03/09/2021 |
|||||
|
Novavax (Nuvaxovid) |
40.0 |
51.0 |
05/11/2020 |
Protein |
✔ |
19/1/2022 |
|
11.0 |
11/12/2020 |
|||||
|
Moderna (Spikevax) |
25.0 |
26.0 |
13/05/2021 |
mRNA |
✔ |
09/08/2021 |
|
1.0f |
1/9/2021 |
|||||
|
Total number of doses purchased (millions) |
315.3 |
|||||
|
Total number of doses purchased that received TGA approval (millions) |
264.3 |
|||||
Note a: Agreements include advance purchase agreements with vaccine manufacturers and agreements with nations to purchase additional vaccine stock.
Note b: The University of Queensland vaccine did not proceed past human trials in December 2020.
Note c: 513,630 Pfizer doses purchased from the COVAX facility.
Note d: 500,000 Pfizer doses were ‘swapped’ with Singapore in August 2021 and repaid in November 2021.
Note e: Four million Pfizer doses were ‘swapped’ with the United Kingdom in September 2021 and repaid in late 2021.
Note f: One million Moderna doses were purchased from European Union member states in September 2021.
Source: ANAO analysis of Health documents and press releases by the Minister for Health and Aged Care and the Prime Minister.
Australia’s COVID-19 vaccine rollout
1.15 Health has been responsible for managing Australia’s COVID-19 vaccine rollout.
1.16 Health published the Australian COVID-19 vaccination policy on 13 November 2020, which included key principles relating to the vaccine rollout.21 On 7 January 2021, based on advice from the Australian Technical Advisory Group on Immunisation (ATAGI), Health published Australia’s COVID-19 vaccine national roll-out strategy, which outlined a three-phase rollout strategy commencing with priority populations.22
1.17 Australia’s COVID-19 vaccine rollout was launched on 22 February 2021 with the provision of the Pfizer vaccine to individuals in Phase 1a (which included quarantine, border and frontline healthcare workers and aged care and disability care residents and staff).23 The AstraZeneca vaccine was made available from March 2021, with the first dose of AstraZeneca administered in Australia on 5 March 2021.
1.18 In early March 2021, reports began to circulate globally of a rare but potentially serious side-effect of AstraZeneca. ATAGI monitored these reports and published several updates. On 8 April 2021, ATAGI updated its advice, recommending the use of Pfizer as the preferred vaccine for eligible people under 50 years of age.24 As a result, Health ‘recalibrated’ the rollout, including by changing the recommended age ranges for some population groups.
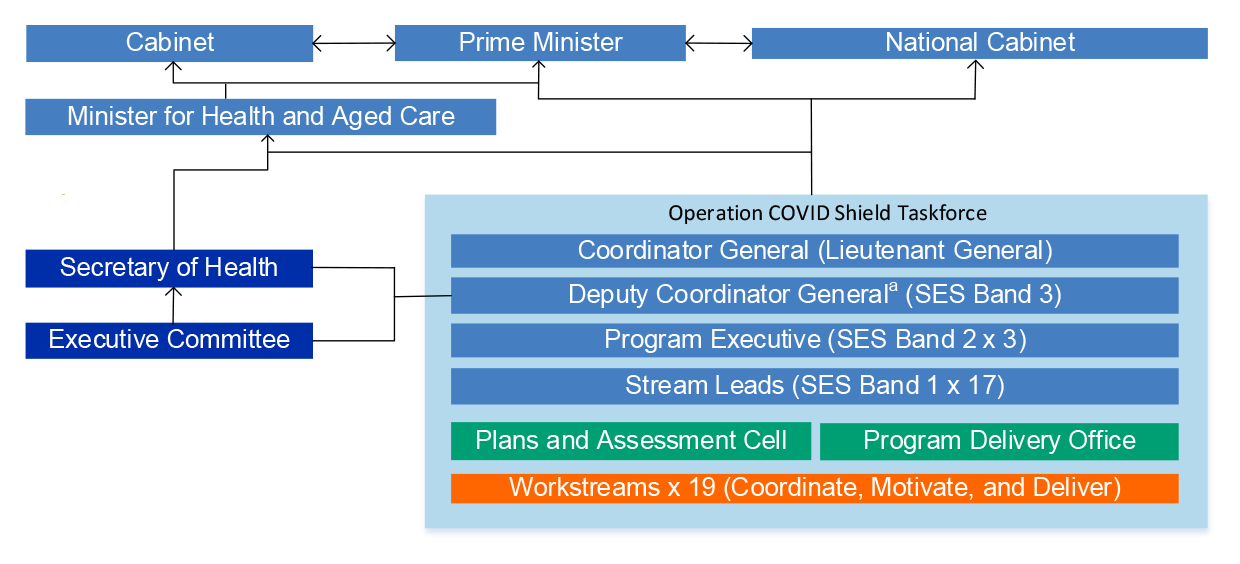
1.19 Until June 2021, the rollout was administered by a taskforce within Health. In June 2021, the Prime Minister appointed Lieutenant General John (JJ) Frewen DSC AM as the Coordinator General of Operation COVID Shield.25 The Coordinator General assumed control of the taskforce and was directed by the Prime Minister to report directly to him and the Minister for Health and Aged Care, while working closely with the Secretary of Health.
1.20 By 31 December 2021, all people aged 12 years and above were eligible, and encouraged, to receive a COVID-19 vaccine. On 8 November 2021, the Australian Government began a vaccine booster program, for people to receive an additional dose of vaccine to provide additional protection. This was initially targeted to priority groups most at risk, such as the elderly and the immunocompromised. From 10 January 2022, the Australian Government expanded eligibility for the program to include those aged five to 11 years.26
1.21 As at 30 June 2022:
- 60.3 million vaccine doses had been administered, 39.0 million of these by Australian Government providers such as pharmacies, general practitioners (GPs) and contracted providers; and
- 19.8 million people aged 16 or over (over 95 per cent) had received at least two vaccine doses.
1.22 Figure 1.1 is a timeline key events that occurred in the lead up to, and during, the vaccine rollout.
Figure 1.1: COVID-19 vaccine rollout key events, 1 August 2020–31 December 2021

