Browse our range of reports and publications including performance and financial statement audit reports, assurance review reports, information reports and annual reports.
Pesticide and Veterinary Medicine Regulatory Reform

Please direct enquiries relating to reports through our contact page.
The objective of the audit was to assess the effectiveness of the Australian Pesticides and Veterinary Medicines Authority’s (APVMA’s) implementation of reforms to agvet regulation and the extent to which the authority has achieved operational efficiencies and reduced the cost burden on regulated entities.
Summary and recommendations
Background
1. Across Australia, over $3 billion of agricultural chemicals, such as insecticides, herbicides and other pesticides, and veterinary medicines (together, agvet chemicals) are sold each year. The sale and use of these chemicals is regulated through a National Registration Scheme, which is established under Commonwealth, state and territory legislation. Under the scheme, the Australian Pesticides and Veterinary Medicines Authority (APVMA or the Authority) is responsible for regulating the supply of agvet chemicals up to the point of retail sale. The key regulatory activities that are undertaken by the APVMA include: assessing and registering agvet products; approving active chemical constituents; issuing permits and licences; monitoring compliance with registration, permit and licence conditions; and investigating suspected non-compliance.
2. In July 2014, a range of legislative reforms came into effect with the aim of improving the efficiency and effectiveness of the APVMA’s regulatory activities. The legislative reforms were wide-ranging and required the Authority to introduce a range of new guidance and assessment procedures, administrative requirements, and timeframes.
Audit objective, scope and criteria
3. The objective of this audit was to assess the effectiveness of APVMA’s implementation of reforms to agvet regulation and the extent to which the authority has achieved operational efficiencies and reduced the cost burden on regulated entities.
4. To form a conclusion against this objective, the ANAO adopted the following high-level criteria:
- Have the regulatory reforms been effectively implemented?
- Are regulatory activities being delivered with greater efficiency and reduced regulatory burden on industry?
- Were sound governance arrangements established to support legislative and business process reform?
Conclusion
5. The Australian Pesticides and Veterinary Medicines Authority’s implementation of agvet chemical legislative reform has been mixed. While key legislative reforms were implemented by the legislated timeframe of July 2014, the full scope of the reform program is yet to be implemented more than four years since the legislative amendments were developed. Further, the Authority is not well placed to determine the extent to which reform objectives have been met in the absence of a robust set of performance measures. There is considerable scope for the APVMA to improve its management of major reform projects, particularly in the context of the Government’s decision to relocate the Authority over the next two years.
6. Projects to support the delivery of key reforms with legislated deadlines—the provision of enhanced guidance to industry, the establishment of pre-application assistance, and the introduction of an online application lodgement system—were prioritised by the APVMA and delivered on time. However, project outcomes required ongoing remediation. Reforms that did not have legislated deadlines for implementation, such as the risk-based regulatory framework and upgrades to internal IT systems to support the achievement of legislative objectives, are yet to be completed. The ongoing assessment of agvet product and chemical applications in the post-reform period has not been supported with fit-for-purpose workflow management systems and a robust quality control framework.
7. The APVMA has not established a robust performance measurement framework to measure the effectiveness of the reform program in achieving greater efficiency of its activities and in reducing the regulatory burden on industry. While performance measures that have been established by the Authority provide insights into the delivery of regulatory activities—for example the timeliness of decision-making—they do not clearly indicate the extent to which reform objectives are being achieved. The limited performance information retained by the APVMA indicates that it has not achieved greater efficiencies in the delivery of its regulatory activities and, overall, the regulatory burden on industry has not been reduced since the reforms were implemented.
8. The APVMA’s governance of the delivery of the reform program was not effective, with significant weaknesses in oversight, planning and risk management arrangements. In particular, the absence of an up to date implementation plan meant that committees established to oversee the implementation of the reform program were not well placed to effectively monitor the progress of projects and hold project managers to account. Further, while implementation risks were considered at an individual project level, they were not aggregated and integrated into an overarching risk management framework. Additionally, engagement with industry in relation to the reform program was undertaken when operational changes were yet to be finalised, which ultimately limited its effectiveness.
Supporting findings
Implementation of the reform program
9. The APVMA implemented key regulatory reforms in accordance with legislated delivery timeframes. The Authority reviewed, reprioritised and re-scoped its reform projects on a number of occasions over the course of implementation to target its efforts towards the minimum legislative requirements of the reform program. Weaknesses in project monitoring, however, meant that the APVMA lacked a clear picture of the extent to which all reform related work had been completed.
10. Key reforms, such as the provision of guidance to industry, pre-application assistance and online application lodgement, did not meet industry or internal business requirements when first implemented. The APVMA has recognised weaknesses in its arrangements for the delivery of these reforms and has undertaken remedial work to improve their effectiveness. The Authority has also revised its governance arrangements for the management of major projects.
11. The APVMA has made progress towards the establishment of a risk-based approach to the delivery of its regulatory activities including the introduction of notifiable variations for administrative registration changes and the establishment of a risk-based prioritisation process for chemical reviews. However the Authority has further work to do as it is yet to implement a risk-based assessment decision framework to target its regulatory activities.
12. While assessment decisions are, in the main, appropriately documented and based on sound evidence, they are not timely—forty per cent of the assessments examined by the ANAO were completed outside the statutory timeframes for decision-making. The establishment of a robust assurance framework for decision-making would better place the Authority to determine whether assessments are being conducted in a consistent manner and in accordance with legislated requirements.
Reform outcomes
13. The APVMA has established a corporate planning framework based on performance strategies, key result areas and performance measures. These measures established by the APVMA cover a broad range of activities but in aggregate do not enable a robust and well-rounded assessment of overall performance over time. The Authority has established appropriate monitoring arrangements in relation to the timeliness of assessments, with improved reported performance in the period 2014–2016, followed by a decline in the six months to March 2017. These fluctuations in the timeliness of assessments have taken place while a backlog of overdue assessments has grown during 2016.
14. The APVMA has not demonstrated greater efficiencies in the delivery of regulatory activities following its implementation of the regulatory reform program. While the Authority has not established a robust means to assess the extent to which efficiencies have been achieved, the ANAO’s analysis of available performance data and industry feedback indicates a decline in efficiency since 2014.
15. Overall, feedback provided by industry stakeholders indicates that the regulatory burden on industry has not reduced following the implementation of regulatory reform, while noting some improvements in relation to individual measures such as pre-application assistance and online lodgement. The absence of specific performance measures to assess regulatory burden makes it more difficult for the APVMA to ascertain changes in regulatory burden arising from the roll-out of reform initiatives.
Governance arrangements
16. The oversight arrangements to monitor the implementation of the reform program were not effective. The APVMA’s governance forums lacked continuity and did not facilitate assurance on the progress of work, ultimately contributing to poor or incomplete implementation outcomes for some projects.
17. The APVMA did not establish an effective planning framework to guide the implementation of the reform program. In the absence of such a framework, the numerous governance committees that were established over the course of reform program implementation lacked an appropriate basis on which to oversee progress and hold project managers to account.
18. The risks to the effective implementation of the reform program were poorly managed by the APVMA. While an appropriate risk management framework is in place, high level business risk reviews were not conducted regularly and risks to the implementation of the reform program were not adequately managed. Further, risk management at the individual project level was inconsistent and treatments for risks in relation to the retention of staff capability have not been effective.