Source: ANAO from Health documents.
Rationale for undertaking the audit
1.23 The COVID-19 pandemic and the pace and scale of the Australian Government’s response impacts on the risk environment faced by the Australian public sector. This performance audit was conducted under phase two of the ANAO’s multi-year strategy that focuses on the effective, efficient, economical and ethical delivery of the Australian Government’s response to the COVID-19 pandemic.27
1.24 The distribution and delivery of COVID-19 vaccines have been one of the largest exercises in health logistics in Australian history. The COVID-19 vaccine rollout has required rapid and flexible planning, decision-making and implementation, to respond to the changing health, social and economic impacts of COVID-19, as well as the effective and timely acquisition and distribution of vaccines once they were created. The audit was conducted to provide independent assurance to the Parliament that Australia’s COVID-19 vaccine rollout was planned and implemented effectively. The audit was identified by the Joint Committee of Public Accounts and Audit as an audit priority of the Parliament.
Audit approach
Audit objective, criteria and scope
1.25 The objective of the audit was to assess the effectiveness of the planning and implementation of the COVID-19 vaccine rollout.
1.26 To form a conclusion against this objective, the following high-level criteria were adopted.
- Has the approach to planning Australia’s COVID-19 vaccine rollout been effective? (Chapter 2)
- Have effective governance arrangements been established to manage the COVID-19 vaccine rollout? (Chapter 3)
- Has the COVID-19 vaccine rollout been effectively implemented? (Chapter 4)
1.27 The scope of the audit did not include:
- the procurement of the vaccines selected for use in Australia;
- the administration of vaccinations by state and territory governments, including state and territory vaccine immunisation hubs;
- the safety or effectiveness of vaccines purchased by the Australian Government, or any other matters requiring clinical expertise;
- the administration of vaccines to Australian Government employees overseas (such as Department of Foreign Affairs staff); or
- the donation of vaccines by the Australian Government to countries in the Pacific and Southeast Asia.28
1.28 The audit focused on the period from the first occurrence of COVID-19 in Australia until 31 December 2021. For that reason, there is limited examination of the implementation of the booster program, which began late in 2021, or the implementation of the rollout for children aged five to eleven, which did not begin until 2022.
Audit methodology
1.29 The audit involved:
- reviewing submissions and briefings to the Australian Government;
- reviewing Health’s documentation including meeting papers and minutes, policies, procedures and correspondence;
- analysing data held in systems used by Health;
- meetings with officers from relevant business areas within Health;
- reviewing 22 submissions and meeting with stakeholders in the health sector, state and territory governments and COVID-19 advisory groups29; and
- reviewing 507 contributions received from organisations and individuals via the ANAO’s ‘contribute to this audit’ facility.
1.30 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $845,000.
1.31 The audit team members for this audit were Julian Mallett, Laura Trobbiani, Anne Kent, Sarah Koehler, Olivia Robbins, Elizabeth Wedgwood, Matthew Rigter, Alexander Wilkinson and Daniel Whyte.
2. Vaccine rollout planning
Areas examined
This chapter examines the effectiveness of the Department of Health and Aged Care’s (Health’s) approach to planning Australia’s COVID-19 vaccine rollout.30
Conclusion
Health’s approach to planning Australia’s vaccine rollout became more effective as the rollout progressed. Health undertook largely appropriate planning to establish administration channels and logistics for the rollout and developed a fit-for-purpose communication strategy. Initial planning was not timely, with detailed planning with states and territories not completed before the rollout commenced, and Health underestimated the complexity of administering in-reach services to the aged care and disability sectors. Further, it did not incorporate the government’s targets for the rollout into its planning until a later stage. Health identified and continually reassessed risks to the rollout, adapting its planning in response to realised risks.
2.1 As noted in paragraph 1.24, the distribution and delivery of COVID-19 vaccines have been one of the largest exercises in health logistics in Australian history. When an initiative is urgent, planning and implementation may need to occur quickly or in stages, prioritising critical foundations and building on them later. One of the most pressing priorities is to reduce risk by seeking expert implementation advice and experience as soon as possible in the delivery phase and adjusting policy and/or delivery settings as necessary.
2.2 This chapter examines whether rollout planning was conducted in a timely fashion and based on evidence. It also examines the assessment of risks to the rollout, the logistics and ‘channels’ used to deliver and administer vaccines, and whether a fit-for-purpose communication strategy was developed.
Was planning timely and informed by appropriate evidence?
The commencement of planning for the rollout was not timely and early planning did not include target dates for the rollout. Detailed engagement with the states and territories on rollout planning did not begin until November 2020 and Jurisdictional Implementation Plans were not agreed until February 2021, by which time the rollout of COVID-19 vaccines had already commenced. Initial planning on the prioritisation of vaccinations to vulnerable and at-risk groups was based on advice from the Australian Technical Advisory Group on Immunisation, an expert committee. Planning continued throughout the rollout. In June 2021, the Prime Minister announced the replacement of Health’s rollout taskforce with new Operation COVID Shield and a new Op COVID Shield National COVID Vaccine Campaign Plan was released in August 2021. Health subsequently developed a series of sub-plans which contained lower-level target dates for vaccinating specific priority groups and sectors.
2.3 On 7 August 2020, the Prime Minister announced that Australian governments31 had ‘strongly welcomed the Commonwealth Government’s COVID-19 Vaccine and Treatment Strategic Approach that provides a framework for securing early access to safe and effective vaccines and treatments’.32
2.4 Australia’s COVID-19 vaccine and treatment strategy (the vaccine and treatment strategy), released on 18 August 2020, stated that the government was ‘working in five areas to deliver safe and effective COVID-19 vaccines and treatments’: research and development; purchase and manufacturing; international partnerships; regulation and safety; and immunisation administration and monitoring.33
2.5 On 13 November 2020, Health published the Australian COVID-19 vaccination policy (the vaccination policy).34 The vaccination policy set out ‘the key policy parameters and approach to providing COVID-19 vaccines’, including high-level information on:
- roles and responsibilities of the Australian, state and territory governments and other key stakeholders in a COVID-19 pandemic vaccination program;
- information on the vaccines purchased by the Australian Government;
- key features of the vaccination program, including how doses would be made available to priority population groups and where and how vaccination would take place;
- how vaccine safety would be monitored;
- how data would be collected and reported; and
- how information on COVID-19 vaccines and vaccination would be made available to consumers and clinicians.
Rollout planning to June 2021
2.6 The vaccine and treatment strategy (released on 18 August 2020) stated that ‘Australian Government agencies are working with states and territories on transportation, storage and distribution plans. This will ensure vaccines, syringes and needles can be moved and stored securely and distributed rapidly’. However, although Health advised that it had monthly meetings between July and November 2020 with states and territories about ‘COVID planning’, the evidence shows that detailed engagement with the states and territories on rollout planning did not begin until November 2020.
2.7 At a meeting of state and territory health authority chief executive officers (CEOs) on 6 November 202035, Health outlined a number of key principles and assumptions for the rollout, which were:
- the rollout would not initially be administered under the National Immunisation Program (NIP)36;
- the rollout would be free for all Australian citizens, permanent residents, and most visa-holders37;
- vaccination would not be mandatory, but encouraged and likely incentivised;
- vaccination would be rolled out on the basis of identified priority populations, linked to delivery schedules, with scope for outbreak response; and
- the Australian Government would oversee the rollout, with defined responsibilities for the Australian, state and territory governments and shared governance.
2.8 A document prepared for the meeting emphasised that ‘connectivity of implementation plans and clear responsibilities are crucial’ and stated that individual Jurisdictional Implementation Plans (JIPs) were intended to be developed between the Australian Government and each state and territory government. The aim was to have JIPs with all jurisdictions agreed by early December 2020.
2.9 Between 18 and 23 November 2020, Health held a series of bilateral meetings with each state and territory health authority to ‘design jurisdictional implementation plans that are aligned with the Vaccination Policy’. Topics discussed included:
- vaccination locations38, workforce and training requirements;
- vaccine transport, delivery and storage;
- monitoring and reporting on vaccine stock, and minimising wastage;
- coordinating safety monitoring and surveillance of adverse events; and
- communication.
2.10 ‘Early indicative drafts’ of the JIPs were sent to each state and territory health authority on 29 November 2020. The ANAO saw evidence of a lengthy consultative and redrafting process during December 2020 and January 2021, with several versions of JIPs being circulated. The JIPs were agreed by state and territory governments in February 2021.39
2.11 The JIPs repeated the respective responsibilities of the Australian, state and territory governments that had been stated in the vaccination policy (see paragraph 2.5). They also contained detailed information about the topics which had been discussed during the earlier bilateral meetings (see paragraph 2.9).
2.12 While some arrangements for the rollout could not be finalised until it was known which vaccines were available40, the statement in the August 2020 vaccine and treatment strategy that ‘Australian Government agencies are working with states and territories on transportation, storage and distribution plans’ was not accurate. More than two and a half months elapsed between the release of the vaccine and treatment strategy (on 18 August 2020) and the first meeting between Health and state and territory health authority CEOs on 6 November 2020 at which Health first put forward the ‘proposed responsibilities for States and Territories’.
Determining priority populations
2.13 Both the vaccine and treatment strategy and the vaccination policy stated that ‘the Australian Technical Advisory Group on Immunisation (ATAGI) is preparing advice to support planning for the allocation and use of safe and effective vaccines’.41
2.14 ATAGI issued two sets of advice ‘aimed at supporting planning by the Australian Government in the development of a strategy for the procurement of COVID-19 vaccines and program delivery’. The preliminary advice (summarised in Table 2.1) was provided to the Australian Government in August 2020 and recognised that ‘when vaccines are available, supplies will initially be limited and priority groups for vaccination will need to be identified’ and that ‘prioritisation should be based on evidence influenced by the prevailing epidemiology when specific vaccines become available’.42 Following further considerations of the progress of clinical trials and international approvals, the advice was finalised and publicly released on 13 November 2020.
Table 2.1: ATAGI preliminary advice on priority population groups
|
Risk factor |
Priority population |
|
Those who have an increased risk of developing severe disease or dying from COVID-19. |
Older people. |
|
People with pre-existing underlying select medical conditions. |
|
|
Aboriginal and Torres Strait Islander people. |
|
|
Those who are at increased risk of exposure and hence of being infected with and transmitting SARS-CoV-2 to others at risk of severe disease or are in a setting with high transmission potential. |
Health and aged care workers. |
|
Other care workers (such as group residential care and disability care workers). |
|
|
People in other settings where the risk of virus transmission is increased (such as correctional and detention facilities, sea and airports and meat processing plants). |
|
|
Those working in services critical to societal functioning. |
Select essential services personnel (such as public health personnel, police, emergency services and defence forces). |
|
Other key occupations required for societal functioning (such as workers in distribution of essential goods and services such as food, water, electricity, telecommunication and other critical infrastructure). |
Source: Department of Health, ATAGI – Preliminary advice on general principles to guide the prioritisation of target populations in a COVID-19 vaccination program in Australia [Internet], 13 November 2020.
2.15 On 24 December 2020, ATAGI provided the Australian Government with supplementary advice which added to its preliminary advice and more specifically identified which population groups should be accorded priority.43 Based on ATAGI’s advice, Health prepared Australia’s COVID-19 vaccine national roll-out strategy (the rollout strategy)44, which was released on 7 January 2021 and announced by the Prime Minister at a press conference on the same day.45 Table 2.2 shows the rollout phases, priority populations and the estimated number of people in each population.
Table 2.2: Rollout strategy: priority populations (as identified by Health)
|
Phase |
Priority population |
Number (est.) |
|
1a |
Quarantine and border workers |
70,000 |
|
Frontline healthcare worker subgroups for prioritisation |
100,000 |
|
|
Aged care and disability care staff |
318,000 |
|
|
Aged care and disability care residents |
190,000 |
|
|
Sub-total |
678,000 |
|
|
1b |
Elderly adults aged 80 years and over |
1,045,000 |
|
Elderly adults aged 70 to 79 years |
1,858,000 |
|
|
Other health care workers |
953,000 |
|
|
Aboriginal and Torres Strait Islander people over 55 |
87,000 |
|
|
Younger adults with an underlying medical condition |
2,000,000 |
|
|
Critical and high risk workers including defence, police, fire, emergency services and meat processing |
196,000 |
|
|
Sub-total |
6,139,000 |
|
|
2a |
Adults aged 60 to 69 years |
2,650,000 |
|
Adults aged 50 to 59 years |
3,080,000 |
|
|
Aboriginal and Torres Strait Islander people 18 to 54 |
387,000 |
|
|
Other critical and high risk workers |
453,000 |
|
|
Sub-total |
6,570,000 |
|
|
2b |
Balance of adult population |
6,643,000 |
|
Sub-total |
6,643,000 |
|
|
3 |
Less than 18 (if recommended by ATAGI) |
5,670,000 |
|
Sub-total |
5,670,000 |
|
|
Total |
25,700,000 |
|
Source: Department of Health, Australia’s COVID-19 vaccine national roll-out strategy.
AstraZeneca recalibration
2.16 The initial stages of the rollout were heavily reliant on the AstraZeneca vaccine, with AstraZeneca comprising 80 per cent of the allocation of doses to sites over the first 12 weeks of the rollout.46
2.17 As discussed at paragraph 1.18, in early March 2021, reports began to circulate internationally about a rare but potentially serious side-effect47 of the AstraZeneca vaccine which appeared to be more prevalent in younger people. ATAGI monitored these reports and published several updates. On 8 April 2021, ATAGI updated its advice, recommending the use of Pfizer as the preferred vaccine for eligible people under 50.48
2.18 In April 2021, Australian governments considered options for the ‘recalibration’ of the vaccine strategy. Issues discussed included limited current supplies of Pfizer, changes to aged-based eligibility, the rollout to remote communities and increasing negative community sentiment towards AstraZeneca.49
2.19 On 22 April 2021, the Prime Minister announced that Australian governments had approved key changes to the COVID-19 vaccination rollout strategy.50 Seven of the key changes are listed in Table 2.3. Health implemented some changes, such as vaccine eligibility, in April 2021 and implemented other changes between May and August 2021.
Table 2.3: ANAO assessment of Health’s implementation of key changes under the recalibrated rollout strategy
|
Description of planned change |
Date implementedb |
Implementation complete |
|
Restricting the Pfizer vaccine to those under 50 years (with a few agreed exceptions).a |
22 April 2021 |
✔ |
|
Utilising the AstraZeneca doses by bringing forward eligibility for those over 50 years of age. |
4 May 2021 |
✔ |
|
Increasing access to Pfizer by:
|
17 May 2021 16 June 2021 |
✔ |
|
Providing more doses of the AstraZeneca vaccine to general practitioners (GPs) as demand permits through reallocation and re-directing doses within jurisdictions where it makes sense to do so. |
21 June 2021 |
✔ |
|
States and territories to continue to operate AstraZeneca sites where required, and open these sites up to those eligible in Phase 1a and 1b. |
24 May 2021 |
✔ |
|
Permitting state and territory operated vaccination sites to operate Pfizer and AstraZeneca services from the one site where practical. |
21 May 2021 |
✔ |
|
Encourage state and territories to incorporate community pharmacies into their rollout plans in rural and remote areas. |
11 August 2021 |
✔ |
Note a: ATAGI continued to monitor national and international safety data around AstraZeneca and on 17 June 2021 updated its advice to recommend the use of Pfizer as the preferred vaccine for eligible people under 60.
Note b: Date implemented is recorded as the date when the change was implemented in at least one jurisdiction or channel as relevant.
Source: ANAO analysis of Health documentation.
2.20 Health also acquired additional Pfizer doses to address the potential vaccine shortfall, consistent with the recalibrated rollout strategy. Between April 2021 and September 2021, Health ordered an additional 25.5 million Pfizer doses for delivery in 2021. These doses were delivered from mid-August with the majority to be available from September 2021.51
2.21 The change in ATAGI advice on AstraZeneca resulted in limited supply of vaccines preferred for people under 60 until September 2021.52 Health adapted the plan for the vaccine rollout and implemented these changes in a timely manner. This included encouraging the use of existing stocks of AstraZeneca while waiting for Pfizer suppply to increase from September 2021.
Sectoral vaccination program implementation plans
2.22 As noted in Table 2.2, people in residential aged care, Aboriginal and Torres Strait Islander people over 55 years and people with disabilities were three of the priority groups for vaccination under Phases 1a and 1b. In addition, Health identified people from culturally and linguistically diverse (CALD) communities as a group which, while not identified by ATAGI as a priority population, might need greater assistance or support due to language and cultural issues.
2.23 Health developed specific vaccination program implementation plans for each of these groups in early 2021, which contained tailored sector-specific information as well as information about:
- Australian, state and territory government responsibilities (and other parties where relevant);
- workforce and training requirements;
- arrangements for safety monitoring and surveillance of adverse events53;
- minimising wastage, monitoring stock and reporting; and
- communication to people in the sector.
2.24 The plans for aged care, Aboriginal and Torres Strait Islander people and CALD communities were published on Health’s website in February and March 2021. The plan for people with disabilities was not published.54
Establishment of target dates in planning
2.25 The vaccine and treatment strategy and the vaccination policy (see paragraphs 2.4 and 2.5) did not contain any target dates or indication when the rollout would start or finish. At the time that these documents were published, it was not known when vaccines would become available. Jurisdictional Implementation Plans (see paragraphs 2.8 to 2.12) contained ‘indicative timelines’ for when the phases referred to in Table 2.2 would commence but not when they might be completed.
2.26 In late 2020 and early 2021, the Prime Minister, the Minister for Health and Aged Care and the Secretary of Health made a number of public statements and commitments about:
- when the rollout would commence;
- the date by which people could expect to be (or be given the opportunity to be) vaccinated; and
- when priority or vulnerable groups would be vaccinated.55
2.27 Health did not include target dates for the rollout as part of its planning prior to June 2021. In March 2021, the COVID-19 vaccination program was the subject of an Implementation Readiness Assessment (IRA)56 commissioned by the Department of Finance, which noted that achievement of the ‘policy commitment to complete the vaccination program by end October 2021’ was ‘highly unlikely’ and found:
the Program has adopted a just-in-time approach to planning, and has an acute focus on the next four weeks. The review team has seen only a high-level plan and no supporting detail for the medium term (until end October). Further, the vaccination deployment modelling beyond April is high level and incomplete. With the delivery of large quantities of vaccine by CSL (Seqirus) by end March, there is no justification for not having a model and delivery schedule for the entire program. The review team finds that the lack of this model and schedule is impeding preparations for future rollout, including by states and territories, that may require considerable lead times. The lack of a model and schedule for the medium term also means that it is not possible to have confidence in achievement of an end-October target.
2.28 The IRA’s recommendations included:
- Consider the use of intermediate objectives that demonstrate key public health outcomes prior to the October target. An example would be ‘all vulnerable Australian are protected’, as at the end of Phase 1a and 1b.
- Consider modifying the Program’s public objective to a more achievable version such as ‘all eligible Australians will be offered the vaccine and will have the opportunity to commence vaccination by end-October’.
- Develop a vaccination deployment model and associated plan for the next 8-12 months…
2.29 Health ‘noted’ these recommendations and its response was contained in an internal document which was not prepared until July 2021, after the establishment of Operation COVID Shield. The response to the recommendation on intermediate objectives read:
To support the recalibration following National Cabinet’s decision on April 16, the overarching narrative and guiding communications principles of the roll out have been revisited and the revised strategic approach supersedes the intent of this recommendation.
The revised approach includes focus groups for key cohorts, the exploration of partnerships to reach specific audiences and a refresh of the communications campaign. Vaccine Communications are also working closely with [the Department of the Prime Minister and Cabinet] to ensure the approach remains responsive to public sentiment. Key artefacts including Stakeholder Engagement and Communications Plan and External Communications Playbook have been revised.
2.30 Following the transition to Operation COVID Shield, lower-level targets were developed for some aspects of the rollout (see paragraph 2.37). Health’s reporting against these targets is discussed at paragraph 3.36 and achievement of targets is discussed in Chapter 4 and Appendix 4.
Rollout planning from June 2021 (Operation COVID Shield)
2.31 At a press conference on 4 June 2021, the Prime Minister announced the appointment of the Coordinator General of Operation COVID Shield, stating:
Lieutenant General Frewen will have direct operational control across numerous government departments for the direction of the national vaccination program and all of those working in that program, from communications to dealings with states, to the distribution and delivery of vaccines and all of these matters, and the ramp-up, the scale-up, the working with the GPs, pharmacists and others, this will all come under the direct control of Lieutenant General Frewen… I think that very direct command and control structure that has proved to be so effective in the past will add a further dimension and assistance as we step up in this next phase.57
2.32 The ANAO sought Health’s comments on the reasons for the move to a ‘command and control’ model and how that differed from the previous arrangements. Health said:
Operation COVID Shield used a command and control structure to coincide with an influx of new supply, and a need to increase the size and speed of the rollout. The command and control structure included direct operation control of all relevant assets and resources across all Commonwealth Government departments and agencies engaged in the direction and implementation of the national COVID vaccination program. [Operation COVID Shield] introduced a military led planning team and an assessment cell to support the command and control structure of the operation. The plans and assessment cells differ from traditional structures as they span all of the areas of the taskforce and aim to consolidate planning and tracking across all branches with a direct report to Coordinator General, as opposed to decentralised planning normally undertaken within the branches.
Operation COVID Shield campaign plan
2.33 On 3 August 2021, the Coordinator General released the Op COVID Shield National COVID Vaccine Campaign Plan (the campaign plan)58, which was stated to cover the period from 1 July 2021 to 31 December 2021. The plan described several challenges existing at that time, including:
- changed guidance from ATAGI in April 2021 (see paragraph 2.17) in relation to potentially dangerous side-effects associated with the AstraZeneca vaccine, which resulted in reduced confidence in the AstraZeneca vaccine and a surge in demand for the Pfizer vaccine;
- limited global supply of the Pfizer vaccine;
- delays in delivery to some priority groups due to global supply challenges, domestic supply shortfalls and ‘scale up’ challenges with vaccine administration service providers59; and
- changes made by states and territories to agreed vaccine eligibility criteria.
The campaign plan was divided into three stages (shown in Figure 2.1) which were based on anticipated vaccine supply and linked to and balanced with the progressive introduction of administration channels (discussed further at paragraph 4.8).
Figure 2.1: Operation COVID Shield campaign plan stages
Source: Operation COVID Shield campaign plan (Department of Health).
2.34 To assist vaccination providers (including those for which the states and territories were responsible) with their own planning, in June 2021, Health published ‘COVID Vaccination Allocations Horizons’ which showed the notional allocations for each state and territory by vaccine and channel for July to August 2021 (Horizon 1), September 2021 (Horizon 2) and October to December 2021 (Horizon 3). Later editions of the ‘Horizons’ were published in July and September 2021, with allocations updated based on modelling of future supply.
2.35 With regard to priority populations, the campaign plan stated that:
There are populations that have circumstances that require unique consideration to ensure equity of access and confidence in the vaccine rollout. To safeguard the health and wellbeing of the Australian population, it is critical that these priority groups have access to vaccines and are supported in receiving them.
2.36 The ten priority populations identified in the campaign plan are listed in Table 2.4.
Table 2.4: Operation COVID Shield campaign plan: priority populations
|
Priority populations |
|
|
Aged care |
Culturally and linguistically diverse communities |
|
Disability accommodation |
Aboriginal and Torres Strait Islander people |
|
Regional, Rural and Remote rollout |
External territories |
|
Quarantine and border workers |
12-15-year olds |
|
Health care workers |
Other priority populationsa |
Note a: This includes people who are homeless, those in prison, those requiring drug and alcohol support, those in mental health facilities and social housing.
Source: Department of Health Operation COVID Shield campaign plan.
Sub-plans
2.37 To support the campaign plan, Health developed a series of sub-plans, including ‘acceleration plans’, which focused on specific priority groups and sectors. While the content of the sub-plans varied according to topic, they generally set out specific tasks and lower-level target dates for priority groups and sectors and were more consistent with the intention of the recommendation in the March 2021 Implementation Readiness Assessment (see paragraph 2.27). Examples of sub-plans are shown in Table 2.5.
Table 2.5: Examples of Operation COVID Shield sub-plans
|
Name |
Plan type |
Date approved |
Target set? |
|
Aboriginal and Torres Strait Islander acceleration plan |
Acceleration |
21/9/2021 |
80% of Aboriginal and Torres Strait Islander people 12 or over receive at least one dose by 31/10/2021. |
|
Youth vaccination (12-18) |
Sub-plan |
21/9/2021 |
All youth are given opportunity to receive at least first dose by 20/12/2021. |
|
Pharmacy rollout |
Sub-plan |
21/9/2021 |
On-board suitable pharmacies for Moderna administration in six week period commencing September 2021. |
|
Residential disability in-reach |
Sub-plan |
21/9/2021 |
Two doses offered to residents of all 6,311 residential disability sites by 31/10/2021. |
|
NDIS participants and workers |
Acceleration |
15/10/2021 |
80% of NDIS participants 12 or over and workers fully vaccinated by 31/10/2021. |
|
Booster in-reach program for aged care and disability residents |
Sub-plan |
3/11/2021 |
Program to commence 8/11/2021. |
|
Regional and remote |
Sub-plan |
Not signed or dated |
Not seta |
Note a: The sub-plan did not set a specific target: it outlined ‘efforts to ensure that regional and remote communities are able to reach 70% and 80% fully vaccinated targets in line with the general population’.
Source: ANAO from Health documents.
Were risks to the rollout identified and regularly reassessed?
Risks to the rollout were identified from June 2020. In January 2021, Health established a comprehensive risks and issues register which was used throughout 2021 to identify risks to the rollout. Risks were kept under regular review and, where necessary, corrective action was determined. The most significant risks and issues were referred to the taskforce leadership for review.
2.38 Under the Public Governance Performance and Accountability Act 2013 (PGPA Act), the accountable authority60 must establish and maintain appropriate systems of risk oversight, management, and internal control for the entity.61 The Commonwealth Risk Management Policy sets out the government’s risk management expectations for Australian Government entities in undertaking the business of government.62
2.39 There was early awareness that vaccination of the entire Australian population would be attended by many risks. For example, advice provided to government in June 2020 observed that the most significant risk was that there may never be a COVID-19 vaccine. As it became clear that a number of potentially effective vaccines would likely be available, internal documents show that Health started considering rollout risks in the latter part of 2020.
2.40 The August 2020 vaccine and treatment strategy committed the government to building a ‘diverse global portfolio of investments to seek to secure early access to promising vaccines and treatments, using local manufacturing wherever possible’. Two of the vaccines initially selected for purchase, the University of Queensland vaccine and AstraZeneca, were intended to be manufactured in Australia. At the time that advance purchase agreements for COVID-19 vaccines were signed, none of the selected vaccines had reached Phase 3 clinical trials and hence achieved TGA approval (see paragraph 1.8), which is a pre-requisite for use in Australia. In September 2020, an internal Health document identified a risk that ‘Domestic manufacture [is] not possible because negotiated candidate is not approved, further reducing dose volume’. This risk was subsequently realised (see case study 1).
|
Case study 1. Realisation of risks to domestic manufacture |
|
In October 2020, following Phase 1 clinical trials, the University of Queensland identified that its vaccine could produce false positive readings with certain HIV screening tests. Though there was no health risk, the potential for a false positive HIV result posed the risk that people may be deterred from being vaccinated.a Health sought the views of SITAG which agreed on 8 December 2020 that the University of Queensland vaccine should not continue to Phase 2/3 clinical trials. Consequently, the contract with the University of Queensland was terminated in December 2020. As a result, Health needed to address the potential shortfall of an anticipated 51 million doses for Australia’s vaccine portfolio. Health responded to the University of Queensland termination and on 10 December 2020, put to government a proposal to:
|
Note a: On 11 December 2020, the University of Queensland reported: ‘There is no possibility the vaccine causes infection, and routine follow up tests confirmed there is no HIV virus present. With advice from experts, CSL and UQ have worked through the implications that this issue presents to rolling out the vaccine into broad populations. It is generally agreed that significant changes would need to be made to well-established HIV testing procedures in the healthcare setting to accommodate rollout of this vaccine’.
Note b: AstraZeneca and Novavax had each indicated they could provide sufficient doses to cover the whole of the Australian population. The additional doses for both vaccines were announced on 11 December 2020. Negotiations for the additional doses of AstraZeneca and Novavax concluded on 24 December 2020, and 31 December 2020 respectively.
Risks and issues log
2.41 In December 2020, Health created a ‘risks and issues log’ which contained both a risk register and an issues log. With respect to risks, the log showed:
- the date that the risk was logged and who raised it;
- the risk owner and impacted workstream;
- the impacted stakeholder group63;
- the risk name, description and impact (if it were to occur);
- the current risk level (likelihood and consequence);
- the control assessment and treatment plan (if necessary); and
- the target risk rating.
2.42 The issues log was similarly detailed. Its purpose was to record what action had been taken with respect to identified risks.
2.43 One of the risks identified in the risks and issue log was that ‘uptake is below desired levels due to consumer sentiment’.64 This risk was also realised with the AstraZeneca vaccine (discussed at paragraphs 2.17 to 2.21).
2.44 The earliest version of the risks and issues log that the ANAO located (dated 15 January 2021) contained 79 risks. Some examples included:
- adverse event management — incomplete and inaccurate data;
- lack of suitably safe vaccine candidates;
- shortages of critical materials required for vaccinations;
- COVID-19 outbreak occurs impacting rollout; and
- immunisation workforce not sufficient, ready or appropriately skilled.
2.45 The latest version of the risks and issues log examined by the ANAO was dated 6 December 2021. It contained 301 risks, with risks added progressively throughout 2021.
2.46 Health instituted a process by which ‘top risks and issues’ were the subject of a ‘deep dive’ to assess the risk and consider whether any specific action was necessary. Risks rated ‘high’ or ‘extreme’ were then presented to a weekly meeting of the taskforce leadership group65, which reviewed the proposed corrective action and whether it was satisfied with the outcome. The ANAO reviewed examples of this process and found there was robust consideration of key rollout risks.
2.47 The consistent use of a risk register and a documented process to respond to risks as they arose demonstrates good practice.
Were administration channels and logistics considered and established?
Health worked with state and territory authorities and relevant stakeholders to consider and establish a variety of administration channels that were suitable for priority groups and the population as a whole. Two logistics providers were engaged to transport and deliver vaccines throughout Australia. Health underestimated the magnitude and complexity of rolling out in-reach services for the residential aged care and disability sectors and did not engage sufficient in-reach providers early in the rollout.
Administration channels
2.48 With more than 20 million people in Australia needing to be given at least two doses of vaccine66, it was clear from the outset that as many administration channels as possible would need to be used.67 The vaccination policy, published in November 2020, stated:
Vaccination sites will be agreed by the Australian Government and the States and Territories through their jurisdictional implementation plans. There are a number of likely vaccination locations. All vaccines must be administered in accordance with the relevant legislation, best practice, and the guidelines and recommendations the Australian Immunisation Handbook. Vaccination locations must facilitate the safety of vaccines, staff, and consumers; be adequately staffed with appropriately trained personnel; have the facilities and protocols in place to ensure data is reported in an accurate and timely way; and be able to manage high volumes of vaccinations.68
2.49 Other factors that influenced decisions about administration channels were:
- the difficulty some people (such as the elderly and people with disabilities) would have in attending a location such as a general practice;
- availability of vaccines;
- distribution capacity of logistics providers;
- the remoteness of some smaller areas of population; and
- the necessity to keep some vaccines ultra-cold at all times from manufacture until administration.
2.50 Under long-standing arrangements, responsibility for the administration and funding of health care facilities in Australia is shared between the Australian and state and territory governments. For example, while in 2017–18, the Australian Government provided 39 per cent of funding for public hospitals69, responsibility for their administration rests entirely with states and territories. Consequently, arrangements for establishing which locations would administer vaccines was one of the issues negotiated during the development of JIPs (see paragraph 2.9). Table 2.6 shows the administration channels that were used for the rollout and which government was responsible for the administration of vaccines for each.70
Table 2.6: Vaccine administration channels: responsible governments
|
Administration channel |
Government responsible |
Comment |
|
General practice |
Australian |
Registered medical practitioners providing medical care for individuals, families and communities. |
|
Commonwealth vaccination clinics (CVCs) |
Australian |
Originally established to assess and test people with respiratory symptoms. |
|
Aboriginal Controlled Community Health organisations (ACCHOs) |
Australian |
Funded by the Australian Government and operated by local Aboriginal and Torres Strait Islander communities. |
|
Community pharmacies |
Australian |
Part of the community they serve (as distinct from consultant, hospital and industrial pharmacies). |
|
In-reach |
Australian |
Companies engaged to supply qualified providers to visit and administer vaccines on site. |
|
Hospital hubs |
State and territory |
Hubs established within, or on the campus of, hospitals. |
|
Mass vaccination hubs |
State and territory |
Fixed sites such as stadiums or conference centres. |
|
Community hubs |
Australian and state and territory |
Such as pop-up clinics in places of worship and mobile clinics for the homeless. |
|
Royal Flying Doctor Service (RFDS) |
Australian |
The RFDS was engaged under contract to distribute vaccines to selected rural and remote areas. |
Source: Health.
2.51 While some administration channels were established specifically to cater to particular priority groups71, people in those groups were not limited to only those administration channels. If they were able to, or preferred to, they could use another administration channels (such as their own general practitioner). Table 2.7 links the priority groups with the administration channels shown in Table 2.6. The introduction of administration channels was staged based on the priority populations they were targeting and Health’s modelling of expected vaccine supply. The implementation of administration channels is discussed in Chapter 4.
Table 2.7: Priority groups and administration channels
|
Population |
In-reacha |
Primary Care |
State and territory hubs |
Other |
|||
|
|
|
GP |
CVC |
ACCHO |
Pharmacy |
|
|
|
Aboriginal and Torres Strait Islander people |
◇ |
◇ |
◇ |
◆ |
◇ |
◇ |
◇b |
|
Residential aged care facility (RACF) residents |
◆ |
◇ |
◇ |
◇ |
◇ |
◇ |
|
|
Residential disability facility residents |
◆ |
◇ |
◇ |
◇ |
◇ |
◇ |
|
|
RACF and residential disability workers |
◆ |
◇ |
◇ |
◇ |
◇ |
◆ |
◇c |
|
Non-residential people with a disability |
◇ |
◆ |
◆ |
◇ |
◆ |
◆ |
|
|
Non-residential older people |
|
◆ |
◆ |
◇ |
◆ |
◆ |
|
|
Frontline healthcare and border workers |
|
◇ |
◇ |
◇ |
◇ |
◆ |
|
|
People with pre-existing medical conditions |
|
◆ |
◆ |
◇ |
◆ |
◆ |
|
|
Regional and remote populations |
|
◆ |
◆ |
◇ |
◆ |
◆ |
◆b |
|
Remainder of population aged 12 or over |
|
◆ |
◆ |
◇ |
◆ |
◆ |
|
|
KEY: ◆ main administration channel/s for the population ◇ able to access vaccines through the administration channel |
|||||||
Note a: The majority of in-reach was performed by contracted Vaccine Administration Service providers (VAS providers). This is discussed further at paragraphs 4.12 and 4.13.
Note b: The Royal Flying Doctor Service delivered and administered vaccines in regional and remote communities, including to Aboriginal and Torres Strait Islander communities.
Note c: RACF and residential disability workers were eligible to access specific pop-up clinics.
Note: People can belong to multiple priority groups, for example an Aboriginal and Torres Strait Islander frontline healthcare worker. These people could access administration channels for both priority groups.
Source: ANAO analysis of Health records.
Operational planning for in-reach services
2.52 Most administration channels listed in Table 2.7 utilised existing infrastructure and processes, such as vaccines being administered by GPs. Health needed to establish new arrangements for in-reach services to the residential aged care and residential disability sectors, which required detailed operational planning.
2.53 Following an approach to market in December 2020, in late January 2021 Health engaged two Vaccine Administration Service (VAS) providers to conduct in-reach visits to residential aged care facilities. Health issued the first work orders under these contracts in early February 2021 and the providers began administering vaccines at residential aged care facilities when the rollout began on 22 February 2021. These work orders included detailed operational requirements, such as site requirements and RACF locations. Health engaged additional VAS providers later in the rollout when it identified the current arrangements were at high risk of not meeting its target dates for this area. The additional VAS providers were engaged under similar arrangements, as discussed in paragraphs 4.37 and 4.47.72
2.54 To plan the administration of vaccines to the residential disability sector, Health contracted a VAS provider (Aspen Medical) on 19 February 2021 to engage with key stakeholders and co-design the delivery model. This was provided to Health on 26 February 2021, and Health confirmed the model and approach for the rollout to the residential disability sector on 10 March 2021, more than one month later than for the residential aged care sector and after the rollout had commenced. On 1 April 2021, Health executed a work order with the same VAS provider used in the residential aged care rollout to provide in-reach services to the residential disability sector. Health engaged an additional VAS provider in May 2021, when existing arrangements proved to be insufficient. Health supplemented its arrangements for in-reach with operational policies and procedures, such as the excess dose policy.
2.55 The need to engage additional VAS providers later in the rollout indicates that Health underestimated the magnitude and complexity of the in-reach rollout to aged care and residential disability facilities. This issue is discussed further at paragraphs 4.35 and 4.47.
Logistics
2.56 In December 2020, Health entered into contracts with DHL and Linfox to co-design arrangements for the delivery of vaccines and ancillary consumables such as syringes throughout Australia. Logistical services contracts were signed with both providers in February 2021. Each provider was allocated responsibility for deliveries to particular geographical regions of states and territories. The providers were required to report to Health monthly on:
- cold chain compliance;
- data integrity;
- completeness and timeliness of deliveries;
- reporting and remediation of incidents; and
- lost, damaged or stolen vaccines and consumables.
2.57 Distribution and logistics services commenced in February 2021 for DHL and March 2021 for Linfox. Implementation of the logistics arrangements is discussed further at paragraphs 4.3 to 4.5.
Was a fit-for-purpose communication strategy for the COVID-19 vaccine rollout developed?
Health developed a fit-for-purpose strategy for communicating the vaccine rollout. The communication strategy and its supporting advertising strategy identified target audiences and was tailored to addressing the concerns of these groups as determined through market research. The advertising strategy complied with government guidelines for advertising campaigns and had a plan to monitor progress.
2.58 Public communication can include advertising campaigns73, information campaigns and a variety of other media, information and marketing activities. Effective public communication is particularly important during a crisis such as the COVID-19 pandemic, as governments seek to convince the public to take prompt action to protect public health.
2.59 Health developed two strategies to guide communication of the COVID-19 vaccine rollout:
- the Communication strategy: COVID-19 vaccines and treatments (communication strategy), developed in October 2020, which outlined Health’s overarching strategy for communicating the vaccine rollout; and
- the COVID-19 Vaccines Campaign Advertising Campaign Strategy (advertising strategy), approved on 11 January 2021, which addressed the public relations and advertising for the rollout.
Communication strategy (October 2020)
2.60 Health developed a draft communication strategy for Australia’s COVID-19 vaccine and treatments strategy in October 2020. Health was unable to provide evidence that the draft strategy was approved. Health informed the ANAO that the strategy was a living document and was updated throughout the vaccine rollout.
2.61 To assess whether Health’s communication strategy was fit-for-purpose, the ANAO examined whether it:
- outlined its objectives and strategic approach;
- was evidence-based;
- identified its target audience; and
- considered risk.74
Objectives and strategic approach
2.62 The objective of the communication strategy was to:
Provide timely, transparent and credible information to inform and educate the Australian public about the Government’s COVID-19 Vaccine and Treatments Strategy. This will build confidence in the regulatory processes for COVID-19 vaccines and treatments, keep Australian’s up to date on progress of and vaccine candidates, including international developments and local investment in research and ensure implementation plans for a national vaccination program are clearly communicated to support high uptake.
2.63 To achieve the communication strategy’s objective, Health developed six principles for communication:
- be the authoritative source of information;
- meet specific information needs;
- address known motivators;
- create genuine engagement with the health sector;
- take preventative measures; and
- support implementation.
2.64 The ANAO assessed that Health’s communication principles collectively addressed the principles for effective communication in public health outlined in the World Health Organisation’s (WHO’s) Strategic Communication Framework for effective communications.75
2.65 Health’s communication strategy identified 16 communication channels to achieve its objective, including Australian Government websites, paid advertising, webinars and newsletters to health professionals76, and outlined high level key messages to be used on these channels. The strategy also established four phases of activity which aligned with the phases of the vaccine rollout (see Table 2.8).
Table 2.8: Planned communication phases for the vaccine rollout
|
Communications phase |
Focus of communication activities |
Vaccine Rollout Phase |
|
Phase 1 |
Purchase announcements, policy announcements |
Pre-rollout |
|
Trial progress and TGA milestones |
||
|
Establishing authoritative, enduring sources of information |
||
|
Preparatory work with providers and priority groups |
||
|
Phase 2 |
Campaign activity begins |
Phase 1a/1b |
|
Safety and effectiveness messages specific to approved vaccine |
||
|
Supporting priority groups in uptake |
||
|
Phase 3 |
Campaign focuses on uptake call to actions |
Phase 2a/2b |
|
Access information, dose completion and consumer monitoring |
||
|
Responsive to consumer questions and concerns |
||
|
Reiterating COVIDSafe behaviours as well as vaccination |
||
|
Phase 4a |
Addressing specific barriers to uptake |
Phase 2b/3 |
|
Campaign targets under-vaccinated groups |
||
|
Encouraging dose completion |
||
Note a: Phase 3 and Phase 4 of communications were consolidated into one phase in December 2020.
Source: Health.
Evidence-based
2.66 The development of the communication strategy was informed by market research on vaccines and COVID-19, including monitoring of public sentiment.77 For the research conducted for the vaccine rollout, the focus groups contained a sample across age, gender, income, cultural and linguistic background and indigeneity. The research identified the public’s likelihood to get vaccinated as well as motivators and barriers for vaccination.
2.67 Health used information from market research to generate targeted messaging. For example, Health’s market research in December 2020 identified that, although support for vaccines was high, 30 per cent of Australians were hesitant to receive a COVID-19 vaccine. Health developed communication activities to target these hesitant Australians. This included regular media engagement by senior officials and advertising materials addressing barriers to vaccination.
2.68 The communication strategy identified key stakeholders to provide expert advice on communication materials and identify appropriate channels for target audiences. These stakeholders include health and community sector peak bodies and health advisory groups.
Target audience
2.69 The communication strategy identified the Australian public and the health sector as the primary audiences for information on the vaccine rollout. Health also identified specific populations to target including: culturally and linguistically diverse communities; Aboriginal and Torres Strait Islander people; people with a disability; older Australians; and hesitant segments of the Australian population.
Risk
2.70 The communication strategy considered communication and rollout risks.78 The communication risks and their mitigations are shown in Table 2.9. The mitigations focused on communication activities.
Table 2.9: Summary of communication risks identified for the vaccine rollout and mitigations
|
Risk |
Proposed mitigation |
|
Disinformation and misinformation |
|
|
Active negative sentiment against vaccination |
|
Source: Health.
Advertising strategy (January 2021)
2.71 The advertising strategy was approved by the Australian Government on 11 January 2021 and supported the communications strategy by addressing advertising and public relations for the vaccine rollout. Consistent with the Australian Government Guidelines on Information and Advertising Campaigns by non-corporate Commonwealth entities (the guidelines) the Australian Government also approved amendments to the advertising strategy and the advertising concepts for each of the three phases of the advertising campaign.
2.72 The guidelines set out five principles and the approval and evaluation process which advertising by all non-corporate Commonwealth entities must comply with. The ANAO assessed that Health’s advertising for the vaccine rollout followed the process included in the guidelines and complied with principles one to four.79 These four principles require advertising to be: relevant to government responsibilities; presented in an objective, fair and accessible manner; objective and not directed at promoting party political interests; and justified and undertaken in an efficient, effective and relevant manner.
2.73 Health did not include measures for success in the communication strategy but the supporting advertising strategy outlined how it would measure its success. Many of these measures of success tracked high level desired impacts, such as public sentiment towards COVID-19 vaccines. This allowed Health to also monitor communication more broadly.
3. Governance
Areas examined
This chapter examines whether the Department of Health and Aged Care (Health) established effective governance arrangements to manage the COVID-19 vaccine rollout.80
Conclusion
The final governance arrangements established to manage the COVID-19 vaccine rollout have been largely effective. Following the commencement of Operation COVID Shield in June 2021, senior level oversight of the program substantially increased. Health regularly consulted with four sector-specific stakeholder advisory groups. Health put in place effective monitoring and reporting arrangements using the best available data. However, it did not undertake sufficient reporting against targets, and it does not have adequate assurance over the completeness and accuracy of the data and third-party systems.
Area for improvement
The ANAO made two recommendations aimed at Health: obtaining greater assurance over data quality and IT controls in externally managed systems; and conducting a comprehensive review of the COVID-19 vaccine rollout.
3.1 Appropriate governance arrangements need to be established early in any emergency response or rapid implementation process to optimise their value. At the whole-of-government level, this can be achieved by mobilising standing governance and coordination arrangements outlined in crisis preparedness frameworks and plans. At the entity level, accountable authorities need to determine what is fit-for-purpose in the specific circumstances facing their entities and ensure that governance considers impacts on business-as-usual activities and changes to risk tolerance levels and risk control measures.
3.2 To assess whether Health established effective governance arrangements to manage the COVID-19 vaccine rollout, the ANAO examined whether: fit-for-purpose governance arrangements were established; key responsibilities were assigned; effective arrangements were put in place for monitoring and reporting progress; and fit-for-purpose stakeholder engagement was undertaken.
Were fit-for-purpose governance arrangements established and key responsibilities adequately assigned?
Fit-for-purpose governance arrangements were established for the vaccine rollout following the commencement of Operation COVID Shield in June 2021. Senior level oversight of the program also substantially increased. Responsibilities of the Australian, state and territory governments and other stakeholders were documented in the Australian COVID-19 vaccination policy, Jurisdictional Implementation Plans and sector specific implementation plans.
Health governance arrangements
3.3 As noted at paragraph 1.19, the administration of the vaccine rollout had two phases. Until June 2021, the rollout was administered by a taskforce within Health. In June 2021, the Prime Minister established Operation COVID Shield and appointed a Coordinator General for the rollout. An Office of the Coordinator General was created, with some reorganisation of responsibilities. This section deals with each phase separately.
Governance arrangements to June 2021
3.4 Health initially established a vaccine strategy taskforce in June 2020, headed by an officer at the Senior Executive Service (SES) Band 2 level (First Assistant Secretary). The structure is shown in Figure 3.1.
Figure 3.1: Initial Health high level organisation and governance chart to June 2021

Note a: SRO means Senior Responsible Officer.
Note b: The Chief Medical Officer is an officer of Health and is also the principal medical adviser to the Minister for Health and Aged Care.
Note c: PwC were contracted to establish and run a Program Delivery Office to assist with the central coordination and delivery of the rollout program.
Note d: The number of SES Band 1 officers increased over the rollout from 6 officers in February 2021 to 11 officers in June 2021.
Source: Health.
3.5 Within the taskforce, a total of seven workstreams were initially established, each headed by an SES Band 1 level officer (Assistant Secretary). The number of workstreams increased over time to nine in February 2021 and eleven in June 2021. The workstreams as at February 2021 and the responsibilities assigned to each workstream are shown in Table 3.1.
Table 3.1: Vaccine strategy taskforce workstreams assignment of responsibilities as at February 2021
|
Workstream |
Assigned responsibility |
|
Sourcing |
Obtaining COVID-19 vaccines and other key inputs (consumables) through advance purchase arrangements. |
|
Distribution |
Identifying of distribution options, establishing of contracts with logistics service providers, and developing of inventory management and administration processes. |
|
Vaccination |
Mobilising a certified and trained immunisation workforce; establishing appropriately equipped vaccination sites. |
|
Post vaccination/ Therapeutic Goods Administration (TGA) |
Monitoring and evaluating the effectiveness of COVID-19 vaccines on vaccinated Australians through active and passive surveillance (delivered in partnership with the TGA). |
|
Aged Care and Disability |
Implementation of the vaccine rollout to residential aged care and disability facilities. |
|
Policy and funding |
Supporting development of key policy decisions and funding arrangements, as well as whole-of-government strategic outputs and jurisdiction interactions. |
|
Communications and stakeholder engagement |
Establishing communication practices with stakeholders; executing strategic communications strategies. |
|
Data and digital |
Designing and implementing technology solutions to support vaccine rollout. |
|
Delivery governance |
Overseeing and coordinating program delivery and escalating key matters for decision at relevant governance forums. |
Source: Health.
Operation COVID Shield governance arrangements after June 2021
3.6 At a press conference on 4 June 2021, the Prime Minister announced the appointment of the Coordinator General of Operation COVID Shield. He said:
Some years ago, you might recall, there was an operation called Operation Sovereign Borders, I put in place at that time with Prime Minister Abbott, a completely new organisational structure to for getting a whole of government effect on a very big problem. It worked on that occasion and I think moving that footing now will further improve how we’re working in the vaccination program. Lieutenant General Frewen will have direct operational control across numerous government departments for the direction of the national vaccination program and all of those working in that program, from communications to dealings with states, to the distribution and delivery of vaccines and all of these matters, and the ramp-up, the scale-up, the working with the GPs, pharmacists and others, this will all come under the direct control of Lieutenant General Frewen.
3.7 At a later press conference in February 2022, the Prime Minister said:
on the vaccination programme, if I had my time over, I would have put it under a military operation from the outset and not later in the year. But we’d all worked up the plan together. Going through cabinet, our cabinet, been through the National Cabinet and set out the timetables. We’d had the goal of ensuring that everyone who wanted a vaccine, could be offered one by October, the record that was achieved on the 25th of October. And as we went through those early months and we had the challenges that we have with the health department and us dealing with many, many issues, I took the decision to send in General Frewen and changed the way we did it and set up a change in the command structure, how logistics were managed, how it was planned, and it worked. But I wish we’d done that earlier, and that’s a lesson.
3.8 The Prime Minister’s comments suggest that the vaccine rollout governance arrangements that existed prior to the establishment of Operation COVID Shield were not considered fit for purpose.
3.9 In his letter of appointment in June 2021 to the Coordinator General, the Prime Minister said:
As Co-ordinator General, you will have direct operational control of all relevant assets and resources across all Commonwealth government departments and agencies engaged in the direction and implementation of the national COVID vaccination program. Among other things, you will be responsible for communication around the vaccine rollout, engagement with states and territories, distribution and delivery of vaccines, engagement with health providers including hospitals, GPs, Aboriginal Health Services and pharmacies, and engagement with key community stakeholders, business groups, and unions.
In performing these functions, you will be responsible directly to me and the Minister for Health. Accordingly, communications should be jointly addressed to both of us and coordination should occur with both of our offices simultaneously.
3.10 In terms of governance arrangements within Health, the Prime Minister also asked that the Coordinator General ‘work closely with both the Secretary of the Department of Health, Dr Brendan Murphy, and Australian Public Service Commissioner, Peter Woolcott AO, to ensure that you have the support you require’.81
3.11 The Coordinator General was supported by a taskforce which became known as the National COVID Vaccine Taskforce. Health advised the ANAO that the majority of staff were drawn from the previous taskforce. In discussion with the ANAO, the Coordinator General stated that he engaged with the ‘key players’ in Health to understand how the rollout was working and then developed the command structure he wanted. This is shown at Figure 3.2.
Figure 3.2: Operation COVID Shield organisation and governance chart after June 2021

Note: Light blue denotes decision-making; dark blue denotes advisory and green and orange denote working teams.
Note a: The Deputy Coordinator General was an SES Band 3 (Deputy Secretary) level officer who holds the necessary delegations from the Secretary of Health for financial approvals under the Public Governance, Performance and Accountability Act 2013 (PGPA Act).
Source: Health.
3.12 The Coordinator General retained a workstream structure. The revised workstreams were broadly similar in terms of responsibilities to workstreams in place before June 2021. Some workstreams were added to reflect the status of the rollout in June 2021 and others were added specifically relating to priority groups. In total, there were 19 workstreams grouped into three ‘themes’:
- coordinate: to develop the framework and governance for a coordinated approach between the Australian Government, jurisdictions and the private sector;
- motivate: to shape positive public sentiment and drive public action via targeted communications and incentives; and
- deliver: to establish the capacity to build on existing vaccination efforts with additional channels and ‘points of presence’, and ‘dynamic reallocation’ of supply across channels, to drive a ‘step up’ in vaccination supported by progressively increased supply.
3.13 The relationship between themes, workstreams and responsibilities is shown in Table 3.2.
Table 3.2: Operation COVID Shield: workstream assignment of responsibilities as at September 2021
|
Theme |
Workstream |
Assigned responsibility |
|
Coordinate |
Digital and Service Design |
Designing and implementing technology solutions to support vaccine rollout. |
|
Policy and Program Design |
Support development of key policy decisions and funding arrangements, as well as whole-of-government strategic outputs and jurisdiction interactions. |
|
|
Data and Intelligence |
Establishing reporting to inform leadership and guide program priorities. |
|
|
Industry Partnerships |
Establishing relationships with industry partners to enable increased accessibility to vaccines. |
|
|
Workforce Procurement |
Recruitment and allocation of vaccination workforce. |
|
|
Planning and Assessment Cell |
Forward planning and assessment of campaign milestones. |
|
|
Program Delivery Office |
Governance and cadence for workstreams and the program overall; monitoring and maintaining program schedule, risks and issues, and status reporting. |
|
|
Motivate |
Communications |
Establish communication practices with stakeholders; executing communications strategies. |
|
Commonwealth Engagement |
Establishing and facilitating engagement between Australian Government and state and territory governments. |
|
|
Deliver |
Sourcing/TGA |
Facilitating purchase of vaccines and consumables through advance purchase arrangements; monitoring and evaluating the effectiveness of vaccines through active and passive surveillance. |
|
Logistics |
Identifying distribution options, establishing contracts with logistics service providers, and developing inventory management and administration processes. |
|
|
Operations |
Mobilising a certified and trained immunisation workforce; establishing appropriately equipped vaccination sites. |
|
|
General Practice |
Coordinating planning and enabling functions to support rollout of vaccinations by General Practice. |
|
|
Commonwealth Vaccination Clinics (CVCs) |
Coordinating planning and enabling functions to support rollout of vaccinations by CVCs. |
|
|
Aboriginal Controlled Community Health Organisations (ACCHOs) |
Coordinating planning and enabling functions to support rollout of vaccinations by ACCHOs. |
|
|
Rural and Remote |
Coordinating planning and enabling functions to support rollout of vaccinations in rural and remote areas, such as the Royal Flying Doctor Service. |
|
|
Pharmacy |
Identifying how and in what circumstances community pharmacies will be engaged to assist the rollout. |
|
|
Aged Care |
Coordinating planning and enabling functions for rollout to residents and workers of residential aged care facilities as part of Phase 1a. |
|
|
Disability |
Coordinating planning and enabling functions for rollout to residents and workers of residential disability facilities as part of Phase 1a. |
|
Source: Health.
3.14 The number of workstreams increased markedly from seven shown in Table 3.1 to 19 shown in Table 3.2. There was a concomitant significant increase in the number of Senior Executive Service officers overseeing the rollout as shown in Table 3.3.
Table 3.3: Health taskforce and Operation COVID Shield: senior staffing
|
SES level (or equivalent) |
Taskforce as at February 2021 |
Operation COVID Shield as at October 2021 |
|
SES Band 3/Associate Secretary |
1a |
2b |
|
SES Band 2 |
1c |
3d |
|
SES Band 1 (workstream leads)e |
6 |
17 |
|
Total |
8 |
22 |
Note a: In the Taskforce, the Senior Responsible Officer was the department’s Associate Secretary who retired just prior to the establishment of Operation COVID Shield.
Note b: In Operation COVID Shield, the Coordinator General was a Lieutenant General which is approximately equivalent to an SES Band 3. As noted in Figure 3.2, the Deputy Coordinator General was also an SES Band 3 officer.
Note c: In the Taskforce, the SES Band 2 officer was the Program Director.
Note d: In Operation COVID Shield, the SES Band 2 officers comprised the ‘COVID-19 Vaccine Program Executive Leadership’.
Note e: Workstreams were headed by SES Band 1 officers. The number of officers does not match the number of workstreams because some officers were responsible for more than one workstream.
Source: ANAO from Health documents.
Australian, state and territory government governance
3.15 Due to the need for effective coordination between the Australian Government and state and territory governments, the peak governance mechanism for the rollout was the national cabinet, which had been established in March 2020, replacing the former Council of Australian Governments.
3.16 To support the implementation of the rollout there were a number of key coordination or engagement bodies comprising senior representatives of the Australian, state and territory governments. These are listed in Table 3.4.
Table 3.4: Key Australian and state and territory government coordination or engagement bodies
|
Name |
Membership/representation |
Assigned responsibility |
|
First Secretaries Group |
Secretaries/Deputy Secretaries of Premiers/Chief Minister’s departments |
Support rapid issue resolution across departments in relation to the rollout program. |
|
First Deputies Group |
||
|
Health CEOs/CHOs |
Chief Executive Officers, Chief Health Officers/Chief Medical Officers |
Discuss implementation across jurisdictions and resolve cross-jurisdictional issues or questions. |
|
COVID-19 Vaccination Program Principals Committee |
Representatives from Health and each state and territory health department |
Engagement with states and territories to ensure alignment with the COVID-19 Vaccination program. |
|
COVID-19 Vaccine Program Managers |
Coordinator General, Deputy Coordinator General, senior officers from each state and territory health department |
|
|
Vaccine Operations Centre (VOC)a Stand Up |
Representatives from Health and each state and territory health department operational team. |
Forum for discussion of operational matters and events. |
|
Vaccine Operations Centre Clinical Advisory working group |
Health, TGA, ATAGI, NCIRSb and subject matter experts nominated by states and territories. |
Identify and address clinical and other relevant issues. |
|
Communication working group |
Representatives from Health and each state and territory health department communications team. |
Discuss and align vaccine rollout communication activities. |
Note a: The Vaccine Operations Centre (VOC), located in Health, coordinated Australian and state and territory government efforts to manage the rollout. It also liaised with manufacturers and logistics providers and monitored vaccine distribution.
Note b: The National Centre for Immunisation Research and Surveillance (NCIRS) was established by Health in August 1997. Its purpose is to lead and support collaborative research and to advance immunisation policy and practice.
Source: Health.
Roles and responsibilities
3.17 The high-level responsibilities of the Australian, state and territory governments were set out in the Australian COVID-19 vaccination policy (the vaccination policy), which was published on 13 November 2020 (see Table 3.5). These responsibilities were also stated in the respective Jurisdictional Implementation Plans (see paragraph 2.11).
Table 3.5: Australian COVID-19 vaccination policy: Australian, state and territory government responsibilities
|
Australian Government |
State and territory governments |
|
|
Source: Department of Health, Australian COVID-19 vaccination policy, 13 November 2020, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australian-covid-19-vaccination-policy [accessed 20 April 2022].
3.18 The vaccine policy also identified shared responsibilities for specific priority groups in the following terms:
In addition, the Australian, State and Territory governments will work together to ensure that the needs of the following groups are met: residential aged care and residential disability settings; Aboriginal and Torres Strait Islander people; culturally and linguistically diverse communities; and vulnerable groups. This will be done in consultation with relevant stakeholders including the Aboriginal and Torres Strait Islander Community Controlled Health Organisations (ACCHOs). The Australian, State and Territory governments will also work together to ensure doses of vaccine are distributed to where they are most needed, based on live information on need and uptake at vaccination locations.82
3.19 Further detail about shared responsibilities of the Australian Government, state and territory governments and relevant non-government stakeholders were subsequently included in the Jurisdictional Implementation Plans (discussed at paragraph 2.8) and the sector-specific implementation plans (discussed at paragraph 2.22).
Were effective arrangements in place for monitoring and reporting progress on the vaccine rollout?
Health put in place systems to monitor the vaccine rollout but does not have adequate assurance over the completeness and accuracy of the data and third-party systems. Health provided decision makers and the public with regular and detailed updates on the vaccine rollout that were tailored and developed using the best available data. These reports did not include progress against targets until August 2021.
3.20 The November 2020 vaccination policy noted that the ‘effective and efficient roll-out of COVID-19 vaccine(s) will require significant coordinated data and reporting mechanisms’ to track the progress of the rollout. Health advised that it did not establish a framework to monitor and report on the progress of the vaccine rollout and that reporting developed as the rollout progressed.
Systems and data
3.21 Health used a number of key systems to manage and monitor the vaccine rollout.
- Australian Immunisation Register (AIR) — an existing database which records vaccinations. Only recognised vaccination providers83 can input data to the AIR and a small number of fields are recorded.84 Services Australia manages the AIR under a program agreement with Health.85
- Vaccine Administration System (VAS) — an existing system used to order vaccines for the National Immunisation Program. VAS required manual input and was used to order and track the number of vaccines delivered through in-reach vaccine administration in the residential aged care and residential disability sectors.
- COVID-19 Vaccine Administration System (CVAS) — a new system that Health brought online on 8 March 2021 to manage the ordering, allocation, delivery and receipt of COVID-19 vaccines and record any wastage.
- Vaccine Data Solution (VDS) — a new system that Health brought online on 15 February 2021 to collate data from the above systems to produce reports on vaccine deliveries and immunisations.
3.22 These systems were configured to share data to provide reporting on the entirety of the vaccine rollout (see Figure 3.3).
Figure 3.3: COVID-19 vaccine rollout data and system flow