19. Industry stakeholders were engaged during the implementation of the reform program via a range of information and training sessions, but delays in the finalisation of reform projects limited the extent to which specific changes at the operational level were communicated. The feedback provided by industry stakeholders to the ANAO on the APVMA’s engagement approach was mixed.
Recommendations
Recommendation No. 1
Paragraph 2.47
The Australian Pesticides and Veterinary Medicines Authority should implement an internal quality framework to provide an appropriate level of assurance that its assessments are undertaken in a consistent manner and made in accordance with agvet chemical legislation.
Australian Pesticides and Veterinary Medicines Authority's response: Agreed.
Recommendation No. 2
Paragraph 3.28
The Australian Pesticides and Veterinary Medicines Authority should establish and monitor an appropriate set of measures and targets to assess the extent to which it is improving the effectiveness and efficiency of its regulatory activities through its ongoing reform agenda.
Australian Pesticides and Veterinary Medicines Authority's response: Agreed.
Recommendation No. 3
Paragraph 4.14
The Australian Pesticides and Veterinary Medicines Authority should improve its governance of the implementation of major reforms, including the maintenance of an oversight body with clearly defined responsibilities and robust project monitoring arrangements.
Australian Pesticides and Veterinary Medicines Authority's response: Agreed.
Recommendation No. 4
Paragraph 4.38
The Australian Pesticides and Veterinary Medicines Authority should implement a structured and systematic approach to identifying and responding to emerging business risks.
Australian Pesticides and Veterinary Medicines Authority's response: Agreed.
Summary of entity response
20. The APVMA’s summary response to the report is provided below, while its full response is at Appendix 1.
The agency welcomes the audit by the ANAO of the effectiveness of implementation of reforms to agricultural and veterinary (agvet) chemical regulation which came into effect in July 2014. The agency acknowledges the findings and areas for improvement identified in the ANAO Report. The agency notes, however, that for the scale of reform undertaken the implementation timeframes were challenging and resourcing required to fully deliver within these timeframes was limited.
The agency notes the transition from pre-July 2014 to post-July 2014 reform arrangements was achieved without significant disruption to service delivery and involved an ongoing program of business improvement. Having moved through the transition period, the reforms were moving into a more mature phase of implementation in mid-to-late 2016, with 78 per cent of product applications processed within timeframe in the June quarter 2016 and 83 per cent in the September quarter 2016.
The agency accepts all four recommendations with action already taken or underway to implement improvements consistent with the recommendations. This includes improvements in quality assurance processes for application assessment; better documentation of business processes to support consistency; and strengthening risk management, governance and performance monitoring frameworks. The agency is also implementing a major program of business process reform to improve the efficiency of its service delivery.
1. Background
Introduction
1.1 Across Australia, over $3 billion of agricultural chemicals, such as insecticides, herbicides and other pesticides, and veterinary medicines (together, agvet chemicals) are sold each year. The supply and use of these chemicals is regulated through a National Registration Scheme, which is established under Commonwealth and state and territory legislation.1 Under the scheme, the:
- Australian Government’s Australian Pesticides and Veterinary Medicines Authority (APVMA or the Authority) regulates the supply of agvet chemicals up to the point of retail sale;
- states and territories are responsible for controlling the use of agvet chemicals once they are sold; and
- Australian Government Department of Agriculture and Water Resources (Agriculture) is responsible for legislative and policy oversight.
Australian Pesticides and Veterinary Medicines Authority
1.2 The APVMA’s purpose is to ‘evaluate the safety and performance of chemicals intended for sale in Australia to ensure that the health and safety of people, animals, crops and the environment are protected’. The role of the APVMA is governed by a legislative framework (see Table 1.1 on the following page). The key regulatory activities undertaken by the Authority include: assessing and registering agvet products; approving active chemical constituents; issuing permits and licences; monitoring compliance with registration, permit and licence conditions; and investigating suspected non-compliance.
1.3 As at February 2017, the APVMA had registered over 13 000 products and chemicals for over 900 businesses, individuals and organisations. The APVMA receives around 5000 applications each year, with approximately 70 per cent of applications seeking to vary an existing registration or approval, or produce a copy of an existing product. The APVMA has around 200 staff, including around 100 regulatory scientists, 30 legal and compliance staff, 40 corporate staff and 30 executive and administrative staff. The Authority’s revenue in 2016–17 was $36.4 million, with funding primarily obtained through cost recovery arrangements.
Table 1.1: Agvet chemical legislative framework
|
Legislation |
Purpose |
|
Agricultural and Veterinary Chemicals (Administration) Act 1992 |
Establishes the APVMA as an independent statutory authority of the Commonwealth responsible for the regulation and control of agvet chemicals in Australia up to the point of retail sale. |
|
Agricultural and Veterinary Chemicals Code Act 1994 [Agvet Code] |
Enables the APVMA to: evaluate, approve or register and review active constituents and agvet products; issue permitsa; license the manufacture of chemical products; and conduct compliance and enforcement activities with respect to the Agvet Code. |
|
Agricultural and Veterinary Chemicals Act 1994 |
Enables the Agvet Code to have effect and provides that the Agvet Code is to apply as a law of the participating jurisdictions. |
|
Agricultural and Veterinary Chemical Products (Collection of Levy) Act 1994 |
Contains measures that allow for the assessment and collection of levies in regard to agvet products registered for use in Australia. |
|
Agricultural and Veterinary Chemicals (Administration) Regulations 1995 |
Prescribes the fees for export certificates and the form of search warrant to be used when there is a suspected offence in relation to the importation, manufacture or exportation of agvet chemicals. |
|
Agricultural and Veterinary Chemicals Regulations 1999 |
Prescribes functions that enable the Director of Public Prosecutions of the Commonwealth to bring prosecutions and proceedings for offences against the Agvet Code or the Agvet Regulations. |
|
Agricultural and Veterinary Chemicals Code Regulations 1995 |
Prescribes detailed provisions of the Agvet Code. |
|
Agricultural and Veterinary Chemical Products (Collection of Levy) Regulations 1995 |
Prescribes the state laws under which an agvet product is registered under the Collection of Levy Act 1994 and specifies the rate of levy applicable. |
Note a: The APVMA may issue permits for non-commercial minor use, emergency and research purposes.
Source: APVMA.
Legislative reform
1.4 Agvet chemical regulation has been subject to a series of reviews and legislative reforms over recent years, with these reforms designed to improve the effectiveness and efficiency of regulatory activities.2 Following the 2010 federal election, the Government directed Agriculture to consult with the agvet chemical industry on the development of measures to improve the efficiency and effectiveness of regulatory arrangements and provide better protection for human health and the environment. To inform the development of new measures, Agriculture released a discussion paper and met with industry stakeholders.3
1.5 The subsequent report prepared by Agriculture, Better Regulation of Agricultural and Veterinary Chemicals, highlighted a range of issues including:
- the absence of: a clear risk-based regulatory framework; statutory timeframes for the review of registered products and chemicals; and intermediate enforcement measures between the extremes of warning letters and criminal prosecution;
- inefficient preliminary assessment arrangements; and
- delays to the completion of assessments due to applicants providing additional information during the assessment process.