Source: ANAO analysis of Health documents.
Systems assurance
3.23 Health has outsourced the data collection and IT management for systems used in the vaccine rollout to other parties, including:
- Services Australia (AIR);
- Salesforce (VAS and CVAS);
- Accenture (reporting dashboards); and
- Amazon Web Services (CVAS).
3.24 Health is responsible under various Acts86 for the confidentiality, privacy and security of the data collected using these systems and cannot outsource these responsibilities. Health does not have assurance that third parties have IT controls in place to ensure the confidentiality, integrity and availability of data.87 Health relies on point of time assessments, contractual obligations and management statements from entities. These are not sufficient to demonstrate that IT controls have been implemented and were operating effectively over the vaccine rollout. This increases the risk that third party providers do not have appropriate IT controls in place over the security of this data. The ANAO did not assess the IT controls of the third parties.88
Recommendation no.1
3.25 The Department of Health and Aged Care establish processes, including during public health emergencies, to ensure it regularly obtains and reviews assurance over the data quality and IT controls in place in externally managed systems on a risk basis, including IT security, change management and batch processing.
Department of Health and Aged Care response: Agreed.
3.26 The Department acknowledges it has responsibility for maintaining appropriate IT controls, including quality and assurance of data for Commonwealth contracted vendor managed systems. The externally managed systems of other jurisdictions, medical software industry and private practitioner and providers, that interface with Commonwealth managed systems, are outside the control of the Department and adoption of data standards and interoperability across these systems remains a challenge for data quality and completeness. The Department will undertake an independent review of its IT controls and application of its internal quality assurance framework.
Availability of data
3.27 The data that Health has used to monitor the progress of the vaccine rollout has increased over time. However, there have been gaps in the data relating to the vaccination status of some priority and target groups (see paragraph 3.30 for more detail).
- Health did not have accurate data on the vaccination rate of critical and high-risk workers, which were a priority group under Phase 1a of the vaccine rollout, as occupation data was not collected in the AIR.
- In July 2021 the Australian Government mandated residential aged care facilities report the vaccination status of their residents and workers and created a new dataset to address a gap in monitoring.89
Accuracy of data
3.28 Auditor-General Report No.5 of 2021–22 Improving Immunisation Coverage found:
Health does not gain assurance about the quality of the data it uses for monitoring and reporting immunisation coverage and has not clarified responsibility for data quality [in the AIR].
3.29 Health has not formally reviewed the data entered into any of the systems listed in paragraph 3.21. This has resulted in undetected and undisclosed inaccuracies in the data, particularly in the AIR and CVAS systems which are distributed systems and require manual input from many users.90
3.30 The most recent National Centre for Immunisation Research and Surveillance study on AIR data in 2018 identified an error rate of 14 per cent.91 In February 2021, prior to the vaccine rollout commencing, the Australian Government mandated that all COVID-19 vaccinations be reported in the AIR to improve the completeness of the data.92 While this may have improved the error rate, there is no evidence available to support this.
3.31 Despite known inaccuracies in the collection of data, Health has used the available data as the most reliable data source for planning and supporting the vaccine rollout. In its use of this data, Health has identified anomalies and rectified these through its providers. However, Health has not quantified the inaccuracies in its internal or external data processes in the period examined.
Other monitoring activities
3.32 Health advised the ANAO that all levels of the vaccine rollout were monitored and that it developed dashboards and reports for some operational areas to identify and action issues. For example, the residential aged care branch developed a dashboard to generate reporting. Health also used this dashboard to monitor vaccination rates of aged care workers and target corrective action at facilities or companies with low vaccination rates.
Reporting arrangements
Reporting to decision-makers
3.33 Health produced daily and weekly reports on the vaccine rollout for decision-makers, which addressed progress for all priority or targeted groups that Health was able to track (see paragraph 3.27 and Appendix 3).93 Health also reported on the program management of the rollout, which included progress against milestones, such as on-boarding vaccine administration sites. Table 3.6 summarises the reports provided to decision makers in August 2021 when the vaccine rollout was at a peak time of activity.94
Table 3.6: Number of regular reports on the progress of the vaccine rollout received by senior decision makersa
|
Type of report received |
Prime Minister |
Minister for Health and Aged Care |
Coordinator General of Operation COVID Shield |
Operation COVID Shield Senior Officials |
|
Program management report |
1 |
1 |
1 |
1 |
|
Tailored reportingb |
1 |
1 |
3 |
2 |
|
Updates on progress of rollout |
3 |
4 |
6 |
4 |
|
Updates on progress of the rollout to priority group/s |
0 |
1 |
2 |
1 |
|
Total regular reports received |
5 |
7 |
12 |
8 |
Note a: Includes daily and weekly reports in August 2021.
Note b: Tailored reporting includes summary reports for specific audiences, such as the Prime Minister or Coordinator General of Operation COVID-19 Shield.
Source: ANAO analysis of Health documents and reporting.
3.34 Health frequently provided briefing for the Prime Minister and the Minister for Health and Aged Care. Between April 2020 and December 2021, Health provided 105 formal briefs related to the vaccine rollout to the Minister for Health and Aged Care and the Prime Minister. These formal briefs addressed issues requiring formal ministerial approval, such as requesting approval to request additional funding for vaccine advertising from the Prime Minister. The weekly program management report provided to the Minister for Health and Prime Minister also included the key risks to the rollout for the week and the status of key program milestones. For example, the 10 March 2021 report identified the slow rollout to residential aged care facilities as a key risk, and noted agreeing the contractual approach to VAS providers in residential disability settings was ‘at risk’.
3.35 Health’s reports on the vaccine rollout were often tailored to suit the specific audience. For example, Health provided a weekly update to the Prime Minister on the vaccine rollout, which was developed in April 2021 in response to feedback from the Prime Minister through his office, the Department of the Prime Minister and Cabinet and Health staff. As a result of feedback from these stakeholders, Health included additional data, and changed how some information was presented to better suit the intended audience.
Reporting against targets
3.36 As noted at paragraph 2.27, Health did not include high-level targets in its planning prior to June 2021. However, government ministers and senior officials made a number of public statements which committed to targets for the vaccine rollout. These targets included the Prime Minister’s statement on 7 January 2021 that he hoped that Australia would administer four million vaccines by the end of March 2021 and the Minister for Health and Aged Care’s statement on 5 November 2020 that all Australians who sought vaccination will be vaccinated within 2021 (see Appendix 4 for a list of the key public targets). Health did not clearly report against these targets to decision makers.
3.37 In August 2021, Health released the Op COVID SHIELD National COVID Vaccine Campaign Plan (the campaign plan), with the purpose to detail the mechanisms to achieve the vaccination targets in the National Plan to Transition Australia’s National COVID Response of 70 and 80 per cent double vaccinated.95 Health also produced a number of sub-plans which included lower-level targets (discussed in paragraph 2.37).96 From 10 August 2021, Health reported against these new targets in weekly reports to the Coordinator General and senior departmental officers. These reports were developed by an ‘assessments cell’ established by the Coordinator General in June 2021. Health made amendments to the rollout based on its progress against targets and issues identified during this process.
Public Reporting
3.38 Health also published regular data-focused updates on the vaccine rollout. This was guided by the Prime Minister’s commitment of 6 April 2021 to greater data transparency, the data available and public interest, and was consistent with the approach taken by other countries.97 From 9 April 2021 the Australian Government published daily and weekly updates on the vaccine rollout on its website (see Figure 3.4).98 The content included in these updates increased over time. This reporting did not assess progress against public targets for the vaccine rollout until 16 August 2021, when Health began reporting against the 70 and 80 per cent double vaccinated targets.
Figure 3.4: Health’s public reporting on the vaccine rollout
|
Type of Information |
Information reported |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
Summary |
Doses administered |
|
|
|
|
|
|
|
|
|
|
|
|
|
Doses administered through delivery channels |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comparison to international rollouts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Doses by age and gender |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Doses utilisationa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jurisdictional |
Vaccination % by state and territory |
|
|
|
|
|
|
|
|
|
|
|
|
|
Jurisdictional breakdown on doses administered |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Priority groups |
Aged care vaccination rates |
|
|
|
|
|
|
|
|
|
|
|
|
|
Disability vaccination rates |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indigenous vaccination rates by geographic area (LGA and SA4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indigenous vaccination rates |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delivery channel |
Doses delivered by primary care |
|
|
|
|
|
|
|
|
|
|
|
|
|
Geographic region |
Vaccination rates by geographic region |
|
|
|
|
|
|
|
|
|
|
|
|
Key:
Note a: Dose utilisation is the number of doses delivered versus doses administered and wasted.
Source: ANAO analysis.
Was there fit-for-purpose stakeholder engagement?
Health developed a stakeholder engagement plan for the rollout, which assessed stakeholders based on their needs, influence, and impact on the program. Health received advice on the rollout from four sector-specific advisory groups that were established for the COVID-19 response. Feedback on the vaccine rollout from key stakeholders contacted by the ANAO was mixed.
Stakeholder engagement arrangements
3.39 Health identified that the COVID-19 vaccine rollout, as one of the largest national vaccination programs in Australia’s history, ‘has a stakeholder ecosystem that spans millions of Australians, thousands of external stakeholder groups and dozens of government bodies’. The time critical nature of the rollout meant that clear stakeholder governance was required to ensure resources were utilised effectively and stakeholders were engaged in a timely and appropriate manner.
3.40 In February 2021 Health documented its planned approach to stakeholder engagement, which included identifying stakeholders and establishing a clear stakeholder governance framework to support engagement effort.99
3.41 Health identified stakeholders according to their needs, their influence on the program and the impact of the program on them. A broad range of stakeholders from government, medical and scientific fields, industry, and the community sectors were considered, categorised and prioritised (see Figure 3.5).
Figure 3.5: Health’s COVID-19 vaccination rollout stakeholder identification matrix

Source: Health documentation.
3.42 In addition to the stakeholders identified in Figure 3.5, Health also identified a range of state and territory engagement forums and primary care engagement forums (some of which, such as the Health CEOs/CHOs group, are mentioned in Table 3.4).
3.43 Of particular relevance for rollout governance were four advisory groups that were identified as key stakeholders for the vaccine rollout (the roles and membership of the four advisory groups are outlined in Table 3.7). These groups were established between March and October 2020 to provide advice and feedback to Health as part of the ongoing national response to COVID-19.
Table 3.7: Sector-specific advisory groups
|
Name |
Role (summary) |
Membershipa |
|
Aboriginal and Torres Strait Islander Advisory Group on COVID-19 |
Provides advice on Aboriginal and Torres Strait Islander health aspects and communities related to COVID-19 and contributes to the flow of information with the Aboriginal and Torres Strait Islander Health sector. |
Includes representatives from Aboriginal Health Services, NACCHO, the National Indigenous Advancement Agency and state and territory government health representatives. |
|
Advisory committee for the COVID-19 response for people with disability |
Provides advice on the health care needs of people with a disability, their families and the disability sector including access to COVID-19 screening, prevention and healthcare. |
Includes representatives from the aged care sector, infection control, emergency preparedness, public health response from across state and territory governments, residential aged care, research sector, and medical practitioners. |
|
Aged Care Advisory Group |
Provides advice to the Australian Health Protection Principal Committee to support the national response to COVID-19 in the aged care sector. |
Includes representatives from the aged care sector, infection control, emergency preparedness and public health response including GPs, peak bodies and colleges. |
|
Culturally and Linguistically Diverse Communities COVID-19 Health Advisory Group |
Provide advice on the impacts on culturally, ethnically and linguistically diverse populations with a specific focus on health matters including mental health, primary care and aged care. |
Includes representatives from a range of non-government bodies from the CALD community, public health and medical experts and civil society organisations. |
Note a: The list of representatives in the table is not exhaustive but included to provide examples of the range of expertise.
Note: Working groups were established as a sub-committee of sector-specific advisory groups to focus on parts of the vaccine rollout. For example, the CALD advisory group established a vaccine working group and a communication working group.
Source: ANAO analysis of Health documentation.
3.44 Each advisory group was chaired by a senior officer from Health.100 Terms of reference were established for each group, including details about the group’s role, membership and areas of expertise, regularity of meetings, meeting protocols, and related committees (as relevant).101
3.45 Meetings were held regularly during 2021, varying from monthly to several times a week as needed. During 2021, a total of 138 meetings were held across the four advisory groups, with the Aboriginal and Torres Strait Islander advisory group, for example, holding 51 meetings during 2021. The ANAO established that records were kept for meetings. Key meeting documents included agendas, meeting outcomes or minutes and communiques.
3.46 Health received advice from the advisory groups when planning the vaccine rollout. For example, in January 2021, advice on communication strategies was provided to Health by the aged care advisory group. In February 2021, the Aboriginal and Torres Strait Islander advisory group considered a paper on barriers and Aboriginal and Torres Strait Islander people’s concerns about vaccination. The advisory groups continued to provide advice to Health throughout the rollout, assisting Health to respond to issues in a timely manner.
ANAO requests to key stakeholders for feedback
3.47 In October 2021, the ANAO wrote to 38 organisations and individuals seeking their feedback on the rollout, including:
- representatives of advisory groups102;
- all state and territory health departments;
- Primary Health Networks in each state and territory;
- major health peak bodies; and
- logistics providers.
3.48 Twenty-two responses were received, providing mixed feedback on the rollout overall.
- For example, one of the major peak bodies stated:
Australia’s vaccine rollout has not been perfect, but in general the [peak body] feels that the campaign was well planned and implemented — recognising that Australia was competing with the rest of the world for vaccines in what have been extraordinary times.
- By contrast, another stakeholder stated:
serious deficiencies have emerged in relation to the planning and execution of the COVID-19 vaccine rollout for people with disability in Australia.
3.49 It was clear from the feedback that many stakeholders had views about improvements that could be made and lessons that could be learned from the rollout in the event that another rollout becomes necessary in the future.
3.50 During the audit, Health drafted a document entitled Catalogue – learnings from the Covid vaccination rollout. The document was dated December 2021, was in draft form and stated:
it is also important to capture what our stakeholders learnt from the vaccination activities which were rolled out into the States and Territories. On-the-field experience is valuable and should be documented so if similar interventions were required in the future, they could be rolled out quickly and be more effective.
3.51 Although the document foreshadowed a publication date of 31 March 2022, Health advised on 22 March 2022 that it ‘is only an early draft, which was not progressed further. The Program Delivery Office has a lessons learned log as part of the management of the Program, this log will not be finalised until the program is closed’.
3.52 In May 2022, Health advised that it had commenced a Post Operations Review to consider the effectiveness of the vaccine rollout. The value of such a review is enhanced if it is broadly based. Health’s Stakeholder Engagement Framework states that Health is:
Committed to two-way open communication that involves listening to our stakeholders, keeping them informed and being clear about how their contributions are being used.
3.53 The framework also says that Health will ‘identify and enable the participation of those people and organisations who contribute to, influence, or are affected by our work.’
3.54 Given the range of comments that stakeholders contacted by the ANAO expressed about the rollout, the ANAO considers that it is critical that they be afforded the opportunity to be involved in a review of the rollout and that and that such an exercise be undertaken without delay, while lessons are fresh in people’s minds.
Recommendation no.2
3.55 Before 31 December 2022, the Department of Health and Aged Care conduct a comprehensive review of the COVID-19 vaccine rollout which:
- invites contribution from all key government and non-government stakeholders;
- examines all aspects of the COVID-19 vaccine rollout;
- identifies what worked well and what did not; and
- makes recommendations to the Australian Government about opportunities for improvement in the event of a future vaccination rollout.
Department of Health and Aged Care response: Agreed.
3.56 The Department accepts the need to undertake a comprehensive review of the COVID-19 rollout. Since the audit was undertaken, a Post Operations Review of the National COVID-19 Vaccine Taskforce has been completed. The Department also notes the intention of Government to undertake a deep review of the overall COVID response (including the vaccine roll out). The review of the COVID-19 vaccine rollout would logically form part of this broader review. The timing of the broader review is still to be agreed by Government.
4. Implementation
Areas examined
This chapter examines whether the Department of Health and Aged Care’s (Health’s) implementation of the COVID-19 vaccine rollout has been effective.103
Conclusion
Health’s implementation of the COVID-19 vaccine rollout has been partly effective. While vaccines were delivered with minimal wastage, Health’s administration of vaccines to priority populations and the general population has not met targets. The vaccine rollout to residential aged care and residential disability were both slower than planned, and the vaccination rate for Aboriginal and Torres Strait Islander people has remained lower than for the Australian population. Health implemented its communication strategy, but its advertising campaign has not yet been evaluated.
4.1 Vaccines are an important tool to reduce the risk from severe illness and death as a result of COVID-19, and were a key part of the Australian Government’s plan to minimise illness and fatalities while removing COVID-19 restrictions as set out in the National Plan to Transition Australia’s National COVID-19 Response released on 2 July 2021. On 31 January 2021, prior to the commencement of Australia’s vaccine rollout on 22 February 2021, the Minister for Health and Aged Care stated that ‘we aim to have the country vaccinated before the end of October’.104
4.2 Rapid implementation will generally require entities to draw on the full range of human, physical and ICT resources available to them, and may involve entities looking externally for relevant resources. Misalignment between resources and needs at the rollout stage creates a significant risk to the delivery of expected outcomes in the required time period. This chapter examines whether Health effectively: implemented vaccine logistics arrangements and administration channels to administer vaccines to the Australian population; administered vaccines to targeted populations as planned; and communicated information on the vaccine rollout.
Have the logistics and administration channels been effective in vaccinating the Australian population?
Vaccines were delivered within agreed logistics timeframes and with minimal wastage, with 60 per cent of vaccines administered through Australian Government managed channels. Health had a principle of allocating vaccines to states and territories on a proportional basis, however issues such as logistical constraints and responses to COVID-19 hotspots meant that allocations were not proportional in the early stages of the rollout. Health did not meet the government’s original target to ‘have the country vaccinated’ before the end of October 2021.
Logistics
4.3 The Australian Government has been responsible for the transport of vaccines and related consumables to storage and administration sites within each state and territory.105
4.4 As discussed in paragraph 2.56, Health engaged DHL and Linfox in February 2021 as the logistics providers for the vaccine rollout.106 Both logistics providers self-reported that they consistently delivered more than 99 per cent of vaccines and consumables within the timeframes set by Health. Health advised that it agreed with the logistics providers’ self-assessments in the period examined.
4.5 Health advised the ANAO that it monitored the timeliness and wastage levels of logistics providers during the vaccine rollout and used this information to resolve issues (vaccine wastage is discussed in paragraphs 4.15 and 4.16). In March 2021 Health identified issues with one of the logistics providers relating to delivery delays, cold chain breaches and poor responsiveness to Health’s requests. In response, Health reduced the deliveries allocated to the provider until it gave evidence it had addressed the identified issues.107
Distribution of vaccine stock
4.6 In early 2021, Health had limited vaccine supply and did not have precise forecasts on when supply would arrive.108 Figure 4.1 shows Health delivered vaccines to administration sites in a timely manner once they became available. Not all doses received by Health were immediately delivered. Health held some vaccines in contingency to ensure it could provide second doses if there were supply shocks. The number of vaccines held in contingency varied over time. The initial contingency with the Pfizer vaccine was calculated as the amount required for second doses.
Figure 4.1: Cumulative vaccine supply received by Health compared to vaccines delivered to administration sites

Note: Vaccine supply refers to deliveries of vaccines received by Health from both international and domestic manufacturers.
Source: ANAO analysis of Health documents.
Administration channels
4.7 Once vaccines were received by the Australian Government, Health administered vaccines through a variety of vaccine administration channels. Some administration channels were used to target priority groups and others were aimed at the general population (see Table 2.7). All administration channels other than state and territory clinics were managed by Health.109 To support the safe administration of vaccines, all administration channels had to meet the site requirements for COVID-19 vaccination clinics developed by Australian Technical Advisory Group on Immunisation (ATAGI).110
4.8 As the rollout progressed and more people became eligible, additional administration channels were brought online. Health advised that as at 31 December 2021, there were 10,154 active vaccine administration providers.111 Figure 4.2 shows that the majority (60 per cent) of vaccines were administered by Australian Government channels. This was mainly through primary care administration channels.
Figure 4.2: Monthly vaccines administered by Australian Government and state and territory administration channels in 2021

Note: Australian Government administration channels include general practitioners, community pharmacists, Aboriginal Community Controlled Health Organisations, Commonwealth Vaccination Clinics, in-reach and the Royal Flying Doctor Service.
The data used to develop this and other figures in this chapter was largely derived from Health’s systems and subject to data quality issues raised in paragraphs 3.29 and 3.30. The ANAO does not have assurance on the completeness and accuracy of this data. This was the best data available during the vaccine rollout and was used by Health to make decisions. Therefore, the ANAO has used this to assess the effectiveness of the rollout. The ANAO confirmed that Health’s processes to extract data from its systems to provide to the ANAO were appropriate. Health updated this data retrospectively where required and as a result the figures used by ANAO may vary from Health’s reporting.
Source: ANAO analysis of Health data.
Primary care
4.9 Primary care includes general practitioners (GPs), community pharmacies, Aboriginal Community Controlled Health Organisations (ACCHOs) and Commonwealth Vaccination Clinics (CVCs). Fifty-seven per cent of vaccines were administered by primary care channels in 2021.
4.10 Health brought primary care sites online in a staggered approach, beginning with CVCs and ACCHOs to improve access in remote and regional areas. Potential GP and pharmacy sites were identified through multiple opt-in expression of interest processes.112 Sites were on-boarded in a phased approach based on readiness, the density of priority populations in the area, and geographic coverage.
4.11 The vaccine rollout released public messages early, which was consistent with the Australian Government’s strategy for the COVID-19 response (the communication of the vaccine rollout is discussed in paragraphs 4.66 to 4.76). The Prime Minister frequently addressed the media to announce policy changes shortly after decisions were made in meetings with state and territory leaders. As a result, some policy changes relating to the vaccine rollout were announced publicly before they were communicated to operational staff and key stakeholders. Stakeholders in the primary care sector advised the ANAO that coordination bodies and individual primary health administrators were not always prepared to implement the changes or respond to patients’ questions. Primary care stakeholders advised this reduced public trust and created other difficulties for administration providers.113
In-reach services
4.12 Health ran in-reach vaccination clinics for residential aged care facilities and residential disability facilities from February 2021, which accounted for three per cent of vaccines administered.114 As discussed in paragraphs 2.52 to 2.55, the in-reach administration channel primarily used contracted Vaccine Administration Service (VAS) providers who travelled to facilities to administer vaccines to recipients. Health advised that from August 2021, primary care providers were also able to provide in-reach vaccinations.
4.13 During in-reach visits, residents were vaccinated first and, if there were any vaccines remaining, they could be offered to staff and any other people on site (such as family of residents) to minimise wastage.115 Residential aged care facilities (RACFs) and residential disability facility in-reach clinics were scheduled based on geographic and logistics considerations such as cold chain requirements. In-reach vaccination clinics primarily used Pfizer as Health wanted VAS providers to only use one vaccine.116
Other administration channels
4.14 The Australian Government established additional vaccine channels to improve access to vaccines, increase uptake and target priority groups. These channels administered less than one per cent of doses in the vaccine rollout and included:
- a workplace vaccination program (Health established a pool of immunisation providers able to provide on-premise vaccination at workplaces, which workplaces could choose to engage);
- in-reach clinics for remote and very remote communities through the Royal Flying Doctor Service; and
- priority group specific vaccination hubs (Health established hubs for aged care and residential disability care workers, and other essential workers to increase vaccine uptake).
Vaccine wastage
4.15 The World Health Organisation (WHO) defines vaccine wastage as ‘the sum of vaccines discarded, lost, damaged or destroyed.’117 The WHO states that 15 per cent is an acceptable indicative wastage rate for multi-dose vials as part of a vaccine campaign.118 Noting Australia’s well-developed infrastructure for administering vaccines, the ANAO used the National Immunisation Program’s (NIP’s) acceptable wastage rate of 10 per cent for new vaccines as a guide. Table 4.1 shows that the highest cumulative rate of vaccine wastage during 2021 was within the NIP’s 10 per cent acceptable wastage rate (9.2 per cent). Table 4.1 also shows that in late 2021, as vaccine supply increased and demand decreased, the rate of vaccine wastage also increased.
Table 4.1: Cumulative vaccine wastagea by administration channel or logistics provider from February to December 2021
|
Month |
Percentage logistics provider wastage |
Percentage primary care wastageb |
Percentage of other Australian Governmentc wastage |
Percentage residential aged care facility wastage |
Percentage states and territories wastage |
Total percentage of wastage |
Below 10% wastage rate? |
|
Jan-21 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
✔ |
|
Feb-21 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
✔ |
|
Mar-21 |
0.00 |
0.00 |
0.01 |
0.00 |
0.00 |
0.01 |
✔ |
|
Apr-21 |
0.00 |
0.03 |
0.04 |
0.00 |
0.00 |
0.07 |
✔ |
|
May-21 |
0.00 |
0.08 |
0.07 |
0.00 |
0.00 |
0.15 |
✔ |
|
Jun-21 |
0.02 |
0.16 |
0.10 |
0.00 |
0.00 |
0.28 |
✔ |
|
Jul-21 |
0.03 |
0.27 |
0.12 |
0.00 |
0.00 |
0.42 |
✔ |
|
Aug-21 |
0.03 |
0.44 |
0.13 |
0.00 |
0.01 |
0.61 |
✔ |
|
Sep-21 |
0.03 |
0.74 |
0.15 |
0.00 |
0.03 |
0.95 |
✔ |
|
Oct-21 |
0.03 |
2.16 |
0.17 |
0.00 |
0.09 |
2.45 |
✔ |
|
Nov-21 |
0.04 |
5.62 |
0.23 |
0.00 |
0.24 |
6.13 |
✔ |
|
Dec-21 |
0.04 |
8.60 |
0.25 |
0.01 |
0.31 |
9.21 |
✔ |
Note a: The percentage of vaccines wasted was calculated using the number of vaccines delivered. The number of vaccines delivered was calculated from the sum of vaccines wasted and vaccines administered. This does not consider vaccines delivered but not yet administered. Health advised this calculation is different from the method used by Health because of data quality issues and therefore the figures may not be the same as Health’s publicly reported figures.
Note b: Health advised that primary care had a higher wastage rate for a number of reasons, including the number of primary care sites, that vaccines were thawed at delivery, reducing shelf-life to four weeks and because primary care used a higher proportion of AstraZeneca and Moderna which used larger vials resulting in more unused doses in an open vial at the end of the day.
Note c: Other Australian Government wastage includes wastage in the Royal Flying Doctor Service, workplace vaccinations (discussed in paragraph 4.15) and other Australian Government controlled channels, and wastage not attributed to a specified location or administration channel when reported. These accounted for less than three per cent of the total wastage.
Source: ANAO analysis of Health data.
4.16 In addition to its indicative wastage rate for multidose vials, the WHO recommends a maximum acceptable wastage of one per cent for unopened vials. This unopened vial wastage often occurs in the transport or storage of vaccines. The wastage rate for logistics (0.04 per cent) was below the WHO’s acceptable wastage rate for unopened vials. This was likely impacted by the strict processes Health required logistics providers to comply with to ensure vaccines were stored within the required temperature ranges to minimise wastage.119
Allocation of vaccines across jurisdictions
4.17 To provide equitable access to vaccines for all states and territories, the Australian COVID-19 Vaccination Policy (the vaccination policy) stated that the Australian Government would allocate vaccines based on the proportion of the eligible population in the state or territory and vaccine supply. The vaccination policy also noted additional doses could be allocated to address COVID-19 outbreaks.120
4.18 Health advised the ANAO that it determined weekly allocations to states and territories based on the proportionality principle outlined in the vaccination policy and that it used the population proportions in Table 4.2.
Table 4.2: Proportion of Australian population in states and territories used to determine COVID-19 vaccine allocations
|
State or territory |
NSW (%) |
Vic (%) |
Qld (%) |
WA (%) |
SA (%) |
Tas (%) |
NT (%) |
ACT (%) |
|
Proportion of Australian populationa |
31.8 |
25.9 |
20.2 |
10.4 |
6.9 |
2.1 |
1.0 |
1.7 |
Note a: These proportions were calculated on Australian Bureau of Statistics (ABS) data. Health advised it originally used 2020 ABS data and updated this to the 2021 data used in this table once released in September 2021.
Source: Health and ABS.
4.19 As there were no supply constraints for AstraZeneca from April 2021, Health advised the ANAO that it focussed on assessing the proportionality of allocations of mRNA vaccines (Pfizer and Moderna) from this point on. Figure 4.3 shows that cumulative allocations of mRNA vaccines were initially not proportional to population. However, from September to November 2021, as the supply of mRNA vaccines increased, Health rebalanced cumulative allocations to within 0.6 percentage points of each jurisdiction’s proportion of the Australian population.121 Health advised the ANAO that the deviations from population proportions in the earlier stages of the rollout (to September 2021) were due to differences in the proportion of priority populations relative to total population in each jurisdiction122, logistical constraints123 and the occurrence of outbreaks.
Figure 4.3: Percentage point difference between proportion of population and proportion of cumulative mRNA vaccines allocated to states and territories

Source: ANAO analysis of Health data.
4.20 During the rollout, Health established processes to allocate additional vaccines to address issues, such as COVID-19 hotspots124, and maximise the use of available vaccines. These processes are summarised in Table 4.3.
Table 4.3: Vaccine allocation processes used in addition to the per capita allocations
|
Allocation processd |
Description of process |
Reason for process |
Month implemented |
|
‘Bringing forward’ doses |
States and territories could request early access to vaccines. Health assessed requests based on need and the available supply in the contingency (see paragraph 4.6).b If approved, Health reduced the state or territory’s allocation in future weeks to replenish the contingency. |
Flexibility to manage stock levels and respond quickly to changing circumstances. |
April 2021 |
|
Dynamic reallocation |
A weekly process to allocate unordered vaccines between administration sites.a This largely reallocated vaccines between administration sites within the same state or territory. |
To minimise underordering and prevent potential wastage, as not all sites ordered all vaccines allocated each week. |
May 2021 |
|
Securing additional doses |
The Australian Government allocated additional vaccines purchased or secured after receiving Health advice.c These could be allocated on a per capita basis or in response to other events, such as increased COVID-19 cases. |
To allocate additional vaccines in response to changing circumstances. |
July 2021 |
|
Doses reallocated from other states |
States and territories could request additional doses be reallocated from other jurisdictions. This required agreement by all state and territory governments. |
Flexibility to respond quickly to changing circumstances. |
Health advised this was not used. |
Note a: This was particularly used for AstraZeneca vaccines, which were consistently under-ordered from mid-May 2021.
Note b: Health did not approve all requests for early access to vaccines made by the states and territories.
Note c: Additional vaccines secured refers to vaccines secured in addition to purchase agreements with vaccine manufactures. In 2021 the Australian Government purchased or arranged vaccine swap arrangements with the Republic of Poland, Singapore and the United Kingdom.
Note d: Health communicated these processes, excluding securing additional doses, to the states and territories.
Source: ANAO analysis of Health documents.
Responding to COVID-19 outbreaks
4.21 The Australian Government used the allocation processes described in Table 4.3 to help control COVID-19 outbreaks. State and territory governments were able to request additional doses (outside of the agreed weekly allocation) in response to increasing COVID-19 cases from May 2021. Health established a process to assess these requests based on the need, timeframe for delivery, and impact on future vaccine stock levels if approved.
4.22 Health advised the ANAO that from May 2021, additional doses of AstraZeneca and Pfizer vaccines were formally requested on 12 occasions by four states and territories experiencing COVID-19 community transmission.
- Victoria requested additional doses on five occasions from May 2021.125 Three of the requests were for additional AstraZeneca doses (185,000 in total), and two requests were for additional Pfizer doses (106,990 in total). Health advised the ANAO that all of the requests were approved, and the Pfizer doses were approved using the ‘bringing forward’ process. The AstraZeneca doses were provided from existing stock as supply was not restricted.
- New South Wales requested additional doses on four occasions from June 2021.126 Three of the four requests (ranging from 183,690 to 500,000 doses per request) were approved by Health. One request (made on 2 July 2021) was rejected due to a lack of vaccine Pfizer supply (additional Pfizer doses not being available on top of what had already been allocated). Health advised the ANAO that the additional vaccine doses were provided using the ‘bringing forward’ process. The AstraZeneca doses were provided from existing stock as supply was not restricted.
- Queensland requested additional Pfizer doses on two occasions in July and August 2021.127 Health advised the ANAO that both requests were approved, and the doses were provided using the ‘bringing forward’ process.
- Australian Capital Territory requested additional Pfizer doses on one occasion in August 2021.128 Health advised the ANAO that the request was approved, and the doses were provided using the ‘bringing forward’ process.
4.23 The ANAO requested that Health provide documentation to support these processes. However, Health could not provide complete records, including evidence of requests, assessments, responses, and repayment of doses provided using the ‘bringing forward’ process. Consequently, the ANAO could not provide assurance that all requests for additional doses to respond to outbreaks were considered and approved using consistent processes.
4.24 In addition to the ‘bringing forward’ process, on 15 August 2021, the Prime Minister announced that the Australian Government had purchased an additional 1,000,350 Pfizer doses from the Republic of Poland.129 Of these doses, 530,010 were prioritised to New South Wales areas with high COVID-19 numbers.130 The decision to prioritise News South Wales, by allocating it 53 per cent of the additional doses, was made by the Prime Minister based on advice from the Chief Medical Officer.
Vaccine administration outcomes
International benchmarks for the vaccine rollout
4.25 The Australian Government began the vaccine rollout on 22 February 2021. This met the target in the Minister for Health and Aged Care’s media release of 5 November 2020, that the rollout would begin in the first quarter of 2021, and later targets for the rollout to begin in either March or late February (see Appendix 4 for a list of key government targets for the vaccine rollout). As shown in Figure 4.4, Australia’s vaccine rollout began later than other countries such as the United Kingdom and the United States.
Figure 4.4: International comparison of percentage of population fully vaccinated since the vaccine rollout began

Note: The population fully vaccinated is based on public reporting and can vary between countries if this is the proportion of the total population or eligible population. For Australia this is the proportion of the population 12 years and over.
The international benchmarks were selected to provide a variety of geographic regions, number of COVID-19 cases, and populations, with at least one country comparable to Australia in each category.
Source: ANAO analysis of Health data and international data from Our World in Data, Coronavirus (COVID-19) Vaccinations [Internet], Global Change Data Lab, available from https://ourworldindata.org/covid-vaccinations [accessed 15 April 2022].
4.26 Figure 4.5 shows Australia’s vaccine rollout followed a similar trend to other countries with relatively low COVID-19 case numbers (New Zealand and Thailand) in the first four months of their respective vaccine rollouts.
Figure 4.5: International comparison of percentage of population fully vaccinated since first vaccine administered

Note: Month one since first vaccine administered for the United States, United Kingdom, Canada and Singapore was January 2021. For Australia, New Zealand and Thailand this was March 2021. Month 10 since the rollout began for Australia was 31 December 2021.
The milestone of the population being fully vaccinated is based on public reporting and can vary between countries, including if this is the proportion of the total population or eligible population. For Australia this is the proportion of the population 12 years and over.
Source: ANAO analysis of Health data and international vaccination data from Our World in Data, Coronavirus (COVID-19) Vaccinations [Internet], Global Change Data Lab, available from https://ourworldindata.org/covid-vaccinations [accessed 15 April 2022].
Vaccine administration to the Australian public
4.27 The Australian Government set a number of targets for the vaccine rollout in 2021, which changed over time (see Appendix 4 for a list of the key public targets and ANAO’s assessment of whether Health achieved them). Table 4.4 shows that Health met three out the nine key public targets for the vaccine rollout to the Australian population.
Table 4.4: ANAO’s assessment of Health’s performance against key public targets for the vaccine rollout to the Australian population
|
Target |
Date target set |
Target met? |
|
‘Australians who sought vaccination will be vaccinated within 2021.’ |
5/11/2020 |
Unknowna |
|
Hope to start the vaccine rollout with around 80,000 vaccinations a week. |
07/01/2021 |
■ |
|
‘Have reached some 4 million population’ by March 2021. |
07/01/2021 |
■ |
|
‘[The] four million position’ will be achieved in early April 2021. |
25/01/2021 |
■ |
|
Completion of the vaccine rollout is on-track for October 2021. |
25/01/2021 |
■ |
|
The Australian Government has a plan to ‘offer COVID-19 vaccines to all Australians by the end of October 2021.’ |
21/02/2021 |
◆ |
|
Every adult Australian to receive at least one dose by the end of October 2021. |
24/03/2021 |
■ |
|
‘Motivate eligible people in Australia to receive at least the first dose of the COVID-19 vaccination by 20 December 2021.’ |
03/08/2021 |
■ |
|
The Australian population [aged 16 or over] to reach 70 per cent and 80 per cent double vaccinated by 31 December 2021. |
03/08/2021 |
◆ |
|
70 per cent of Australians, aged 16 or over, vaccinated before the end of 2021. |
09/08/2021 |
◆ |
|
KEY: ◆ target met ■ target not met |
|
|
Note a: The ANAO was unable to make an assessment whether all people who sought vaccination received a vaccine.
Source: ANAO analysis of Health data, transcripts and press releases by the Prime Minister, Minister for Health and Aged Care and Hansard records.
4.28 Figure 4.6 shows the Australian population reached 80 per cent double vaccinated in November 2021. As summarised in Table 4.4, this met the National Plan to Transition Australia’s National COVID Response target of 80 per cent of the Australian population double vaccinated, which was referenced in the August 2021 Op COVID SHIELD National COVID Vaccine Campaign Plan. However, this did not meet the Minister for Health and Aged Care’s target to ‘have the country vaccinated before the end of October’131, as was anticipated in the March 2021 Implementation Readiness Assessment (see paragraph 2.27).
Figure 4.6: Australian vaccination rate over 2021 for the population aged 12 and over

Note: The ANAO calculated the vaccination rate based on the eligible population at 31 December 2021, which included all people aged 12 years or over. Children aged 5 to 11 years were eligible to get vaccinated from 10 January 2021. Health assessed if it met this target using the vaccination rate of people aged 16 and over. The ANAO assessed 80 per cent of people aged 16 and above were doubled vaccinated on 2 November 2021.
The ANAO calculated vaccination rates using 2021 ABS data on the Australian population as at June 2021. This was used to calculate vaccination rates for all figures unless otherwise stated.
Source: ANAO analysis of Health and ABS data.
4.29 Figure 4.6 also shows the beginning of the vaccine booster program in November 2021, which has continued into 2022. As at 28 March 2022, 68 per cent of eligible Australians had received a vaccine booster dose.
Was the implementation of the vaccine rollout to priority groups effective?
Health’s administration of the vaccine rollout to the residential aged care and disability sectors was slower than planned, due to Health initially contracting insufficient vaccine administration providers and other planning and implementation issues. Health’s target for the vaccination rate for Aboriginal and Torres Strait Islander people to be equal to or greater than the national target [80 per cent double vaccinated] has not been met in 2021.
4.30 The vaccination policy stated that the vaccine rollout should provide equitable protection from COVID-19 for all people living in Australia.132 As discussed in paragraph 2.15, the COVID-19 National Vaccine Rollout Strategy (the vaccine rollout strategy) set out prioritised access to vaccines based on risk of transmission and adverse effects. This section assesses the effectiveness of the vaccine rollout to priority groups.
4.31 As discussed in paragraph 3.36, the Australian Government set a number of targets for the vaccine rollout, including for some priority groups (see Appendix 4 for a list of key targets). Table 4.5 shows Health did not meet any of the five key public targets it set for the vaccine rollout to priority groups.
Table 4.5: ANAO’s assessment of Health’s performance against key public targets for the vaccine rollout to priority groups
|
Target |
Date set |
Target met? |
|
The vaccine rollout to residential aged care facilities will take approximately six weeks. |
16/02/2021 |
■ |
|
Quarantine and border workers, and aged care residents are ‘on track to be vaccinated by April 2021’. |
21/02/2021 |
Unknowna |
|
Health is confident it will complete the rollout to residential aged care facilities in May 2021. |
20/04/2021 |
■ |
|
Vulnerable people, including those in residential disability settings, to be vaccinated by the middle of the year (30 June 2021). |
20/04/2021 |
■ |
|
Aboriginal and Torres Strait Islander vaccination rates to meet, or exceed, the national target. |
14/09/2021 |
■ |
|
KEY: ◆ target met ■ target not met |
|
|
Note a: The ANAO was unable to make an assessment on this due to the lack of data on the cohort.
Source: ANAO analysis of Health data, transcripts and press releases by the Prime Minister, Minister for Health and Aged Care and Hansard records.
Phase 1a priority groups
Priority occupation groups
4.32 Health administered vaccines consistent with the prioritisation in the vaccine rollout strategy discussed in paragraph 2.15 and Table 2.2. States and territories led the administration of vaccines to quarantine, border, and frontline healthcare workers eligible under Phase 1a and monitored the progress of this. Critical and high-risk workers were serviced through both primary care, state and territory clinics, and in occupation-specific sites. Health did not have accurate data on the vaccination rates of these groups.133
Residential aged care facility residents
4.33 Health administered vaccines to residents of residential aged care facilities (RACFs) principally through in-reach vaccinations (see paragraph 4.12). Health committed to providing first and second dose clinics for 2,573 eligible RACFs134, which contained approximately 186,350 residents. Health also ran ‘roving’ clinics for residents who were previously unable to be vaccinated or had chosen not to, and, from 8 November 2021, booster clinics.
4.34 The Minister for Health and Aged Care stated in a media release on 16 February 2021 that he anticipated the vaccine rollout to RACF residents would take approximately six weeks, indicating that this priority population would be completed by early April 2021. On 20 April 2021 Health stated it was confident the rollout to RACF residents would be completed in May 2021. Figure 4.7 shows second dose vaccine clinics for RACF residents were completed for 99 per cent of RACFs by June 2021.
Figure 4.7: Percentage of Residential Aged Care Facilities receiving in-reach first and second dose clinics for residents

Note: Of the 2,573 RACFs, Health did not record the date that first and second dose clinics were conducted for five. These were excluded from the total.
Source: ANAO analysis of Health data.
4.35 Health documents indicated the rollout of vaccines to RACFs was slower than planned for a number of reasons.
- There were delays in scheduling visits to RACF sites. Health’s reporting showed the VAS providers it contracted had difficulty scheduling visits and engaging with facilities, which caused confusion and uncertainty at some facilities.
- The Vaccine Administration System used by Health to order and register vaccinations required manual processes that caused delays and errors in the data.
- Health contracted a limited workforce of the VAS providers (which were also contracted to deliver the residential disability rollout).
- One VAS provider contracted by Health early in the rollout underperformed. This poor performance included governance and logistics issues resulting in cancellations of clinics at short notice.
4.36 As the rollout progressed, Health improved governance and reporting processes for the RACF rollout. For example, in March 2021 Health established processes for Primary Health Networks (PHNs)135 to engage with RACFs to identify residents who were not vaccinated during in-reach clinics and connect them with vaccine services. Health also worked with PHNs and VAS providers to improve coordination and to schedule roving clinics to improve vaccination rates.
4.37 As discussed in paragraph 2.53, Health expanded its contracts with existing VAS providers and engaged additional VAS providers in February, March and April 2021 to address the insufficient workforce from the providers it initially contracted.
4.38 By 10 January 2022, 87 per cent of residents in RACFs had received two doses of a COVID-19 vaccine, as a result of the in-reach visits shown in Figure 4.7.
Residential aged care facility workers
4.39 Health made a decision in February 2021 to vaccinate aged care staff separately from residents due to lessons learnt from international vaccine rollouts.136
4.40 From 15 June 2021, residential aged care providers were required to report on the vaccination status of their workers; and all residential aged care workers were required to receive at least a first dose of a COVID-19 vaccine by 17 September 2021.137 Further, to encourage workers to get vaccinated, in July 2021 Health established a grant program to compensate workers for travel costs and lost shifts.138 On 17 September 2021 the Minister for Health and Aged Care reported in a media release that 76.9 per cent of residential aged care workers were double vaccinated (this compared to 44 per cent of the Australian population). Health advised that as at 11 May 2022, 98.9 per cent of residential aged care facility workers were double vaccinated.
Residential disability facility residents and staff
4.41 The rollout to the residential disability sector began on 22 February 2021. As with the aged care cohort, Health also offered a first and a second dose in-reach vaccination clinic for residential disability facility residents and staff.139 Residential disability facilities included both recipients of National Disability Insurance Scheme (NDIS) support and non-NDIS recipients, as not all people with a disability receive NDIS support.140
4.42 On 20 April 2021 the Secretary of Health advised the Senate Select Committee on COVID-19 that Health aimed to have vaccinated vulnerable people, including residential disability residents, by the middle of 2021. By 20 September 2021 Health had held second dose clinics at 71 per cent of residential disability sites, compared to 100 per cent of residential aged care facilities.141 The vaccination rate of NDIS residential disability residents did not reach 80 per cent double vaccinated until 9 November 2021 (see Figure 4.8).142 This was approximately the same time as the Australian population aged 16 years and over (2 November 2021), even though residential disability workers and residents were eligible to get vaccinated six months earlier than the majority of Australians aged 16 years and over.
Figure 4.8: Vaccination rate of NDIS recipients aged 16 and above living in residential disability facilities between May and December 2021