2014 legislative reforms
1.6 The Agricultural and Veterinary Chemicals Legislation Amendment Act 2013 and regulations were designed to address the issues outlined in the department’s review and to ‘encourage the development of newer and safer chemicals by providing more flexible and streamlined regulatory processes with higher levels of transparency and predictability for businesses seeking approval for agvet chemicals to enter the market’. The key reforms that the APVMA was required to implement from 1 July 2014 included:
- new regulatory guidance to industry under the reformed legislative arrangements;
- a structured, upfront pre-application assistance scheme for applicants;
- a system to electronically receive all applications online;
- stricter preliminary assessment arrangements that focus on basic application requirements (restricting the ability of the applicant to rectify a defect in their application during this phase of assessment);
- revised maximum assessment timeframes based on the type of application being made, with increased time for the assessment of certain product and chemical applications;
- additional requirements for the review of registered products and chemicals (such as the development of work plans for each review) and statutory timeframes for completing chemical reviews; and
- procedural, technical and transitional arrangements, including limiting the acceptance of additional material from applicants and introducing requirements to provide notices of certain proposed decisions to applicants.4
1.7 In addition, the new legislation provided two mechanisms for the APVMA to target its resources more effectively. Firstly, by allowing the APVMA to implement a risk-based regulatory framework to guide the conduct of regulatory activities resources can be directed towards areas of high risk. Secondly, the introduction of a range of new enforcement powers supports a more graduated response to non-compliance than was possible previously.
1.8 The APVMA commenced projects to implement the legislation during the development of the legislative reforms. An overview of the timeline for the development and implementation of the 2014 agvet chemical legislative reforms is provided at Figure 1.1.
Figure 1.1: Timeline of the development and implementation of the 2014 agvet chemical legislative reforms
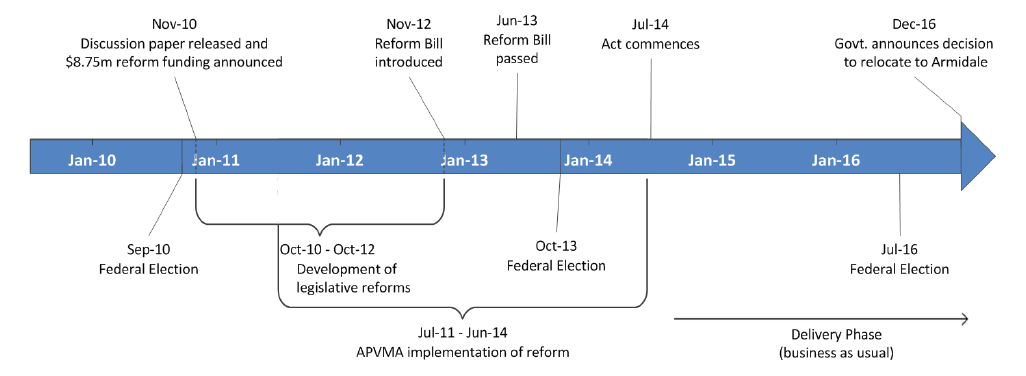
Source: ANAO based on APVMA and departmental information.
Recent developments
Agricultural Competitiveness White Paper
1.9 The Government’s 2015 Agricultural Competitiveness White Paper, which sets out the Australian Government’s roadmap of actions to grow the agriculture sector in Australia, noted that agvet chemical regulation imposes a heavy regulatory burden on industry that is often disproportionate to the risks that products pose. The White Paper also outlined the Government’s intentions for further reform including: limiting pre-market assessments of low and medium risk products; recognising assessments from accredited third party suppliers and trusted chemical regulators; and exploring opportunities to improve post-market compliance and national control of chemical use with co-regulators.5 In 2016, the Government allocated $7.3 million over four years to the APVMA to progress these further reforms.
Relocation of the regulator
1.10 On 23 November 2016, the Minister for Finance made a Government Policy Order under the Public Governance, Performance and Accountability Act 2013 to relocate the APVMA from Canberra to a regional community. The Order is supported by additional funding of $25.6 million to relocate the Authority to Armidale, New South Wales, to be completed in 2019.6
Further reviews
1.11 In October 2014, COAG agreed to consider changes to the regulatory framework governing chemicals to improve efficiency. The Department of Industry and Science, on behalf of COAG engaged consultants in 2015 to review Commonwealth chemicals assessment functions with a focus on complementary regulatory and administrative functions. The different regulatory requirements for chemicals and chemical products administered by the APVMA, the National Industrial Chemicals Notification and Assessment Scheme, the Therapeutic Goods Administration, Food Standards Australia New Zealand, and the Australian Competition and Consumer Commission were identified. The report is yet to be finalised, pending further consideration by COAG.
1.12 In November 2015, the Treasurer requested the Productivity Commission to undertake an inquiry into the regulatory burden imposed on Australian farm businesses focusing on regulation with a material impact on domestic and international competitiveness. The Commission’s report of March 2017 recommended that the APVMA increase its use of international evidence in its assessments.7
1.13 In December 2015, Agriculture commissioned a review of the duplication of effort and unnecessary costs on industry associated with their compliance with agvet chemical legislation and the Work Health and Safety Act 2011.8 The review was completed in August 2016 and made four recommendations including that the APVMA: continue to work with Safe Work Australia9 to assist industry on labelling requirements; consider work health and safety labelling as part of any future changes to agvet labelling requirements; and apply discretion where possible to enable veterinary chemical producers to re-label products at the point of supply.
Upcoming reviews
1.14 The Agricultural and Veterinary Chemicals Legislation Amendment Act requires a review of the agvet regulatory reforms to be completed by July 2019. In addition, the Agricultural and Veterinary Chemicals (Administration) Act 1992 requires a comprehensive review of the suite of agvet legislation—over 20 instruments—to be completed by July 2024.
Audit approach
1.15 The objective of the audit was to assess the effectiveness of APVMA’s implementation of reforms to agvet regulation and the extent to which the authority has achieved operational efficiencies and reduced the cost burden on regulated entities. To form a conclusion against this objective, the ANAO adopted the following high-level criteria:
- Have the regulatory reforms been effectively implemented?
- Are regulatory activities being delivered with greater efficiency and reduced regulatory burden on industry?
- Were sound governance arrangements established to support legislative and business process reform?
1.16 The scope of the audit included an examination of the measures established by the APVMA to give effect to the 2014 reforms and their impact on achieving efficiencies, lowering the burden on industry and improving regulatory outcomes. The audit considered the implementation of reform in the context of the Government’s separate policy of relocating the regulator and the APVMA’s management of business continuity risks associated with the move. The audit did not examine functions that do not directly relate to the objectives of the reforms, such as the collection of fees from industry.
1.17 In conducting the audit, the ANAO examined the APVMA’s internal records, systems and procedures relating to the implementation of reform, including governance reviews, engagement with industry, assessment decision-making and project documentation. The ANAO also interviewed APVMA staff, stakeholders, and co-regulators and conducted an online survey of regulated entities to gain an industry perspective on regulation.10
1.18 The audit was conducted in accordance with ANAO Auditing Standards at a cost to the ANAO of approximately $427 000.
1.19 The team members for this audit were Mark Rodrigues, Alicia Vaughan, Vinesh Abbott, Jillian Blow, Jessica Carroll and Sally Ramsey.