Note: Analysis based on regular reports between 25 May and 23 December 2021. The vaccination rate was calculated based on the total number of NDIS recipients in residential accommodation on 17 October 2021.
Data matching between the AIR and National Disability Insurance Agency did not have a common unique identifier and could lead to false matches. Services Australia removed these as they were identified. New processes were also developed to improve data accuracy throughout the rollout. This can result in unexpected variations in the data.
Source: ANAO analysis of Health data.
4.43 As at 11 November 2021, 79 per cent of NDIS screened applicants to work with people with a disability143 were double vaccinated. By 23 December 2021, 87 per cent of NDIS screened applicants were double vaccinated.
4.44 Health engaged with the National Disability Insurance Agency and Services Australia to access and improve data on the vaccination status of NDIS recipients (which represented a large proportion of the residential disability population). This improved Health’s ability to monitor the rollout in the residential disability sector. To administer vaccines safely, Health also required all VAS providers to complete training on managing residential disability facility residents’ concerns and behaviours.
4.45 Similar to the vaccine rollout to RACFs, Health documents indicated the rollout of vaccines to residential disability facility residents was slower than planned due to a number of issues.
- As discussed at paragraph 2.54, Health did not finalise operational planning and engage in-reach VAS providers until after the rollout started.
- As at 21 September 2021, there were 6,311 residential disability facilities with varying numbers of residents. These facilities could support residents with a broad spectrum of disabilities, including physical or developmental.144 This made safely administering vaccines and obtaining valid consent difficult.
- The distributed nature of the sector and lack of complete and accurate data on the vaccination rate of residents and workers made it difficult to plan and monitor the rollout.145
- Health initially engaged VAS providers with a limited workforce. As with the residential aged care rollout, this limited the number of sites that the VAS providers could visit.
- Health prioritised the deployment of contracted VAS providers to aged care facilities due to the higher risk of severe outcomes of COVID-19.146 This delayed deployment to residential disability facilities.
4.46 Most of these issues were identified as risks during the joint planning process undertaken by Health in February 2021 with one of the VAS providers. However, they were not adequately addressed, which impacted the speed of the rollout of vaccines to residential disability facility residents.
4.47 Health did not engage sufficient VAS providers with sufficient capacity to deliver the in-reach administration channel early in vaccine rollout. Health advised that in the early stages of the vaccine rollout, the high demand for vaccine administrators created challenges for the operational readiness of the contracted VAS providers. As discussed in paragraph 2.53, Health engaged additional VAS providers later in the rollout to increase the number of in-reach clinics. Health also arranged additional in-reach visits by primary care (such as GPs) from August 2021 and established vaccination hubs for persons with a disability and residential disability workers from 3 May 2021.
4.48 On 21 September 2021, Health approved a Disability Phase 1a Rollout Plan to accelerate the residential disability rollout with the target to offer first and second dose clinics to all residential disability sites by 31 October 2021.147 Health advised that it met this target on 2 November 2021. 148Due to the poor quality of the data provided the ANAO was not able to make an independent assessment of this. This plan included a number of activities to encourage residents and staff to get vaccinated. For example, Health introduced a grant program for facilities to encourage them to transport residents to an out-of-home vaccination site and residential disability worker vaccination hubs. Health also redirected VAS providers from residential aged care to residential disability facilities to schedule additional in-reach clinics.
Phase 1b priority groups
Aboriginal and Torres Strait Islander people
4.49 Aboriginal and Torres Strait Islander people aged 50 and over were eligible to receive AstraZeneca from 22 April 2021 (unless they were eligible under a Phase 1a priority group category).149 The balance of the Aboriginal and Torres Strait Islander population was eligible to receive a vaccine from 8 June 2021. Aboriginal and Torres Strait Islander people could access vaccines through Aboriginal Community Controlled Health Organisations (ACCHOs), which were engaged to provide localised and culturally safe vaccinations.150
4.50 Figure 4.9 shows that the vaccination rate of Aboriginal and Torres Strait Islander people was consistently lower than that of the non-Indigenous Australian population throughout the rollout. In a media release on 14 September 2021, the Minister for Health and Aged Care stated that Health was ‘committed to seeing Aboriginal and Torres Strait Islander vaccination rates meet if not exceed the national target [of 80 per cent double vaccinated].’ Health did not reach this target in 2021. Seventy-two per cent of the eligible Aboriginal and Torres Strait Islander population was double vaccinated by 31 December 2021 compared to 97 per cent of the non-Indigenous Australian population.
Figure 4.9: Aboriginal and Torres Strait Islander and non-Indigenous vaccination rates for people aged 12 years and over in 2021

Note: The ANAO calculated Indigenous and non-Indigenous vaccination rates using the ABS’s 2021 population estimates based on 2016 census data. The ABS published data on age groups on five-year cohorts, including ages 10–14 years. The ANAO estimated the population of the 12–14 age group, was 60 per cent of the 10–14 year population group.
Source: ANAO analysis of Health data.
4.51 Health advised the ANAO that the lower vaccination rate was influenced by several factors including:
- the predominant use of AstraZeneca in regional and remote areas until May 2021 where 62.6 per cent of the Aboriginal and Torres Strait Islander population live (compared to 27.3 per cent of the non-Indigenous population)151 — the discovery in March 2021 of very rare but potentially fatal side effects of the AstraZeneca vaccine (discussed in paragraph 2.17) increased hesitancy for this vaccine;
- religious based hesitancy and misinformation targeted at Aboriginal and Torres Strait Islander people;
- a lack of urgency to get vaccinated — until August 2021, there had been no significant outbreaks and limited COVID-19 community transmission in Aboriginal and Torres Strait Islander communities, which contributed to complacency and no rush to get vaccinated; and
- delays in family decision-making — the Indigenous Governance Toolkit states ‘Decisions in Indigenous nations, communities or groups are usually made through extensive collective discussion and consultation.’152 Health advised the ANAO that the separation of Aboriginal and Torres Strait Islander people into age-based eligibilities made family decision-making more difficult and may have delayed vaccine uptake.
4.52 Health ran targeted communication activities for Aboriginal and Torres Strait Islander people with the intention of addressing vaccine hesitancy and increasing uptake (discussed further in paragraph 4.74). Health identified primary care sites to provide coverage in regional and remote areas, including those servicing Aboriginal and Torres Strait Islander communities. Health also prioritised ACCHOs to receive doses of Pfizer to provide an alternative for those hesitant to receive AstraZeneca.
4.53 From 14 September 2021, Health began targeted activities to accelerate the COVID-19 vaccine rollout for Aboriginal and Torres Strait Islander people in 30 priority areas through tailored vaccination channels and activities.153 This included running home visits and pop-up clinics in the identified areas.154 These targeted activities were in response to the low vaccination rate of Aboriginal and Torres Strait Islander people relative to the broader Australian population.
4.54 The gap in vaccination rates between Aboriginal and Torres Strait Islander people and the Australian public did not begin to decrease until December 2021 when the non-Indigenous vaccination rate plateaued. The vaccination gap between the populations was 33.8 per cent in November 2021 and 31.3 per cent in December 2021.
Non-residential disability
4.55 Adults with a disability who did not live in a residential disability facility were eligible to receive a vaccine in Phase 1b on 22 March 2021. All NDIS recipients aged 16 and over were eligible to receive a vaccine from 8 June 2021. Figure 4.10 shows that the vaccination rate for NDIS recipients remained lower than the rate for the broader Australian population, despite earlier eligibility to receive a vaccine.
Figure 4.10: Vaccination rate of all NDIS recipients aged 16 or over in 2021 compared to the Australian population
Note: Health accessed regular data on the number of National Disability Insurance Scheme recipients who are either single or double vaccinated from 25 May 2021.
The percentage of the Australian population fully vaccinated is based on the proportion of the population aged 16 years and over to align with the cohort for NDIS recipients in residential disability included in reports.
Source: ANAO analysis of Health documents.
4.56 People with disabilities may have a range of access or sensory requirements155 for vaccination sites, such as wheelchair ramps. Health and the state and territory governments ran vaccination hubs designed for people with a disability and other priority groups to promote the safe uptake of vaccines. Health advised that the first Australian Government vaccination hub for people with a disability occurred on 4 May 2021.
4.57 On 15 October 2021, Health approved the Acceleration of Vaccination to NDIS participants and NDIS screened Workers Plan (the acceleration plan), which had a target of achieving 80 per cent ‘fully vaccinated’ by 31 October 2021. Health reached the acceleration plan’s target of 80 per cent of eligible NDIS recipients double vaccinated on 12 December 2021.
Age-based eligibility
4.58 The Australian Government prioritised the vaccination of people aged 70 and over (not in residential aged care facilities) as part of Phase 1b of the vaccine rollout156, and people aged 50–69 years as part of Phase 2a of the vaccine rollout. In the April 2021 recalibration, Health introduced two additional cohorts in order to stagger eligibility — adding people aged 40–49 years within Phase 2a, and people aged 16–39 years within Phase 2b. Following TGA approval and ATAGI advice in July and August 2021, people aged 12–15 years were eligible to receive a vaccine from 13 September 2021. Age-based groups were serviced through vaccination channels available to the Australian public including primary care and state and territory clinics. There were no specific channels targeted to age cohorts. Health advised all age groups were able to access the eligibility checker and vaccine clinic finder to advise if they were eligible for receive a vaccine and, if so, where appointments were available.157
4.59 Figure 4.11 shows older age groups were vaccinated earlier in the rollout consistent with their earlier eligibility.
Figure 4.11: Rate of double vaccination by age cohort in 2021
Note: People aged 50 years or older were eligible to receive AstraZeneca from 3 March 2021. All age groups could receive any vaccine from 30 September 2021.
People in an age group may have been eligible to receive a vaccine earlier as part of another priority group, such as border and quarantine workers.
Source: ANAO analysis of Health data.
Other targeted groups
Culturally and linguistically diverse communities
4.60 As discussed in paragraph 2.22, culturally and linguistically diverse (CALD) communities were not a priority group to receive a vaccine under the national vaccine rollout strategy. Health accessed population level data on the CALD rollout from the Multi-Agency Data Integration Program from June 2021.158
4.61 In August 2021, people with a recorded measure of cultural and linguistic diversity159 had a lower uptake for vaccination than the national average. As at 21 November 2021, 91 per cent of people aged 12 and over had received at least one vaccine dose. In comparison:
- 84 per cent of people born overseas (excluding the United Kingdom, Ireland or New Zealand) had received at least one dose;
- 83 per cent of people who speak a language other than English at home had received at least one dose; and
- 81 per cent of people with low English proficiency had received at least one dose.
4.62 Health shared information on the CALD vaccination rates with the CALD Communities Advisory Group (see Table 3.7), PHNs and the states and territories from 2 November 2021. Health also ran targeted communication activities to attempt to increase vaccination in CALD communities (discussed further in paragraph 4.74).
Regional and remote locations
4.63 Health relied on primary health channels to increase vaccine coverage in regional and remote locations.160 Health engaged the Royal Flying Doctor Service to support locations with limited access to primary care and hospital vaccination sites, and to transport vaccines when required. Initially, the rollout in regional and remote locations used AstraZeneca because the ultra-cold transport and storage requirements of Pfizer made its use more difficult. However, publicity about the potential adverse side-effects of AstraZeneca led to vaccine hesitancy and reduced uptake. Consequently, on 19 April 2021 Health made all remote or very remote communities eligible to receive any vaccine to encourage uptake.
4.64 As vaccination rates increased across Australia, as at 31 December 2021, there were still a number of regional and remote areas with lower vaccination rates the Australian average. Factors influencing this included:
- limited healthcare workforce capacity in regional and remote areas to administer vaccines, which made it more difficult for recipients to easily access vaccines; and
- difficulty transporting vaccines to remote locations while maintaining cold chain requirements.
4.65 Throughout the rollout, Health on-boarded primary care sites to expand coverage of vaccine sites in remote and regional locations (see paragraph 4.10). As discussed in paragraph 4.52, Health also targeted activities to promote vaccine uptake to Aboriginal and Torres Strait Islander residents in remote and regional areas.
Has communication about the vaccine rollout been effective?
Health’s communication about the vaccine rollout has been largely consistent with its communication strategy, and its monitoring of public sentiment has shown attitudes towards COVID-19 vaccination improved over time. Health implemented communication activities targeted at Aboriginal and Torres Strait Islander people and culturally and linguistically diverse communities, but these activities were not as effective at reaching these groups as communication to the general population. Health has not yet conducted an evaluation of the effectiveness of the advertising campaign as the campaign is ongoing.
Consistency with communications strategy
4.66 As noted at paragraph 2.63, Health’s Communication strategy: COVID-19 vaccines and treatments (communication strategy) outlined six principles to achieve its high-level objective. Table 4.6 shows Health’s communication activities largely addressed these principles (detailed analysis is shown at Appendix 5).
Table 4.6: ANAO’s assessment of Health’s implementation of communication principles
|
Health’s communication principle |
Communication activities addressed principle? |
|
Be the authoritative source of information |
|
|
Meet specific information needs |
|
|
Address known motivators |
|
|
Create genuine engagement with the health sector |
|
|
Take preventative measures |
|
|
Supports implementation |
|
|
Key: = largely addressed
|
|
Source: ANAO analysis of Health documents.
4.67 Health implemented communication activities which fully addressed five of its principles, and largely addressed its principles for ‘meets specific information needs’. Health’s communication activities did not recognise the economic significance of a COVID-19 vaccine in advertising materials or key messages, which was included under their principle to ‘meet specific information needs’. Health’s market research identified that economic concerns were not a strong driver for vaccine uptake in 2021, and as such focused messaging on the health benefits of vaccines.
4.68 As noted at paragraph 3.38, Health published a large range of information on the progress of the vaccine rollout on its website throughout the COVID-19 pandemic.161 From 9 April 2021 this included daily public updates and regular weekly updates. The information communicated in these updates increased over time (see Figure 3.4) as a result of public interest, availability of data and the Prime Minister’s public commitments to increasing data transparency.
4.69 Health used the communication channels identified in its communication strategy (see paragraph 2.60) to communicate information on the vaccine rollout. Health started communication for Phase 1 on 22 January 2021 before the vaccine rollout began and followed the four phases identified in the communication strategy (see Table 2.8).
4.70 Health implemented the mitigation strategies against the communication risks identified in the communication strategy (see Table 2.9). Health did not identify any additional communication risks to the vaccine rollout. As discussed in paragraph 2.46, Health identified and managed whole-of-rollout risks through separate processes.162
Effectiveness of Health’s communication activities
4.71 Health began monitoring sentiment and intent to vaccinate from late 2020 (see paragraph 2.66). Health used this sentiment monitoring to track the effectiveness of vaccine rollout communications against the objectives of the COVID-19 Vaccines Campaign Advertising Campaign Strategy (advertising strategy) and communication strategy (see paragraph 2.73). As at June 2022, Health had not conducted its planned final evaluation of the effectiveness of the advertising strategy as the campaign is ongoing.
4.72 Figure 4.12 shows that over time the proportion of people likely to get vaccinated, or who had already been vaccinated, decreased from 73 per cent in March 2021 to 63 per cent in June 2021 (following the changes in ATAGI advice on AstraZeneca), then increased to 93 per cent in December 2021.
Figure 4.12: Likelihood to get the COVID-19 vaccine and vaccination status

Note: Likelihood to vaccinate was determined from participants rating from 1–10 to ‘You previously noted that you have not yet had the first dose of the COVID-19 vaccine. How likely are you to get it now that it is available?’ A response of 0–1 was assessed as very unlikely, 2–3 as unlikely, 4–6 as neither likely or unlikely, 7–8 as likely and 9–10 as very likely.
Source: ANAO analysis of Department of Heath documents.
4.73 During the vaccine rollout Health adjusted its advertising strategy and activities in response to monitoring and evaluation (see case study 2).
|
Case study 2. Identification and response to hesitancy to the AstraZeneca vaccine |
|
On 17 April 2021, Health paused advertising on the rollout in response to the Australian Technical Advisory Group on Immunisation’s advice on a rare but potentially severe side effect linked to the AstraZeneca vaccine. Health’s communication monitoring in April and May 2021 identified:
On 29 April 2021 Health developed new advertising materials to target Australians aged 50 and over, consistent with the Australian Government’s new approach to the vaccine rollout. On 10 May 2021 Health responded to research on the current barriers to vaccination and amended this advertising to include key messages that a vaccine was voluntary, free and the best way to ‘keep you and your community COVID-free’. In June 2021 the likelihood of eligible participants to get vaccinated stabilised, which included Australians aged 50 and over. |
Communication to target groups
4.74 As discussed in 2.69, the communication strategy identified CALD communities, Aboriginal and Torres Strait Islander people, people with a disability, older Australians and demographics with a high level of vaccine hesitancy and target groups for communication. Table 4.7 summarises the key communication activities targeted to these groups.
Table 4.7: Examples of population specific communication activities in 2021a
|
Targeted group |
Activities implemented |
|
CALD communities |
|
|
Aboriginal and Torres Strait Islander people |
|
|
People with a disability |
|
|
Older Australians including those in RACFs |
|
|
Demographics with a higher level of vaccine hesitancy or regions with low vaccination rates |
From July to December 2021, Health:
|
Note a: This is not a comprehensive list of all targeted communication activities undertaken throughout the vaccine rollout. Health advised it undertook additional activities.
Note b: Not all materials or media were translated into all languages.
Source: ANAO analysis of Health documents.
4.75 Forty-nine per cent of all Australians recalled seeing, hearing or reading vaccine campaign advertising materials in December 2021. However, Figure 4.13 shows recognition was lower for advertising targeting CALD communities (21 per cent) and Aboriginal and Torres Strait Islander people (31 per cent).163
Figure 4.13: Recognition of vaccine rollout advertising targeted to Aboriginal and Torres Strait Islander people and CALD communities compared to recognition of advertising materials by the Australian public

Note: All public advertising refers to the recognition of any advertising materials on the vaccines rollout. CALD targeted advertising includes all media communication through non-English media. Indigenous targeted advertising includes material shown in Indigenous media.
The sample size for Indigenous advertising recognition was consistently smaller than the CALD sample and the contractor noted ‘results by month should be treated with caution due to low sample size’.
Source: ANAO analysis of Department of Heath documents.
4.76 Stakeholders from the Aboriginal and Torres Strait Islander health sector advised the ANAO that, despite the activities listed in Table 4.7, communication was not appropriately targeted to Aboriginal and Torres Strait Islander communities in the early stages of the vaccine rollout.164 Similarly, the final report from Public Hearing 12 of the Royal Commission into Violence, Abuse Neglect and Exploitation of People with Disability stated, although Health developed ‘easy read’ materials which met the technical criteria for accessibility, the materials were not understandable to people with an intellectual disability.165
Appendices
Appendix 1 Entity response