2. Implementation of the reform program
Areas examined
The ANAO examined the Australian Pesticides and Veterinary Medicines Authority’s (APVMA’s or the Authority’s) development of key initiatives to implement legislative reform, including the provision of guidance to industry, changes to business processes and the transparency and timeliness of regulatory decision-making.
Conclusion
Projects to support the delivery of key reforms with legislated deadlines—the provision of enhanced guidance to industry, the establishment of pre-application assistance, and the introduction of an online application lodgement system—were prioritised by the APVMA and delivered on-time. However, project outcomes required ongoing remediation. Reforms that did not have legislated deadlines for implementation, such as the risk-based regulatory framework and upgrades to internal IT systems to support the achievement of legislative objectives, are yet to be completed. The ongoing assessment of agvet product and chemical applications in the post-reform period has not been supported with fit-for-purpose workflow management systems and a robust quality control framework.
Areas for improvement
The ANAO has made one recommendation aimed at strengthening arrangements to support transparent, accountable and consistent decision-making. There is also scope for the APVMA to:
- monitor the outcomes of applications following the use of pre-application assistance to inform in the development of appropriate guidance for industry; and
- develop intelligence collection and analysis arrangements to strengthen the implementation of its graduated compliance and enforcement strategy.
Were key reform requirements identified and implemented on time?
The APVMA implemented key regulatory reforms in accordance with legislated delivery timeframes. The Authority reviewed, reprioritised and re-scoped its reform projects on a number of occasions over the course of implementation to target its efforts towards the minimum legislative requirements of the reform program. Weaknesses in project monitoring, however, meant that the APVMA lacked a clear picture of the extent to which all reform related work had been completed.
2.1 As outlined in Chapter 1, the Department of Agriculture and Water Resources led the process to design the agvet chemical legislative amendments with input from industry and the APVMA. The design process was informed primarily through the development and release of a discussion paper in 2010, with feedback sought from relevant stakeholders.
2.2 The legislative amendments covered a broad range of the APVMA’s regulatory activities. By July 2014, the Authority was to have introduced key requirements including:
- updated guidance for industry;
- a pre-application assistance scheme;
- an online application system;
- stricter preliminary assessment arrangements that focus on basic application requirements (restricting the ability of the applicant to rectify a defect in their application during this phase of assessment);
- revised maximum assessment timeframes;
- work plans for the chemical reviews and new timeframes for those reviews; and
- procedural, technical and transitional requirements with respect to the assessment of applications.
2.3 Other 2014 reforms without a prescribed deadline provided for the APVMA to implement a risk-based regulatory framework and exercise a graduated range of enforcement powers. In anticipation of the resourcing requirements necessary to support the implementation of the reforms and to upgrade relevant IT infrastructure, the Government allocated the APVMA an additional $8.75 million over the period from 2010–11 to 2013–14.11
2.4 The 2014 legislative amendments were introduced into Parliament in November 2012 with a commencement date of July 2013.12 In June 2013, the House of Representatives approved minor amendments to the Bill, including delaying commencement to July 2014. The bill was passed on 28 June 2013.
Prioritisation of reforms
2.5 To prepare for the expected regulatory reforms, the APVMA developed a series of projects in 2011 to be monitored by its executive management committees.13 For the eight key reform related projects planned14, the APVMA has retained limited documentation regarding the progress made during 2012. By July 2013, the projects had been subsumed into five key areas of focus: pre-application assistance; registration assessment; chemical review; re-registration/ re-approval; and compliance. The APVMA identified complementary major projects that were pertinent to the successful delivery of reform across these areas relating to ‘IT transformation’, internal and external regulatory guidance and a leadership development program for senior staff.
2.6 Further revisions to the APVMA’s approach to delivering reform were made in late 2013, with the APVMA identifying reform elements with legislated implementation deadlines and areas where implementation could be delayed. In January 2014, the APVMA considered that ‘core implementation projects’ to support the implementation of the reform program were not on schedule and re-prioritised and re-scoped projects to focus on the minimum requirements that could be achieved within legislated deadlines. In March 2014, the Authority had 13 reform related projects underway. By May, seven streams of work had been identified: pre-application assistance; preliminary assessment; evaluation; application finalisation; permits; reconsideration; and compliance. Table 2.1 outlines the main changes in project coverage over time.
Table 2.1: Iterations of reform project coverage over time
|
Time |
Reform project coverage |
Additional comments |
|
July 2011 |
8 key projects |
Legal Interaction, Expert Advice, Risk Framework, Overseas Assessment, Science Panel, Re-registration, Compliance and Business Improvement |
|
July 2013 |
5 key areas |
Pre-application Assistance, Registration Assessment, Chemical review, Re-registration/Re-approval; and Compliance |
|
November 2013 |
10 key projects |
Regulations Guidelines Content Project, Compliance, Legislative Reform Training Project, Legal Implementation, Pre-Application Assistance, Registration Application Assessment, Reform Communication Implementation, Website Redevelopment, Chemical Review and IT Projects |
|
January 2014 |
13 key projects |
Consultation Project and Permits were added. Legal Implementation was separated into two projects |
|
March 2014 |
13 key projects |
Regulations Guidelines Content Project was considered complete. Case Management was added. |
|
May 2014 |
7 main streams |
Pre-application assistance, preliminary assessment, evaluation, application finalisation, permits, reconsideration, and compliance. |
Source: ANAO analysis based on APVMA documentation.
Implementation status as at July 2014
2.7 The APVMA was unable to provide a complete set of documents relating to the implementation of reform projects. Documentation retained by the APVMA indicates that the Authority had implemented key reforms on time, including the establishment of new assessment procedures, a pre-application assistance service, new regulatory guidance, and online application submission portal. The ANAO’s review of the status of implementation, as at 1 July 2014, is outlined at Table 2.2.
Table 2.2: ANAO analysis of the APVMA’s implementation of reform, as at 1 July 2014
|
Key reforms |
Implementation status at 1 July 2014 |
Evidence supporting the completion of reforms |
|
Stricter preliminary assessment arrangements (new timeframes and ‘shut-the-gate’ provisions) |
Largely complete |
s.6A guideline in place by 1 July 2014. Work instruction for Preliminary Assessment issued 1 July 2014. At June 2015, Decision Maps relating to preliminary assessment remained in development. |
|
Revised maximum assessment timeframes |
Insufficient documentation to verify |
N/A |
|
New chemical review arrangements |
Completed |
New timeframes outlined on website by 1 July 2014. Internal progress monitoring indicates work instructions and work plan templates were updated by 1 July 2014. Stocktake review also noted that work instructions were in place by 1 July 2014. |
|
Pre-application assistance |
Complete |
Internal progress monitoring. Work instruction issued 1 July 2014. |
|
Online application submission portal |
Largely complete |
Post implementation remediation and monitoring. |
|
Regulatory guidance |
Complete |
Internal monitoring indicates new website (including s.6A guidelines) launched by 1 July 2014. |
|
Procedural, technical and transitional requirements |
Insufficient documentation to verify |
Internal monitoring noted 93 per cent completion of Registration Application Assessment project at 7 May 2014 (including work to support implementation such as staff training). s.6A guidelines for re-categorising applications, altering applications and issuing section 159 notices in place by 1 July 2014. Legislative instrument detailing Application Requirements issued 26 June 2014. |
Source: ANAO analysis based on APVMA documentation.
2.8 The limited monitoring of the status of reform projects that had been undertaken by the APVMA in early July 2014 indicates that, while key reforms with a legislated deadline had been implemented, the APVMA did not have a clear picture of the extent to which all reform related work had been implemented.
2.9 In October 2014, the Authority commenced a ‘reform legislation implementation stocktake’ to identify incomplete work and any matters additional to what was required under the new legislative requirements. The stocktake, which was finalised in June 2015, noted the significant work undertaken to implement the key reforms, although some reform related work was not completed on time. These outstanding areas included: updating internal work instructions, procedures and decision maps; and internal IT systems to support reform. The stocktake also identified a lack of documentation to substantiate progress against some reform projects.
Reform activities post July 2014
2.10 As at February 2017, the APVMA had ongoing projects related to the 2014 reforms, including the Lower Regulatory Approaches to Registration Project (continuing from the previous Risk-based Assessment Framework Project), and an Internal IT Portal Upgrade Project. These projects are discussed further in paragraphs 2.22 and 2.28–2.29.
Were key reforms effectively delivered?
Key reforms, such as the provision of guidance to industry, pre-application assistance and online application lodgement, did not meet industry or internal business requirements when first implemented. The APVMA has recognised weaknesses in its arrangements for the delivery of these reforms and has undertaken remedial work to improve their effectiveness. The Authority has also revised its governance arrangements for the management of major projects.
2.11 As outlined earlier, the APMVA was required to implement key reforms by 1 July 2014. For these key reforms, the ANAO reviewed the APVMA’s implementation of new regulatory guidance to industry, a pre-application assistance service, and online application submission tools.
Industry guidance
2.12 To support the implementation of the broader reform program, the APVMA commenced a Regulatory Guidelines Project to replace the previous Manual of Requirements and Guidelines in 2013. The aim of the project was to redevelop website content to provide comprehensive information to industry stakeholders about the registration of products, the approval of chemicals, and management of applications. Draft guidelines were released in January 2014 for public consultation, with the revised guidelines released on the APVMA’s website on 1 July 2014.15
2.13 Further, formal guidelines have been released under s.6A of the Agvet Code which outline, for APVMA officers and industry stakeholders, the principles and processes for the APVMA’s functions and powers under the Agvet Code and the Agvet Code Regulations. As at 1 July 2014, the Authority had developed 13 s.6A guidelines, covering topics such as ‘Preliminary Assessment’ and ‘Altering Applications’.
2.14 In relation to the quality of the guidance material prepared, the APVMA’s 2015 Stocktake noted staff concerns about missing content, errors and the lack of a user friendly structure. The review also found that there were a ‘number of sections of the legislation for which no reference [could] be found on the website’ and a need to update some of the content.16 It is unclear if these issues had been addressed because the Stocktake did not identify which sections of the legislation were absent from the website and the APVMA has not retained documents to demonstrate rectification of these issues.
2.15 The responses to the ANAO’s survey of regulated entities indicated mixed views on the quality of APVMA’s guidance materials. While around one third of respondents considered that the APVMA’s website was easy to navigate and that its guidance to industry was clear, around 40 per cent considered that guidance was unclear. Respondents’ views on the range of coverage of the APVMA’s guidance was more positive, with around 42 per cent considering that the APVMA’s written advice covered a sufficient range of topics (a further 30 per cent not expressing a strong view on the matter).
2.16 The APVMA has continued to develop its guidance for industry into 2017. In December 2016, the APVMA developed a project plan for ‘Transforming the User Experience’, following a website usability review. Key deliverables for the project included improved search and web functionality and tailored guidance to support the ‘top 20’ most common application types.
Pre-application assistance
2.17 From 1 July 2014, potential applicants could obtain assistance from the APVMA to prepare their applications under the Pre-application Assistance (PAA) Scheme. The APVMA reviewed the service in October 2014, following concerns raised by industry stakeholders. The review found that: the PAA had not delivered the service and benefits as intended by the reforms; and the Authority had failed to meet the reasonable expectations of industry. The review also found that the scheme lacked: clarity about the objective; a consolidated statement of internal roles and responsibilities; reporting and monitoring; sound business workflow processes; and a robust quality assurance mechanism.
2.18 The 40 recommendations made by the review were generally addressed by key changes introduced by the APVMA in November 2015, when the Authority:
- introduced of a tiered system to manage different types of requests for assistance;
- offered applicants the option to request face-to-face meetings and the appraisal of trial protocols (which was previously available only through technical assessments); and
- simplified the fee structures.
2.19 The ANAO reviewed APVMA data to determine the extent to which applications for pre-application assistance were finalised within the Authority’s target timeframes. The ANAO’s analysis indicated that of the 146 applications received from November 2015 and finalised by December 2016, 96 applications (66 per cent) were not finalised within established PAA timeframes, and were on average, 43 days overdue. The APVMA has not analysed PAA data to identify the outcomes of applications submitted following use of the scheme. There would be benefit in the APVMA monitoring the progress of applications following applicants’ use of the PAA to better measure the extent to which it is achieving its objective.
2.20 Of the survey responses received by the ANAO, 25 per cent considered that pre-application assistance had significantly or somewhat reduced time or costs for their organisation in 2014, while 29 per cent considered that there was no impact.
Online application lodgement
2.21 From 1 July 2014, applications could be submitted online. However, there were extensive weaknesses in the APVMA’s management of the project. Specifically, core IT project management practices such as technical and user acceptance testing was not undertaken prior to releasing new applications into production. In addition, key project documentation was either not prepared or was incomplete.17
2.22 The APVMA has recognised that there are weaknesses in its governance of ICT projects. To address these weaknesses, the Authority has established new governance arrangements for ICT projects in September 2016 and developed key supporting ICT planning documents such as a Project Management Framework (June 2016), a Project Management Plan (in draft at November 2016), and a new ICT Strategic Plan (October 2016). The most recent internal portal project deliverable, the Permits Module, was completed by the APVMA in May 2016. The module was delivered to scope and on budget.
2.23 As noted in paragraphs 2.37–2.41, the APVMA’s assessment of applications is poorly supported by internal workflow management systems. Current systems in place do not enable the direct transfer of records and data between assessment staff and the tracking of assessment progress is fragmented.
Industry views
2.24 The ANAO received generally positive feedback from stakeholders in relation to the online application lodgement capability. Responses to the ANAO’s survey indicated that the new functionality had significantly or somewhat reduced time or costs for 40 per cent of participating organisations. There were, however, 19 per cent of respondents that reported that the new functionality had somewhat or significantly increased time or costs for their organisation.
Are regulatory activities graduated in proportion to risk?
The APVMA has made progress towards the establishment of a risk-based approach to the delivery of its regulatory activities including the introduction of notifiable variations for administrative registration changes and the establishment of a risk-based prioritisation process for chemical reviews. However the Authority has further work to do as it is yet to implement a risk-based assessment decision framework to target its regulatory activities.