Appendix 2 Improvements observed by the ANAO
1. The existence of independent external audit, and the accompanying potential for scrutiny improves performance. Improvements in administrative and management practices usually occur: in anticipation of ANAO audit activity; during an audit engagement; as interim findings are made; and/or after the audit has been completed and formal findings are communicated.
2. The Joint Committee of Public Accounts and Audit (JCPAA) has encouraged the ANAO to consider ways in which the ANAO could capture and describe some of these impacts. The ANAO’s 2021–22 Corporate Plan states that the ANAO’ s annual performance statements will provide a narrative that will consider, amongst other matters, analysis of key improvements made by entities during a performance audit process based on information included in tabled performance audit reports.
3. Performance audits involve close engagement between the ANAO and the audited entity as well as other stakeholders involved in the program or activity being audited. Throughout the audit engagement, the ANAO outlines to the entity the preliminary audit findings, conclusions and potential audit recommendations. This ensures that final recommendations are appropriately targeted and encourages entities to take early remedial action on any identified matters during the course of an audit. Remedial actions entities may take during the audit include:
- strengthening governance arrangements;
- introducing or revising policies, strategies, guidelines or administrative processes; and
- initiating reviews or investigations.
4. During the course of the audit, the ANAO did not observe changes in Health’s approach to the vaccine rollout.
Appendix 3 Monitoring data availability
Table A.1: Availability of data to track the progress of the vaccine rollout and the vaccination status of priority and target populations
|
Information tracked |
Was data available to Health? |
Date data was availablea |
Data location/ system |
Comments |
|
Vaccination status of Residential Aged Care Facility residents |
◆ |
22/02/2021 |
Myagedcare portal |
Reporting mandated on 27 July 2021. |
|
Vaccination status of Residential Aged Care Facility workers |
◆ |
15/06/2021 |
Myagedcare portal |
Reporting mandated on 15 June 2021. |
|
Vaccination status of residential disability residents |
▲ |
21/05/2021b |
Multiple locations |
Data partially available. Data sets used to assess this held by multiple agencies/departments. |
|
Vaccination status of residential disability workers |
▲ |
18/12/2021 |
Multiple locations |
Reporting not mandated by states and territories until December 2021. |
|
Vaccination status of non-residential disability |
▲ |
21/05/2021b |
Multiple locations |
Data partially available. Datasets used are held by multiple agencies/departments. |
|
Vaccination status of people with an underlying medical condition |
■ |
N/A |
N/A |
Data on medical conditions was not recorded with vaccination status. |
|
Vaccination status of Aboriginal and Torres Strait Islanders |
◆ |
22/02/2021 |
AIR |
Data collected with vaccination status. |
|
Vaccination status of people in regional and remote areas |
◆ |
22/02/2021 |
Australian Immunisation Register |
Data collected with vaccination status. |
|
Vaccination status of culturally and linguistically diverse |
◆ |
17/06/2021 |
AIR data and the Multi-Agency Data Integration Program |
Health integrated AIR data with the Multi-Agency Data Integration Program data held by the Australian Bureau of Statistics to provide estimated whole of population data based on the 2016 census. |
|
Vaccination status of frontline healthcare and border workers |
■ |
N/A |
N/A |
Occupation data was not recorded with vaccination status. |
|
Vaccination status of critical and high-risk workers |
■ |
N/A |
N/A |
Occupation data was not recorded with vaccination status. |
|
Vaccination status of age-based groups |
◆ |
22/02/2021 |
Australian Immunisation Register |
Data collected as part of AIR reporting. |
|
Vaccine wastage |
◆ |
22/02/2021 |
CVAS |
Vaccine wastage was recorded through existing systems before CVAS was brought online. |
|
Vaccines ordered and delivered |
◆ |
22/02/2021 |
CVAS |
Vaccine wastage was recorded through existing systems before CVAS was brought online. |
|
KEY: ◆ data available to Health ▲ data partially available to Health ■ data not available to Health |
||||
Note a: 22/02/2021 reflects that the data was available from the beginning of the vaccine rollout.
Note b: The date Services Australia and National Disability Insurance Agency data was first reported to Health and checked against AIR records.
Source: ANAO analysis of Health documents and press releases by the Minister for Health and Aged Care.
Appendix 4 ANAO assessment of Health’s performance against key public targets
Table A.2: ANAO assessment of Health’s performance against key public targets
|
Target |
Source of target |
Datea |
Was the target met? |
Was the target superseded? |
What was it superseded by? |
Comments |
|
Targets for the beginning of the rollout |
||||||
|
‘135 million units of vaccine are potentially available for Australia, with the most likely timing being the first quarter, probably March, for the first vaccines to roll out.’ |
Minister for Health and Aged Care |
5/11/2020 |
◆ |
Yes |
Rollout beginning March 2021 |
The vaccine rollout began on 22 February 2021. |
|
‘On track to deliver vaccines to Australians, commencing in March of 2021.’ |
Minister for Health and Aged Care |
13/11/2020 |
◆ |
Yes |
Rollout to begin in late February 2021 |
The vaccine rollout began on 22 February 2021. |
|
‘[Australia is] still, though, on track to commence later [the vaccine rollout] this month [February].’ |
Prime Minister |
04/02/2021 |
◆ |
No |
N/A |
The vaccine rollout began on 22 February 2021. |
|
Targets for the Australian population |
||||||
|
‘The goal and the expectation is that Australians who sought vaccination will be vaccinated within 2021.’ |
Minister for Health and Aged Care |
5/11/2020 |
Unknown |
Yes |
Have vaccinated Australia by 31 October 2021 |
The ANAO was unable to make an assessment if all people who wanted to get vaccinated received a vaccine. By 31 December 2021 93 per cent of Australians aged 12 or over had received at least one dose of vaccine. |
|
‘We anticipate optimistically that we would hope to start the vaccination with around 80,000 vaccinations a week.’ |
Prime Minister |
07/01/2021 |
■ |
No |
N/A |
36,539 doses were administered by 28 February 2021, one week after the vaccine rollout began. |
|
‘We hope by the end of February- end of March, I should say, to have reached some 4 million population. That is a target.’ |
Prime Minister |
07/01/2021 |
■ |
Yes |
Four million doses by early April 2021 |
By 31 March 2021, 733,646 vaccines were administered in Australia. |
|
‘The events of recent weeks I think will mean that four million position will be something that is going to be achieved in early April as opposed to late March.’ |
Prime Minister |
25/01/2021 |
■ |
No |
N/A |
By 30 April 2021, 2,025,332 were vaccines administered. Four million vaccines administered was reached on 31 May 2021. |
|
‘We aim to have the country vaccinated before the end of October.’ |
Minister for Health and Aged Care |
31/01/2021 |
■ |
Yes |
Offer all Australians a vaccine by the end of October 2021 |
By 31 October 2021, 87 per cent of Australian aged 12 or over had received at least one vaccine dose. |
|
‘The Australian Government has a comprehensive plan to offer COVID-19 vaccines to all Australians by the end of October 2021.’ |
Minister for Health and Aged Care |
21/02/2021 |
◆ |
Yes |
All eligible Australians can receive a first dose of a vaccine by the end of October. |
From 13 September all people aged 12 or over were eligible to receive a vaccine. By 31 October 2021, 87 per cent of Australians aged 12 or over had received at least one vaccine dose. |
|
‘The one goal that we are absolutely locked into is getting every adult Australian a first dose in the arm by the end of October or earlier.’ |
Secretary of Health |
24/03/2021 |
■ |
No |
N/A |
By 31 October 2021, 87 per cent of Australians aged 12 or over had received at least one vaccine dose. |
|
‘The National COVID Vaccine Taskforce is to coordinate and lead the implementation of the COVID-19 vaccination program and public information campaign that will motivate eligible people in Australia to receive at least the first dose of the COVID-19 vaccination by 20 December 2021.’ |
Coordinator General of Operation COVID Shield (Operation COVID Shield Campaign Plan) |
03/08/2021 |
■ |
No |
N/A |
At 20 December 2021, 93 per cent of the eligible Australian population had received at least one dose. |
|
‘The purpose of [the Operation COVID Shield Campaign Plan] is to detail the mechanisms and arrangements that will lead achievement of vaccination targets which are set out in the National Plan to Transition Australia’s National COVID Response (i.e., ~70% fully vaccinated to move to Phase B and ≥80% fully vaccinated to move to Phase C)’…..[the Operation COVID Shield Campaign Plan] covers the period from 1 July to 31 December 2021.’ |
Coordinator General of Operation COVID Shield (Operation COVID Shield Campaign Plan) |
03/08/2021 |
◆ |
No |
N/A |
On 16 October 2021, 70 per cent of Australians aged 16 or over were double vaccinated and on 30 October 2021, 80 per cent were double vaccinated. |
|
‘Every vaccination saves lives and gets us one step closer to reaching 70 per cent of Australians, aged over 16, vaccinated before the end of the year.’ |
Minister for Health and Aged Care |
09/08/2021 |
◆ |
No |
N/A |
By 31 December 2021, 92 per cent of Australians aged 16 or over had received at least two doses of a vaccine. |
|
Targets for priority groups |
||||||
|
‘It is anticipated the roll out to aged care facilities will take approximately six weeks [completed by 5 April].’ |
Minister for Health and Aged Care |
16/02/2021 |
■ |
Yes |
Aged care rollout to be completed by 31 May 2021. |
Health completed second dose visits to all eligible RACFs on 20 September 2021. |
|
‘Quarantine and border workers and aged care residents are on track to be vaccinated by April 2021.’ |
Prime Minister |
21/02/2021 |
Unknown |
No |
N/A |
The ANAO was unable to make an assessment on this due to the lack of reliable data on the vaccination rate of this cohort. This was also an ongoing effort as people joined on left the sector and was led and tracked by the states and territories. |
|
‘[Health] are very confident that we will complete [the rollout to] aged care in May [2021].’ |
Associate Secretary of Health |
20/04/2021 |
■ |
No |
N/A |
Health completed second dose visits to all eligible RACFs on 20 September 2021. |
|
‘[the Secretary of Health has] said the middle of the year for the vulnerable people [including residential disability]. Obviously, there’s no specific date on that, but the middle of the year is around 30 June.’ |
Secretary of Health |
20/04/2021 |
■ |
No |
N/A |
At 30 June 2021 10 per cent of residents in residential disability facilities were double vaccinated. By 20 September 2021, Health had held second dose clinics at 71 per cent of residential disability sites. |
|
‘[The Australian Government is] absolutely committed to seeing Aboriginal and Torres Strait Islander vaccination rates meet, if not exceed, the national target [of 80 per cent double vaccinated].’ |
Minister for Health and Aged Care |
14/09/2021 |
■ |
No |
N/A |
Not met in 2021. At 31 December 2021, 72 per cent of Aboriginal and Torres Strait Islander people aged 16 or over had received at least two doses of a vaccine. |
|
KEY: ◆ target met ■ target not met |
||||||
Note a: Date of the announcement is the date of the first announcement of the target. Many of the targets, such as the vaccine rollout beginning in March 2021, were stated in multiple media releases or interviews.
Source: ANAO analysis of Health data, transcripts and press releases by the Prime Minister, Minister for Health and Aged Care and Hansard records.
Appendix 5 ANAO assessment of Health’s communication activities
Table A.3: Consistency of Health’s communication activities with the principles and associated actions identified in its Communication Strategy for the vaccine rollouta
|
Principle |
Associated actions |
Implemented actions |
Implemented action/s consistent with those planned |
|
Be the authoritative source of information |
Be transparent. |
Daily progress reports on the vaccine rollout published on Health’s website. |
✔ |
|
Build regular and real-time communication. |
Regular updates and press engagement by the Prime Minister, Minister for Health and Aged Care, and senior Health officials. |
✔ |
|
|
Make use of social media. |
Regular use of official social media accounts. Health published 4,099 vaccine related posts between 1 January and 31 December 2021. This included 76 videos addressing the current ‘top three’ questions made on social media. |
✔ |
|
|
Update website information regularly and rapidly. |
Health advised it regularly updated the Department’s website with new information on the conduct of the rollout. This included the daily updates on the progress of the vaccine rollout discussed in paragraph 3.33. |
✔ |
|
|
Anchor communications where possible to the Australian Government’s COVID-19 Vaccine and Treatments Strategy. |
Key strategic documents were available to the public including the Australian COVID-19 Vaccination Policy and the Operation COVID Shield Campaign Plan. |
✔ |
|
|
Meet specific information needs |
Recognise the economic significance of a COVID-19 vaccine. |
Health’s market research showed that in 2021, economic concerns were not a strong motivator for vaccine uptake, and as such messaging focused on the health benefits of vaccines. Health advised the 2022 vaccine booster communication activities highlight the economic benefit. |
✘ |
|
Promote the process for the rigorous testing and monitoring of vaccines and vaccine safety. |
Information provided by TGA and ATAGI on approvals process and allocation decisions, published on Health’s website. Twenty-five per cent of the Minister for Health and Aged Care, and the Prime Ministers’ media releases between January and December 2021 mention vaccine safety requirements or that safety is a top priority. |
✔ |
|
|
confirm in which ways the vaccine process adheres to regular processes and what exemptions have been made, in particular in the context of vaccine safety and the role of the regulators. |
Consistency of key messages on vaccine safety and efficiency in press releases in 2021 by Health, the Prime Minister and the Minister for Health and Aged Care. Information was also provided by TGA and ATAGI on vaccine development, approvals process and allocation decisions, on Health’s website. Health also published 76 videos in 2021 addressing the ‘top three’ questions made on social media in the previous week. |
✔ |
|
|
Transparency on rollout, ie prioritisation groups, manufacturing capacity and constraints, and volumes/availability. |
Key strategic documents were available to the public including the COVID-19 Vaccine Policy and the Operation COVID Shield Campaign Plan, and vaccine allocation horizons. |
✔ |
|
|
Recognise the public health benefits of a COVID-19 vaccine. |
Sixteen per cent of media releases by the Prime Minister, Minister for Health, Health and ATAGI recognise the community benefits of vaccines. |
✔ |
|
|
Provide information on the development process of vaccines and treatments including clinical trial phases. |
Information provided by TGA and ATAGI on vaccine development, approvals process and allocation decisions, on Health’s website. Press releases by the Prime Minister and Minister for Health and Aged Care providing updates on vaccine purchased and progress through clinical trials. |
✔ |
|
|
Address known motivators |
Acknowledge the link between a vaccine and Australia’s ‘return to normal.’ |
Advertising material promotes vaccination as a return to normal through tailored videos, radio scripts, out of home advertising, and posters. |
✔ |
|
Address community benefits and incentivising disengaged or complacent groups to get vaccinated for the sake of others or a greater good. |
Sixteen per cent of public statements by the Prime Minister, Minister for Health and Aged care and Health referenced the community and family benefits of vaccines. Advertising materials targeted messaging based on barriers to vaccination identified in market research. |
✔ |
|
|
Overcoming any lack of convenience, or obstacles, that exist in the administration model. |
Health advised it developed and regularly updated the vaccine clinic finder online tool and the National Coronavirus to support consumers to find and access vaccines more easily. The vaccine clinic finder and eligibility checker were consistently referenced in communication materials. Health also ran information kiosks in shopping centres and major events to help consumers book vaccination appointments. |
✔ |
|
|
For people who distrust the authorities, meet them where they are and to appeal directly to their sense of social identity or culture. |
Targeted communication to CALD, Aboriginal and Torres Strait Islander and other groups. This included translating materials into multiple Indigenous and non-Indigenous languages and ensuring materials were culturally appropriate. Public relations activities to provide information from trusted sources within the local environment, such as pop-up booths in shopping centres. |
✔ |
|
|
Create genuine engagement with the health sector |
Work with industry to amplify important messages to the sector and to the public. |
Regular GP, Primary Health Network and allied health webinars and newsletters. Primary health sites administering vaccines were responsible for providing clear and accessible information to their patients. |
✔ |
|
Leverage existing mechanisms for peak bodies and healthcare providers to communicate with government, identify opportunities and address concerns. |
Regular engagement with health sector peak bodies including those representing priority groups, such as the National Aboriginal Community Controlled Health Organisation. |
✔ |
|
|
Take preventative measures |
Provide regular updates to ensure Australians that the most up to date information is being promoted proactively. |
Daily and weekly updates on vaccine rollout progress data published on Health’s website and regular press conferences and media releases by the Prime Minister, Minister for Health and Aged Care and senior Health officials. Between 1 January 2020 and 31 December 2021 the Prime Minister held 366 interviews or press conferences which referenced vaccines. The Minister for Health and Aged Care held 169 in the same period. |
✔ |
|
Have a clear and detailed plan to proactively respond to vaccine related issues and emerging concerns. |
Communication strategy considers risk and includes mitigation plans for communication risks. |
✔ |
|
|
Roadmap on rollout, including regular status updates. |
Daily and weekly updates on vaccine rollout progress data published on Health’s website. These updates do not include reporting against targets. |
✔ |
|
|
Influence media partners to cover vaccine related information responsibly. |
Health advised it engaged with social media organisation on coverage of the vaccine rollout. |
✔ |
|
|
Educate about how to spot false and misleading information. |
Regularly updated ‘Is it true?’ page on Health of Health website addressing theories and questions on the vaccine rollout. |
✔ |
|
|
Support implementation |
Support the taskforce’s ‘roadmap’ approach for implementation |
Health included a section on communication in the Australian COVID-19 Vaccination Policy. The sectoral implementation plans released in early 2021 also included sections on communication. Health also included communication as a line of effort in the August 2021 Operation COVID Shield Campaign Plan and its supporting operational level plans. |
✔ |
|
Meet the need for post-immunisation communication and actions |
Weekly public reports on vaccine safety by the Therapeutic Goods Association. |
✔ |
|
|
Promote the two-dose regimen to improve completion rates for the vaccination program |
Health communicated that two vaccine doses were required in some website and social media materials from the beginning of the rollout in March 2021. The ATAGI statements published on Health’s website also stated this. |
✔ |
|
|
Educate the public on post implementation safety and monitoring processes, including avenues to report adverse events. |
Weekly public reports on vaccine safety by the Therapeutic Goods Association. Online vaccine side effect checker. |
✔ |
|
|
Educate the sector on implementation: timelines, storage, administration etc. |
Accessible training developed by the Australian College of Nurses for the storage and administration of vaccines. This was updated 31 times between February and December 2021 to include new clinical advice. |
✔ |
|
Note a: This is not an exhaustive list of all communication activities for the vaccine rollout conducted by Health.
Source: ANAO analysis of Health documentation.
Footnotes
1 Australian Government, Australia’s COVID-19 vaccine and treatment strategy, Department of Health, 18 August 2020, p. 3.
2 During the course of the audit and prior to 1 July 2022, the administering entity was the Department of Health.
3 Further details on the ANAO’s COVID-19 multi-year audit strategy can be found at https://www.anao.gov.au/work-program/covid-19.
4 Biosecurity (Listed Human Diseases) Amendment Determination 2020, 21 January 2020, available from https://www.legislation.gov.au/Details/F2020L00037 [accessed 25 June 2021].
5 Biosecurity (Human Biosecurity Emergency) (Human Coronavirus with Pandemic Potential) Declaration 2020, 18 March 2020, available from https://www.legislation.gov.au/Details/F2020L00266 [accessed 25 June 2020]. The emergency declaration was extended on 15 May 2020, 4 September 2020, 11 December 2020, 3 March 2021 and 11 June 2021.
6 Commonwealth of Australia, Keeping Australia safe, Budget 2022–23 factsheet, available from https://budget.gov.au/2022-23/content/download/glossy_safe.pdf [accessed 27 April 2022].
7 The COVID-19 Emergency Response Plan drew heavily on a pre-existing plan, the Australian Health Management Plan for Pandemic Influenza, which was published in August 2019.
8 Department of Health, Australian Health Sector Emergency Response Plan for Novel Coronavirus, February 2020, p. 17. Although the terms vaccination and immunisation are often used interchangeably, they are not the same. Vaccination is the process of receiving a vaccine to stimulate the body’s immune response against disease. Immunisation is the process by which a person becomes protected against a disease through vaccination.
See: Centres for Disease Control and Prevention, Immunization: The Basics [Internet], September 2021, available from https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm [accessed 14 April 2022]
9 The ANAO has audited aspects of the NIP in two previous audits: Auditor-General Report No.5 2021-22 Improving Immunisation Coverage, and Auditor-General Report No.46 2014-15 Administration of the Australian Childhood Immunisation Register.
10 The 17 diseases are: hepatitis B, diphtheria, tetanus, pertussis (whooping cough), haemophilus influenzae type b (Hib) disease, poliomyelitis, pneumococcal, rotavirus, measles, mumps, rubella, meningococcal, varicella (chickenpox), hepatitis A, human papillomavirus (HPV), influenza and herpes zoster (shingles).
11 Prior to COVID-19, the fastest a vaccine had been developed from viral sampling to approval was four years for the mumps vaccine in the 1960s. (See P Ball, ‘The lightning-fast quest for COVID vaccines — and what it means for other diseases’, Nature, 589, 2021.)
12 During the course of the audit and prior to 1 July 2022, the administering entity was the Department of Health.
13 Therapeutic goods are products used for: preventing, diagnosing, curing or alleviating a disease, ailment, defect or injury; influencing, inhibiting or modifying a physiological process; testing susceptibility to a disease or ailment; influencing, controlling or preventing conception; and testing for pregnancy.
14 The AstraZeneca vaccine is a genetically modified vaccine which also required approval from the Office of the Gene Technology Regulator, which was issued on 8 February 2021.
15 Some countries (such as the United Kingdom, United States and Canada) used ‘Emergency Use Authorisations’ (EUAs) to approve COVID-19 vaccines. The TGA does not have an EUA ‘pathway’ to approval.
16 In June 2020, Health advised the government that there were more than 130 COVID-19 vaccine candidates in pre-clinical development worldwide and 10 had entered clinical trials.
17 SITAG is chaired by the Secretary of Health and its members include the Chief Medical Officer, the co-chairs of the Australian Technical Advisory Group on Immunisation, the Chief Scientist, the Chair of the Pharmaceutical Benefits Advisory Committee and the Chief Executive of the CSIRO. It also included a number of non-government experts.
18 Australian Government, Australia’s COVID-19 vaccine and treatment strategy, Department of Health, 18 August 2020, p. 3. In this context, ‘diverse’ meant vaccine type (viral vector, protein or mRNA).
19 Most vaccines (including AstraZeneca and Novavax) can be stored and transported between 2°C and 8°C. Others (such as Moderna) must be kept frozen at -20°C and a few (such as Pfizer) must be kept ‘ultra-cold’ at - 90°C to -60°C.
20 A vaccine must go through three phases of clinical trials before it can be licensed and registered for human use. Phase 1 trials involve tests of dose level and safety on typically dozens of participants. Phase 2 trials (with hundreds of participants) test immunogenicity (the ability of the vaccine to provoke an immune response in the body). Phase 3 trials (with thousands of participants) are aimed at assessing the efficacy of the vaccine.
See: National Centre for Immunisation Research and Surveillance, Phases of clinical trials [Internet], available from https://ncirs.org.au/phases-clinical-trials [accessed 14 April 2022].
21 Department of Health, Australian COVID-19 Vaccination Policy, 13 November 2020, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australian-covid-19-vaccination-policy [accessed 20 April 2022].
22 Health, Australia’s COVID-19 vaccine national roll-out strategy 7 January 2021, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australias-covid-19-vaccine-national-roll-out-strategy [accessed 21 April 2022].
23 S. Morrison (Prime Minister), First COVID-19 Vaccinations, media release, 21 February 2020.
24 On 17 June 2021, ATAGI again amended its advice, recommending the use of Pfizer as the preferred vaccine for people under 60. This is discussed further at paragraph 2.21.
25 Prior to his appointment as Coordinator General, Lieutenant General John (JJ) Frewen was the Commander Defence COVID-19 Taskforce and was coordinating the Australian Defence Force COVID-19 response. In this report, Lieutenant General John (JJ) Frewen is referred to as the Coordinator General.
26 Children aged five to 11 years were eligible to receive the Pfizer vaccine at one third of the dosage given to people aged 12 years and above.
27 Further details on the ANAO’s COVID-19 multi-year audit strategy can be found at https://www.anao.gov.au/work-program/covid-19.
28 On 31 October 2020, the Minister for Foreign Affairs announced a $500 million vaccine access and health security initiative ‘including supplying safe and effective vaccine doses and delivering technical support to our regional partners.’
Minister for Foreign Affairs, Australian support for COVID-19 vaccine access in the Pacific and Southeast Asia, media release, 31 December 2020, available from https://www.foreignminister.gov.au/minister/marise-payne/media-release/australian-support-covid-19-vaccine-access-pacific-and-southeast-asia [accessed 19 May 2022].
29 The ANAO contacted 38 stakeholders identified by Health as key stakeholders in the vaccine rollout, and health authorities in each state or territory. Twenty-two of these stakeholders provided submissions.
30 During the course of the audit and prior to 1 July 2022, the administering entity was the Department of Health.
31 During the COVID-19 pandemic Australian governments have met regularly through a mechanism called ‘national cabinet’. These meetings have comprised the Prime Minister, state premiers and territory chief ministers. On 29 May 2020, the Prime Minister announced that national cabinet would replace the Council of Australian Governments.
32 Prime Minister, National Cabinet, media release, 7 August 2020, available from https://www.pm.gov.au/media/national-cabinet-7aug2020 [accessed 20 April 2022].
33 Department of Health, Australia’s COVID-19 vaccine and treatment strategy, 18 August 2020, available from https://www.health.gov.au/resources/publications/australias-covid-19-vaccine-and-treatment-strategy [accessed 20 April 2022].
34 Department of Health, Australian COVID-19 Vaccination Policy, 13 November 2020, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australian-covid-19-vaccination-policy [accessed 20 April 2022].
35 This included CEOs of state or territory health authorities or Chief Health Officers or, in some cases, both.
36 Despite the historical strengths of the NIP, Health advised the government that it did not possess some of the functionality that would be required for the COVID-19 rollout. Specifically, the NIP did not have the ability to: prioritise populations for sequential rollout and adjust where required; access real-time data on stock levels at all relevant locations; directly allocate doses from one location to another (including from one state to another); track and trace all doses from port to consumer (including monitoring wastage); understand, in near real time, people who have been vaccinated and any adverse events; and specify the types of locations where vaccination should take place.
37 The government subsequently decided to include everyone in Australia.