2.25 The agvet chemical legislative amendments also provided for the APVMA to implement a risk-based regulatory framework, new arrangements for chemical review and exercise a graduated range of new enforcement powers. The ANAO assessed the extent to which these reforms have contributed to a more graduated regulatory approach in proportion to risk.
Implementation of risk-based approach to regulation
2.26 The APVMA’s work towards implementing a risk-based approach to regulation has progressed slowly over a number of years. A 2011 legislative reform plan produced by the Authority included the development of a ‘fully documented and published Risk Analysis Framework’ to ‘improve transparency of APVMA operations, facilitate the submission of quality applications, enhance stakeholder and community confidence and drive alignment of regulatory effort with the degree of risk’.
2.27 In 2013 the APVMA commissioned a review of the application of risk proportionate regulatory processes for chemicals regulated in Australia including the Therapeutic Goods Administration and Food Standards Australia New Zealand. The review identified options for reducing regulatory burden on industry through the application of risk-based principles. In 2014, the APVMA commissioned the Centre of Excellence for Biosecurity Risk Analysis (CEBRA)18, to develop a conceptual framework underpinning a risk-based assessment decision framework. The resulting report outlined the development and testing of a screening level risk assessment tool for agvet chemicals. The APVMA intended to use the report to develop new risk-based measures and implement ‘low hanging fruit’ by July 2016, however no significant regulatory changes directly arose from the CEBRA engagement.
2.28 To date, a key work to implement a risk based regulatory approach by the APVMA has been through the ‘Lower Regulatory Approaches to Registration’ (LRAR) project in 2015. Key outcomes of this project have been:
- the removal of assessments for five types of variations (for example, changing the manufacturer’s address or storage and disposal instructions)19; and
- streamlined processes (‘fast-tracking’) for low risk applications, such as repack registrations, in prescribed circumstances.
2.29 The LRAR project also aims to: expand fast-track assessments to other application types; develop and publish risk assessment manuals; and develop, in consultation with Agriculture, future legislative reforms to the prescribed application categories under the agvet Code. In addition to the LRAR Project, the APVMA is undertaking two joint projects with Agriculture to improve farmers’ access to chemicals.20 Collectively these projects aim to improve access to chemical products for minor use by streamlining registration requirements and reducing the regulatory effort.
Chemical Review (Reconsideration)
2.30 As part of the 2014 reforms21, the APVMA implemented a documented risk-based approach to the reconsideration of products and chemicals that had been registered. In November 2014, the APVMA held a two-day chemical review prioritisation workshop with Australian, state and territory government co-regulators and compiled a list of 19 chemicals or types of chemicals for review, reduced from the previous number of 39. The remaining 20 chemicals were either no longer considered to require review, or concerns were to be managed using alternate agvet regulatory processes. The APVMA subsequently consulted the public, industry and government entities to prioritise these chemicals, which included an invitation for submissions from April to June 2015. Following consultation with relevant stakeholders, five chemicals were prioritised for detailed scoping and the remainder were scheduled for re-evaluation by December 2016.22
Compliance and enforcement activities
2.31 As noted in Chapter 1, the APVMA is responsible for regulating agvet chemicals up to and including the point of sale. This responsibility involves monitoring compliance with agvet legislation to identify the supply of unregistered products, the use of unapproved labels, unfounded advertising claims, and chemicals that do not conform to standards. The APVMA’s activities to identify non-compliance include post-border inspections, audits of manufacturers and labels, and presumptive chemical testing. The 2014 reforms provided the Authority with an expanded range of compliance and enforcement powers, including penalty infringement notices, enforceable undertakings, substantiation notices, suspensions/cancellations of approval/registration and monitoring and investigation warrants, in addition to letters of warning and criminal prosecution.
2.32 To facilitate the use of its new enforcement powers, the APVMA developed a Compliance and Enforcement Strategy for 2015–17 and a Compliance Plan for 2016–17, which outline a range of graduated education, engagement, compliance and enforcement activities. The Strategy provides for a risk-based approach to compliance, including the use of intelligence analysis to underpin its broader compliance activities, and the establishment of formal arrangements to undertake joint investigations and information sharing with partner agencies, such as states and territories, which are responsible for regulating the use, transport and storage of agvet chemicals.
2.33 However, the APVMA’s compliance and enforcement activities are not supported by a fit-for-purpose investigation case management system and procedures for the collection and use of compliance intelligence. Draft work instructions and intelligence reporting and referral templates were developed in 2014 and 2015 but were not operationalised. Intelligence sharing arrangements with other jurisdictions are also yet to be formalised. As a result, the APVMA’s 230 investigations per year have been largely reactive, in response to reports of non-compliance submitted by the public on its website. The current lack of formal procedures and appropriate systems has the potential to undermine the effectiveness of the APVMA’s compliance program.23
2.34 Notwithstanding these potential limitations to its compliance activities, the APVMA has exercised its expanded enforcement powers since July 2014. In 2015–16, the Authority issued one statutory notice, six formal warnings, two infringement notices (totalling $27 000) and one investigative warrant. The development of appropriate intelligence collection and analysis arrangements would better place the APVMA to implement its graduated compliance and enforcement strategy.
Industry views
2.35 Of the regulated entity responses to the ANAO’s survey, 38 per cent agreed or strongly agreed with the statement: ‘Within its legislative constraints, APVMA’s regulatory effort is generally targeted towards the most important issues’. Of the remaining responses, 2 per cent did not agree with this statement and 30 per cent did not express a strong view on the matter.
Are assessment decisions appropriately documented, evidence-based and timely?
While assessment decisions are, in the main, appropriately documented and based on sound evidence, they are not timely—forty per cent of the assessments examined by the ANAO were completed outside the statutory timeframes for decision-making. The establishment of a robust assurance framework for decision-making would better place the Authority to determine whether assessments are being conducted in a consistent manner and in accordance with legislated requirements.
2.36 Applications for the registration of agvet chemicals are managed by the APVMA’s Case Management and Administration Unit, with all applications to undergo preliminary assessment to determine if basic documentary requirements have been met. The assessment process includes the production of technical reports that may cover toxicology, workplace health and safety, residues, trade, efficacy (crop or host animal safety), environment and chemistry and manufacture.24 The APVMA may determine whether further information from the applicant or a re-categorisation of the application is required at any stage over the assessment process.
Documentation of decision-making
2.37 The retention of appropriate documentation to evidence the decision-making process supports accountability and positions regulators to explain and defend their decisions. The APVMA has recorded assessment outcomes against core application requirements25, but the approval of final assessment decisions was not recorded consistently. Of the 100 major assessments sampled by the ANAO, seven assessments were identified where the decision was not recorded on the decision document (alternative records had been retained to evidence the delegate’s approval). To provide greater assurance regarding delegate decision-making, there would be merit in the APVMA ensuring that delegate decisions are recorded on each decision document.
Assessment delays
2.38 The processing of applications is shared across a number of areas of APVMA including Case Management and Administration Unit, risk managers, delegates and scientific assessment areas. The Authority has not, however, assigned responsibility for end-to-end timeframe management.
2.39 The statutory period in which the APVMA is required to complete assessments ranges from two months to 25 months depending on the application type. Of the 100 assessments reviewed by the ANAO, 60 applications were found to have been completed on time, with 40 overdue—ranging from two weeks to 10 months. While the reasons for overdue assessments varied across the application types and assessment components, more common reasons included:
- delays in receiving and reviewing technical assessments;
- misplaced application material (including, in one case, confidential commercial information26);
- staff absences27; and
- delays in recognising the payments from applicants.28
Workflow management
2.40 The APVMA use a number of automated and manual systems to manage applications, including its internal portal, which is currently subject to a major redevelopment. In general, the assessment of applications is poorly supported by existing systems. In particular, the existing portal does not include sufficient information on the progress of assessments to support effective monitoring. For example, information on the status of technical reviews and whether additional information from the applicant is outstanding is not retained. To confirm the status of applications and to track the progress of assessments, assessors are required to review standalone spreadsheets.
2.41 There are also functional limitations relating to the operation of the external portal. For example, the external portal does not provide sufficient information to applicants to enable them to track the progress of their applications. When contacted, the APVMA responds to applicant queries with general information on the assessment, but does not provide an estimate of the likely completion timeframe or explain those factors that impact on the assessment timeline. As a consequence, applicants are not provided with clarity surrounding expected assessment completion dates, which ultimately makes it more difficult for them to schedule further work to prepare for the release of relevant products.
Requests for further information and re-categorisations
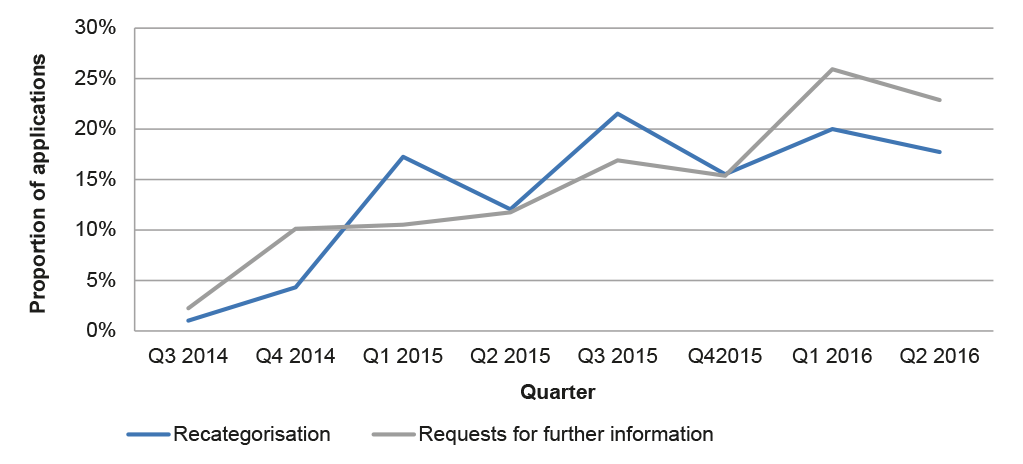
2.42 Almost half of the 100 applications examined by the ANAO were subject to a request for further information (27) or re-categorisation (22).29 Data on all major applications submitted after July 2014 indicates similar rates of requests for further information and re-categorisation, and an increasing trend over time (Figure 2.1).
Figure 2.1: Requests for further information and re-categorisation, 2014–2016
Source: ANAO analysis of APVMA information.
2.43 The increasing proportion of requests for further information and re-categorisations suggests that existing guidance for applicants relating to the application requirements may need to be reviewed. The introduction of additional pre-application guidance to applicants as part of the reform program was designed to better place applicants to submit complete and accurate applications. Two applications in the sample examined by the ANAO involved the use of Pre-application assistance under the pre-November 2015 arrangements (outlined in 2.18). Of these, one exceeded the statutory decision-making timeframe and one was on time but subject to a request for further information. As noted at paragraph 2.19, there would be merit in the APVMA monitoring the rate of requests for further information and re-categorisations to better target its guidance to industry.
Quality control and review
2.44 The APVMA informed the ANAO that some assessment areas within the Authority undertake peer review of technical assessment decisions, although quality review processes are not integrated across assessment streams and documented. The absence of a fit-for-purpose internal quality framework limits the APVMA’s ability to provide assurance that assessments are undertaken in accordance with legislative requirements and are appropriately evidenced. The risk of divergence from legislative requirements is heightened in the context of poor workflow management support systems and lack of staff clarity in the processing of applications.
2.45 In 2014–15 the APVMA established an internal Registration Quality Committee to provide ‘advice on APVMA frameworks to foster excellence in decision making; overseeing quality assurance and the administration of decision making on registrations to ensure that decisions are consistent, timely, transparent and predictable’. In 2016 the Committee considered a project to document the elements of good regulatory decision making, undertake a gap analysis against existing capability and develop a quality assurance framework. This ‘Quality Decision Making’ Project commenced in July 2016, but was suspended in December 2016.30
2.46 The implementation of a quality framework would assist the APVMA to detect and address any inconsistent practices among assessment staff as well as demonstrate the appropriate application of standards and procedures to support rigorous regulatory decision-making.
Recommendation No.1
2.47 The Australian Pesticides and Veterinary Medicines Authority should implement an internal quality framework to provide an appropriate level of assurance that its assessments are undertaken in a consistent manner and made in accordance with agvet chemical legislation.
Australian Pesticides and Veterinary Medicines Authority’s response: Agreed.
2.48 The APVMA agrees the quality assurance framework for agvet chemical assessments can be improved. The APVMA believes the current processes for assessment of agvet chemicals are robust, with appropriate documentation and based on sound evidence, as acknowledged in the ANAO report. This provides for high quality scientific decision making for registration of agvet chemicals in line with the legislative framework.
2.49 Internal governance committees for registration management and science quality are operational within the agency to provide assurance that regulatory decision making is in line with legislative requirements, fit-for-purpose and consistent. The terms of reference for these committees will be reviewed to ensure they reflect action to implement the recommendation.
2.50 The APVMA will support the work of the committees through a program of better documentation of assessment frameworks, targeted training for assessment staff, and business process and IT improvements to standardise application processes as much as possible and improve consistency.
2.51 The APVMA notes the ANAO’s suggestion regarding analysis of pre-application assistance outcomes with a view to developing appropriate industry guidance. The agency agrees improved guidance for industry continues to be an area for improvement and has commenced a process in consultation with industry to develop better guidance material for high volume applications.
2.52 The APVMA notes the ANAO’s suggestion to develop intelligence collection and analysis arrangements to strengthen its compliance and enforcement strategy and will include this suggestion in future strategies.
3. Reform outcomes
Areas examined
The ANAO examined the Australian Pesticides and Veterinary Medicines Authority’s (APVMA’s or the Authority’s) development and use of performance monitoring strategies, and the extent to which it has improved its efficiency and lowered the regulatory burden on industry since the legislative reforms came into effect in July 2014.
Conclusion
The APVMA has not established a robust performance measurement framework to measure the effectiveness of the reform program in achieving greater efficiency of its activities and in reducing the regulatory burden on industry. While performance measures that have been established by the Authority provide insights into the delivery of regulatory activities—for example the timeliness of decision-making—they do not clearly indicate the extent to which reform objectives are being achieved. The limited performance information retained by the APVMA indicates that it has not achieved greater efficiencies in the delivery of its regulatory activities and, overall, the regulatory burden on industry has not been reduced since the reforms were implemented.