38 ‘Locations’ became known as ‘channels’. The Australian Government was responsible for the rollout to general practice, Commonwealth vaccination clinics, Aboriginal Controlled Community Health services, community pharmacies and ‘in-reach’ services to disability and aged care residential facilities. State and territory governments were responsible for vaccinations at hospitals and mass and community vaccination hubs. For more detail, see paragraph 2.48.
39 The plans were prepared for signature by the Australian Government Minister for Health and Aged Care and his state or territory counterparts. Health was not able to supply copies of signed versions for Queensland and South Australia. None of the plans were signed by the Minister for Health and Aged Care.
40 Such as the logistical requirement for ultra-cold transportation and storage for some vaccines.
41 ATAGI was established in February 1998. Its role is to ‘advise the Minister for Health on the medical administration of vaccines available in Australia’. Its members are experts in immunisation and infectious diseases.
See: Department of Health, Australian Technical Advisory Group on Immunisation (ATAGI) [Internet], available from https://www.health.gov.au/committees-and-groups/australian-technical-advisory-group-on-immunisation-atagi [accessed 21 April 2022].
42 Department of Health, ATAGI – Preliminary advice on general principles to guide the prioritisation of target populations in a COVID-19 vaccination program in Australia [Internet], 13 November 2020, available from https://www.health.gov.au/resources/publications/atagi-preliminary-advice-on-general-principles-to-guide-the-prioritisation-of-target-populations-in-a-covid-19-vaccination-program-in-australia [accessed 14 April 2022].
43 In discussions in November 2021 with the ANAO, the co-chair of ATAGI advised that in addition to drawing upon the knowledge and expertise of its members, ATAGI also had regard to the World Health Organisation’s Values framework for the allocation and prioritization of COVID-19 vaccination (published on 14 September 2020, available from https://www.who.int/publications/i/item/who-sage-values-framework-for-the-allocation-and-prioritization-of-covid-19-vaccination) [accessed 21 April 2022].
44 Health, Australia’s COVID-19 vaccine national roll-out strategy 7 January 2021, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australias-covid-19-vaccine-national-roll-out-strategy [accessed 21 April 2022].
45 Prime Minister, press conference, 7 January 2021, available from https://www.pm.gov.au/media/press-conference-australian-parliament-house-12 [accessed 21 April 2022].
46 The first 12 weeks of the rollout ended on 10 May 2021.
47 Thrombosis with thrombocytopenia syndrome or TTS.
48 Department of Health, ATAGI statement on AstraZeneca vaccine in response to new vaccine safety concerns, 8 April 2021, available from https://www.health.gov.au/news/atagi-statement-on-astrazeneca-vaccine-in-response-to-new-vaccine-safety-concerns [accessed 27 May 2022].
49 Health’s monitoring of the community’s intention to vaccinate is discussed at paragraphs 4.71 to 4.73.
50 Prime Minister, media statement, 22 April 2021, available from https://www.pm.gov.au/media/national-cabinet-4 [accessed 29 April 2022].
51 The additional doses acquired included: one million doses purchased from the Republic of Poland announced by the Prime Minister on 15 August 2021; a 500,000-dose swap agreement with Singapore; and a dose sharing partnership with the United Kingdom for 4 million doses to be received from September 2021 and returned by the end of 2021.
52 On several occasions during this period, ATAGI provided updated advice on the use of AstraZeneca. On the 17 June 2021 ATAGI recommended the use of Pfizer as the preferred vaccine for eligible people under 60. On 13 July 2021, in the context of a COVID-19 outbreak where the supply of Pfizer was constrained, ATAGI recommended adults younger than 60 years old who do not have immediate access to Pfizer should re-assess the benefits to them and their contacts from being vaccinated with COVID-19 Vaccine AstraZeneca, versus the rare risk of a serious side effect.
53 As noted at paragraph 1.9, monitoring adverse events (side-effects) is the responsibility of the TGA.
54 Health advised that ‘due to the complexity involved in the disability rollout and the need to be responsive to stakeholder advice, publishing a static implementation plan did not support the requirement.’
55 Further information about these statements and commitments is at Appendix 4.
56 IRAs are short, independent reviews that provide assurance to government for high-risk policy proposals. The IRA for the vaccine rollout assessed its readiness as ‘amber’, meaning that ‘successful implementation of the program/project appears feasible but prompt management attention is required to address issues/risks’.
57 Prime Minister, press conference, 4 June 2021, available from https://www.pm.gov.au/media/press-conference-australian-parliament-house-act-42 [accessed 26 April 2022].
58 At that date, 12.8 million vaccine doses had been administered and, of the population aged 16 or over, 41.9 per cent had received a first dose and 20.2 per cent had received a first and second dose.
59 These implementation challenges are further discussed at paragraphs 4.35 and 4.45.
60 Under the PGPA Act, Health’s accountable authority is the Secretary.
61 Public Governance, Performance and Accountability Act 2013, section 16, available from https://www.legislation.gov.au/Details/C2017C00269 [accessed 22 February 2022].
62 In this context, risk is defined as the ‘effect of uncertainty on objectives’ and risk management as the ‘coordinated activities to direct and control an organisation with regard to risk’. Issues are defined as ‘a risk that has already eventuated – something that is currently being managed’.
63 Stakeholder groups included state and territory governments, health care providers, patients and logistics providers and other external parties.
64 As noted at paragraph 2.25, planning prior to June 2021 did not include reference to targets.
65 In August 2021, with the establishment of the Operation Covid Shield Taskforce the First Assistant Secretary weekly meeting was replaced with a weekly leadership meeting with the Coordinator General.
66 Health’s estimate in the rollout strategy released on 7 January 2021 of the number of people aged over 18 was 20,030,000 (see Table 2.2).
67 In this audit, ‘administration channel’ is used to denote the type of location or means by which vaccines are physically administered. Balancing vaccine supply with the number of administration channels was a key challenge in the rollout: when supply was initially limited, there would have been little point in having (for example) general practitioners making appointments to vaccinate patients if they could not obtain vaccines. Conversely, when supply was more plentiful, there would have been a risk of wastage if there were not enough qualified people to administer vaccinations.
68 Department of Health, Australian COVID-19 vaccination policy, 13 November 2020, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australian-covid-19-vaccination-policy [accessed 26 April 2022].
69 In 2020, the Australian Institute of Health and Welfare reported that of the $57.7 billion spent on public hospitals, the Australian Government contributed $22.7 billion (39 per cent), the state and territory governments contributed $29.9 billion (52 per cent) and $5.1 billion (9 per cent) came from non-government sources such as individuals and private health insurers.
70 The channels shown in Table 2.6 for which the Australian Government is responsible (excluding in-reach) are also known as primary care, which is those services in the community that people attend first for health care (secondary care is delivered by specialists and tertiary care is delivered in hospitals).
71 For example, in-reach was established for residents of aged care and disability facilities who were high risk groups who may not be mobile, and ACCHOs were engaged as a channel because they would be trusted providers for Aboriginal and Torres Strait Islander people.
72 Health advised the two additional VAS providers engaged in late February and March 2021 were assessed under the same process but were not initially engaged to ensure redundancy in a constrained market.
73 An advertising campaign is a planned series of communication activities including paid media placement that share common objectives, target the same audience and have specific timelines and a dedicated budget.
74 These criteria are based on principles from Organisation for Economic Co-operation and Development, OECD Report on Public Communication [Internet], OECD, 2021, p. 22, available from https://www.oecd-ilibrary.org/governance/oecd-report-on-public-communication_22f8031c-en [accessed 15 March 2022].
75 WHO principles aim for communication to be accessible, actionable, credible and trusted, relevant, timely, and understandable.
See: World Health Organisation, WHO Strategic Communications Framework for effective communications [internet], WHO, 2017, p. 3, available from https://www.who.int/about/communications [accessed on 14 April 2022]
76 The remaining communication channels were social media, media engagement, information shared through health professionals, external websites, information shared through peak bodies, online portals used by health professionals, direct outreach, mailing lists, Australian Government apps and portals, advisory group updates, state and territory forums and public relations.
77 Public sentiment research monitors the public’s views towards issues through responses to survey questions as part of a focus group.
78 These included: none of the identified vaccine candidates are approved, rollout delays, adverse events (side effects) and inadequate supply. Health did not include mitigations for whole-of-rollout risks.
79 Principle five of the guidelines addresses procurement of advertising materials and was outside the scope of this audit.
80 During the course of the audit and prior to 1 July 2022, the administering entity was the Department of Health.
81 While the Coordinator General was required to report to the Prime Minister and the Minister for Health and Aged Care, the National COVID Vaccine Taskforce was not established as a separate entity. It remained a functional unit within Health and its staffing and operational expenses were met from Health’s budget.
82 Department of Health, Australian COVID-19 vaccination policy, 13 November 2020, p7, available from https://www.health.gov.au/resources/publications/covid-19-vaccination-australian-covid-19-vaccination-policy [accessed 20 April 2022].
83 The Australian Immunisation Register Act 2015 defines a recognised vaccination provider as ‘a general practitioner or an individual, or body, endorsed to administer vaccines in Australia, if the endorsement is for purposes that include the purposes of the Australian Immunisation Register; and is by the Commonwealth, a State or a Territory’.
84 The AIR records the date and type of vaccine administered, basic identifying information of the recipient, indigeneity, and basic information on the vaccine provider.
85 The ANAO did not examine Services Australia’s management of the AIR.
86 Including the PGPA Act and Privacy Act 1998.
87 Section 16 of the PGPA Act states the accountable authority of a Commonwealth entity must establish and maintain ‘an appropriate system of internal control for the entity.’ This includes IT controls for third parties.
88 The ANAO is aware that Amazon Web Services provides certification of its IT controls. Health did not request and has not reviewed this certification.
89 On 15 June 2021, the Minister for Health and Aged Care required all residential aged care providers to report on the vaccination status of their workers via the myagedcare portal. On 27 July 2021, this was expanded to residential aged care facility residents.
90 Health advised that daily and weekly reconciliations are performed between logistics providers’ warehouse and transport systems and CVAS to identify discrepancies.
91 National Centre for Immunisation Research and Surveillance, Australian Immunisation Register Data Transfer Study, NCIRS, 2018. The National Centre for Immunisation Research and Surveillance is the leading research organisation in Australia that provides objective expert advice on all aspects of vaccine preventable diseases, and other issues related to immunisation, to inform policy and planning for immunisation services in Australia.
92 The Australian Government amended the Australian Immunisation Register (Reporting) Act 2015 and the Australian Immunisation Register Rule 2015 to mandate this. The Australian Government made further amendments to the Australian Immunisation Register Rule 2015 in July 2021 to require vaccination providers report all National Immunisation Program Vaccines delivered from 1 July 2021 in the AIR.
93 Other government departments and agencies produced reporting for senior decision-makers which included information on the vaccine rollout. For example, the Department of the Prime Minister and Cabinet produced a daily report on the COVID-19 response for the Prime Minister.
94 Health advised this was a peak time as between 1.5 million and 2 million doses were being administered weekly in this period, and Health was also procuring and delivering additional vaccines during a COVID-19 outbreak.
95 The campaign plan set out Health’s plan for the vaccine rollout until 20 December 2021.
96 High-level targets referred to targets for the rollout as a whole. Lower-level targets were targets for specific priority groups or sectors.
97 The United States, Canada, United Kingdom and New Zealand regularly published data on the progress of their respective vaccine rollouts. The type and amount of information reported varied between each nation.
98 As at April 2022, Health’s daily updates had been released every day since 9 April 2021, except for 8 and 20 May 2021 (due to administrative error) and 25 and 26 December 2021.
99 Mechanisms for engagement and coordination with key stakeholders were updated in August 2021 in the campaign plan.
100 The Aboriginal and Torres Strait Islander Advisory Group on COVID-19 was co-chaired by a representative of NACCHO.
101 Aboriginal and Torres Strait Islander Advisory Group on COVID-19, available from https://www.health.gov.au/committees-and-groups/aboriginal-and-torres-strait-islander-advisory-group-on-covid-19; Advisory Committee for the COVID-19 Response for People with Disability, available from https://www.health.gov.au/committees-and-groups/advisory-committee-for-the-covid-19-response-for-people-with-disability; Aged Care Advisory Group, available from https://www.health.gov.au/committees-and-groups/aged-care-advisory-group; Culturally and Linguistically Diverse Communities COVID-19 Health Advisory Group, available from https://www.health.gov.au/committees-and-groups/culturally-and-linguistically-diverse-communities-covid-19-health-advisory-group [all accessed 2 May 2022].
102 Including the four sector-specific advisory groups shown in Table 3.7, SITAG and ATAGI.
103 During the course of the audit and prior to 1 July 2022, the administering entity was the Department of Health.
104 Minister for Health and Aged Care, ‘Doorstop interview about new medications added to the PBS for heart disease and ADHD’, media release, Canberra, 31 January 2021.
105 Once a state or territory accepts delivery of COVID-19 vaccines and/or consumables at a designated storage or administration site, further transport is the responsibility of the respective state or territory. This was out of the scope of this audit.
106 Health also used the Royal Flying Doctor Service to deliver vaccines to some remote and very remote communities.
107 Health allocated these deliveries to the other logistics partner during this time. Health advised deliveries to sites in this area were not interrupted.
108 Vaccine manufacturers provided the scheduled number of vaccines to arrive in a three-month period, but not the exact date when vaccines would be shipped. In early 2021, Italy and India did not allow vaccine manufacturers to export all vaccines. This impacted Health’s expected supply of vaccines.
109 States and territories administered vaccines to eligible persons according to the Australian Government’s high-level guidance and state and territory policy decisions. State and territory governments could change the eligibility for vaccines administered through state and territory administration channels. For example, on 17 August 2021, the South Australian Government allowed anyone aged 16 or over to receive any COVID-19 vaccine. At this time, the Australian Government restricted eligibility to priority groups and adults aged 40 or over.
110 These requirements covered the: physical environment and location; infrastructure; workforce requirements; cold chain management; technology and recordkeeping; waste disposal; personal protective and other equipment; accreditation and regulatory requirements; accessibility and cultural safety; management of the clinic; and the ability to store equipment. As at April 2022, compliance with these requirements has relied on self-reporting in line with accreditation requirements and concerns reported by the public. Health plans to start proactive compliance activities in 2022.
111 Between 22 February and 31 December 2021, 27,463 AIR providers administered COVID-19 vaccines. AIR providers can be both individuals, such as GPs, or vaccination sites. Each site could have multiple AIR providers.
112 The first community pharmacies to join the rollout were 49 pharmacies in regional and rural Queensland in June 2021. This was prior to the national on-boarding of pharmacies to the vaccine rollout.
113 Examples cited by stakeholders included ‘heightened confusion, patient anger, and practice turmoil’.
114 Health executed its first work order for residential disability in-reach on 1 April 2021. Prior to this, a small number of visits were conducted using Australian Defence Force medical teams and VAS providers under work orders for the rollout to RACFs.
115 In-reach clinics were also provided for aged care staff.
116 Health chose to use Pfizer over AstraZeneca for this, as it was available earlier than AstraZeneca, and once age-based Pfizer eligibility was introduced, it was able to be used for recipients of all ages. This was particularly relevant for residential disability where most residents are aged under 50 years.
117 Wastage can result from a number of causes including storage at improper temperatures, accidental breakage or destruction of vials or inability to administer all doses once vial is opened.
118 This is based on the WHO’s calculation of vaccine wastage prior to 2019 for multidose vials containing five to 20 doses. In 2019 the WHO introduced a new calculation for vaccine wastage which considered the vaccine type and country of delivery. This was not available for COVID-19 vaccines in planning or early implementation of the vaccine rollout. The three COVID-19 vaccines used in Australia’s vaccine rollout came in multidose vials containing between six and ten doses.
119 This included having each vaccine pallet travel with a temperature monitor, with the provider recording the temperature on acceptance, transport and delivery of vaccines.
120 The vaccination policy states: ‘where a State or Territory has a region or regions where significant COVID-19 community transmission is taking place, additional doses may be allocated to support ring-fencing.’ The Health Promotion Journal of Australia defines ring-fencing as establishing temporary boundaries to restrict the movement of people out of an affected area.
121 The September 2021 ‘COVID Vaccination Allocations Horizons’ document (discussed at paragraph 2.34) outlines a pathway to rebalancing mRNA vaccine allocations to jurisdictional population proportions. As the methodology was based on historical orders rather than historical allocations, cumulative allocations did not precisely rebalance to population proportions (as shown in Figure 4.3).
122 In Phase 1a and 1b of the vaccine rollout, the proportion of the eligible population did not always equal the proportion of the total population. For example, South Australia has 6.9 per cent of the Australian population but has approximately 8.9 per cent of Australia’s residential aged care and residential disability residents.
123 For example, in the early stages of the rollout the smaller jurisdictions (South Australia, Tasmania, Northern Territory, and Australian Capital Territory) received proportionally more mRNA vaccines than their population proportion due to the large size of the Pfizer trays that could not be broken down when frozen. In addition, smaller jurisdictions received a more consistent supply throughout the rollout due to less capacity to rapidly increase their weekly vaccination rate.
124 The Australian Health Protection Principal Committee has not agreed on a national definition of a COVID-19 outbreak. States and territories took different approaches to supress or manage the impact of the virus with different risk tolerance that would have resulted in different thresholds for a declaration of an outbreak. For the purposes of providing Commonwealth support and assistance, Health defined a hotspot as cases that are occurring in a geographic area which has less than 80 per cent vaccination, and/or there is the risk of cases overwhelming the Health system; and a state or territory Chief Health Officer has written to the Commonwealth Chief Medical Officer requesting a Commonwealth hotspot be declared, with an outline of the relevant epidemiology highlighting the need for support.
125 On 8 May 2021, Health declared the Shire of Mornington Peninsula in Victoria as a COVID-19 hotspot.
126 In May 2021, areas in and surrounding greater Sydney began recording increasing cases of COVID-19. On 23 June 2021 Health declared a number of these areas, including the City of Sydney, as COVID-19 hotspots.
127 On 1 August 2021, Health declared six local government areas in South East Queensland as COVID-19 hotspots.
128 On 12 August 2021, Health declared the Australian Capital Territory as a COVID-19 hotspot.
129 On 4 August 2021, NSW requested an additional 500,000 Pfizer doses to respond to the current outbreak.
130 The remaining doses were allocated to the other states and territories as follows: Victoria 175,500, Queensland 136,890, Western Australia 70,200, South Australia 47,970, Tasmania 17,550, Australian Capital Territory 14,040, and Northern Territory 8,190.
131 Minister for Health and Aged Care, ‘Doorstop interview about new medications added to the PBS for heart disease and ADHD’, media release, Canberra, 31 January 2021.
132 The vaccination policy states that this is the underlying principle of ATAGI’s prioritisation advice.
133 The number of people in these occupation groups changed over time as people started or left jobs in the sector.
134 Some state and territory run RACFs identified they would receive vaccines through state and territory channels and were not included in this number.
135 PHNs are independent organisations funded by Health to streamline health services and to coordinate care in health regions.
136 Vaccinating both residents and staff at the same time could create safety issues if the staff had an adverse reaction to the vaccine and were unable to appropriately care for residents.
137 Mandatory reporting obligations for aged care providers were established under the Accountability Principles 2014 which is a legislative instrument of the Aged Care Act 1997. Mandatory vaccination requirements for workers were established under state and territory public health orders.
138 The grant of $80 compensated workers if they were required to travel to an off-site vaccination location and $185 per day if they became unwell following a vaccination.
139 Health defined these as residential disability facilities of two or more people, excluding volunteer carers and disability facilities in hospitals, gaols and short stay or transitional facilities. Unlike the RACF rollout, Health vaccinated disability care residents and staff in the same vaccination clinic. Health advised that this was feasible due to the different staffing arrangements in the comparatively smaller disability facilities where staff generally attend for shorter periods than in RACFs.
140 Health and the Department of Social Services assessed at 1 July 2021 there were 4,946 residential disability sites for NDIS recipients and 1,206 sites for non-NDIS recipients.
141 Some residential disability facilities declined in-reach vaccination clinics. There were a number of reasons for this including that the residents and staff had been vaccinated through other administration channels, such as primary care, or that the residents did not consent to getting vaccinated. Facilities that were offered and declined in-reach were counted as completed.
142 Health did not have access to data on the vaccination rate of people with a disability outside of the NDIS. This is the most accurate data available on this group.
143 A NDIS screened applicant is a person who has made an application for an NDIS worker screening check and who has been verified by an employer as a person who works, or intends to work, in the disability sector. Not all verified NDIS screened applicants are disability support workers. Not all disability support workers are NDIS screened applicants.
144 The majority of residential disability facilities were smaller than residential aged care facilities and had fewer than ten residents.
145 The National Disability Quality and Safeguards Commission is ‘an independent agency established to improve the quality and safety of NDIS supports and services.’ It does not coordinate services outside of the NDIS.
146 Health engaged the same VAS providers for in-reach at residential disability facilities as those for residential aged care facilities.
147 The ANAO could not assess if Health met this the target in the Disability Phase 1a Rollout Plan due to the poor quality of the data provided.
148 Health advised that on 2 November 2021, 96 per cent of residential disability residents had received or been offered in-reach vaccinations. Health advised it later identified the remaining 4 per cent had also been offered in-reach but had not yet reported this.
149 Phase 1a included frontline healthcare and border workers and residents and workers in residential aged care facilities and residential disability facilities. Aboriginal and Torres Strait Islander people who were also in those groups were eligible. Health advised the ANAO that a smaller proportion of Aboriginal and Torres Strait Islander people are employed in those sectors compared to the general public.
150 Aboriginal and Torres Strait Islander people were eligible to access vaccines through other channels including state and territory clinics. The Australian Government provided additional funding to ACCHOs to administer vaccines.
151 The Indigenous and non-Indigenous populations are based on 2016 ABS census data. Regional and remote includes areas classified by the ABS as inner regional, outer regional, remote, and very remote.
152 Indigenous Governance Toolkit, 5.3. Decision making by governing body [Internet], Indigenous Governance Toolkit, available from https://toolkit.aigi.com.au/toolkit/5-3-running-effective-meetings [accessed 28 March 2022].
153 The locations targeted and the activities were developed in consultation with state and territory governments and the National Aboriginal Community Controlled Health Organisation.
154 Health redirected the vaccine administration provider and ACCHO workforce to support these activities where required. Health monitored and reported on its progress improving vaccination rates in these areas weekly.
155 Loud, bright, or otherwise sensory-heavy environments can be inaccessible to people with certain disabilities.
156 Health advised that this group did not receive in-reach vaccinations as it was anticipated they would be able to go to specified central location or medical facilities to receive vaccinations when eligible.
157 There were communication activities targeted to aged-based groups of the vaccine rollout. This was based on both eligibility and vaccination rates.
158 The Multi-Agency Data Integration Project (MADIP) created a data asset managed by the Australian Bureau of Statistics which combines information on health, education, government payments, income and taxation, employment, and population demographics over time. It can provide insights about various population groups in Australia.
159 Measures of cultural and linguistic diversity include whether a person: was born overseas (other than the United Kingdom, Ireland or New Zealand); speaks a language other than English at home; or has low English proficiency.
160 Health uses the Modified Monash Model which it developed to define whether a location is city, rural, remote or very remote. The model measures remoteness and population size on a scale of MM1 to MM7. A community is considered remote or very remote if it is in a MM7 or MM6 area and has a population less than 5,000.
161 Information was published at: https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/numbers-statistics
162 Health advised it used communication as a mechanism to manage emerging whole-of-rollout risks. For example, addressing public concerns through the ‘Top 3’ weekly social media videos and the ‘is it true?’ webpage.
163 Health advised that recognition of broader campaign advertising materials (in contrast to targeted advertising) among CALD communities and Aboriginal and Torres Strait Islander people was similar to that for the Australian public as a whole.
164 Health advised the communications materials targeted to Aboriginal and Torres Strait Islander people were consulted with the advisory group and issues were addressed from the beginning of the rollout.
165 Royal Commission into Violence, Abuse Neglect and Exploitation of People with Disability, Public Hearing Report: Public Hearing 12: The experience of people with disability in the context of the Australian Government’s approach to the COVID-19 vaccine rollout, Attorney-General’s Department, 27 October 2021.