Area for improvement
The ANAO has made one recommendation aimed at improving the benchmarks and targets used by the APVMA to measure the effectiveness and efficiency of its regulatory activities.
Was a robust performance measurement framework established to assess effectiveness and efficiency of regulatory activities?
The APVMA has established a corporate planning framework based on performance strategies, key result areas and performance measures. These measures established by the APVMA cover a broad range of activities but in aggregate do not enable a robust and well-rounded assessment of overall performance over time. The Authority has established appropriate monitoring arrangements in relation to the timeliness of assessments, with improved reported performance in the period 2014–2016, followed by a decline in the six months to March 2017. These fluctuations in the timeliness of assessments have taken place while a backlog of overdue assessments has grown during 2016.
3.1 The APVMA’s performance monitoring arrangements are based on indicators established within its Corporate Plan, Operational Plan, and as outlined in the Regulator Performance Framework.31 The Authority’s corporate planning framework is outlined in Figure 3.1.
Figure 3.1: APVMA’s corporate planning framework
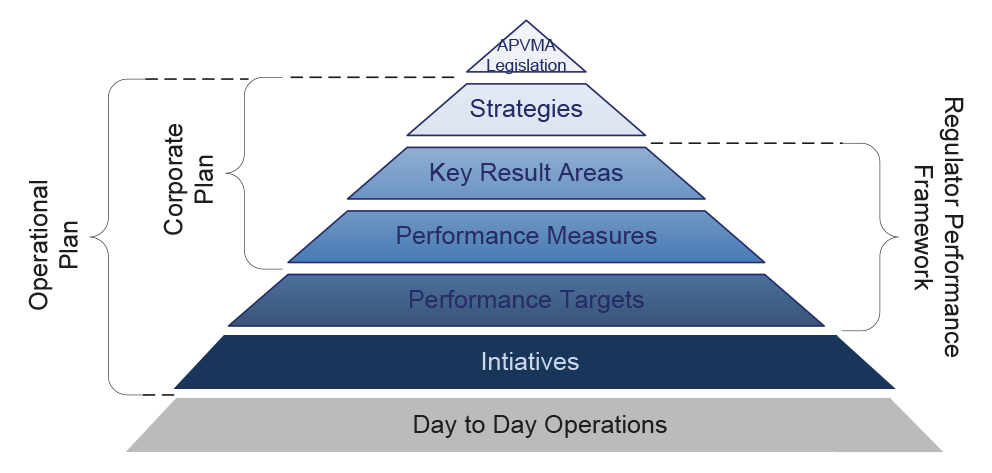
Source: ANAO analysis of APVMA information.
Corporate plan
3.2 The APVMA’s Corporate Plan 2015–20 included the following four key strategies:
- deliver regulatory decisions that are timely, science-based and proportionate to the risks being managed;
- reduce the burden on industry in complying with regulatory requirements;
- build a client focused approach to service delivery, committed to continuous improvement; and
- operate as a contemporary, high performing and efficient organisation.
3.3 These strategies are linked to nine key result areas and 22 performance measures.
Operational Plan
3.4 The APVMA is required to produce an operational plan each year in accordance with s.55 of the Agricultural and Veterinary Chemicals (Administration) Act 1992. The Operational Plan 2015–16 links the strategies, key result areas and performance measures outlined in the Corporate Plan 2015–20 with 70 performance measures and 77 initiatives.32
3.5 Individual performance measures should be relevant and reliable, indicating the desired level of achievement (target) and an expected timeframe. Collectively, performance measures should enable an overall assessment of the extent to which a program or function is achieving its established objectives. While the APVMA’s performance measures cover a broad range of its activities, in aggregate they do not enable a robust and well-rounded assessment of overall performance over time. The ANAO’s assessment of the APVMA’s performance measures is outlined in Table 3.1.
Table 3.1: The ANAO’s review of the APVMA’s performance measures
|
Characteristic |
Assessment |
|
Relevant |
|
|
Reliable |
|
|
Complete |
|
Source: Examples selected extracted from the APVMA information.
Monitoring and reporting
3.6 The APVMA’s Executive Leadership Team (ELT) is responsible for reviewing performance reporting against its operational plans. However, the ELT’s review of performance reports has been ad hoc and generally focused on the second half of each financial year. The operational plan reports that have been provided to the ELT listed each initiative/activity, including the responsible director, milestones, progress indicators and comments on work planned, in progress or undertaken. In late 2015, the APVMA revised its performance reporting processes. Under this process, additional reports against the Operational Plan initiatives that are classified under ‘major projects’ are provided to the Major Projects Board, which was established in October 2015.33
3.7 The APVMA’s performance against its corporate and operational plan measures was reported in its 2015–16 Annual Report. Examples of the APVMA’s reporting against its performance targets are outlined in Table 3.2.
Table 3.2: Examples of the APVMA’s reporting against its performance measures
|
Performance target |
Result reported by the APVMA |
|
Timeframe performance met for 100 per cent product registration |
66 per cent. |
|
Four stakeholder forums held each year to discuss issues affecting regulated entities |
The APVMA held more than four stakeholder forums and meetings with key industry associations throughout 2015–16 to discuss operational and other matters affecting agvet chemical regulation. |
|
Feedback from stakeholders about the quality of guidance material |
There is a feedback mechanism on every web page containing guidance material. All feedback is assessed and referred to business owners for action. A useability review will be completed in 2016–17 and recommendations will inform further improvement to guidance material. |
|
Documented compliance and enforcement strategy, including options for graduated compliance, in place |
The compliance and enforcement strategy, which is underpinned by a risk-based approach is available under ‘Corporate documents’ (apvma.gov.au/node/11026). |
|
Number of corporate training days per full-time equivalent (FTE) |
During the 2015–16 financial year, staff participation in training averaged 5.1 training days per FTE. |
Source: Extracted from the APVMA information.
Timeliness of assessment decision-making
3.8 Timeliness of assessment decision-making is a primary performance metric for the APVMA and of particular interest to industry stakeholders. Delays in the completion of assessments can add to the time required by industry to introduce new and varied products to market and can increase the risk of certain products being unavailable for a cropping season. To help improve timeframe predictability for industry, the 2014 reforms provided new statutory timeframes for assessment decision-making based on a revised methodology. Since July 2014, timeframe performance for new assessments is based on ‘elapsed time’ from lodgement to finalisation, rather than the previous ‘clock time’ methodology, which excluded non-assessment time during which the APVMA waited for applicants to provide additional material.34
3.9 The APVMA’s arrangements for monitoring the timeliness of assessments has gained maturity since 2014 and is now well established, with the publication of quarterly performance reports on its website. The ANAO analysis of APVMA data indicates that, across all applications, the APVMA finalised applications on time in 79 per cent of cases in 2014–15 and 68 per cent of cases in 2015–16. Under the previous ‘clock time’ methodology, prior to the implementation of the reform program, the APVMA reported an overall timeframe performance of 90 per cent of applications finalised within statutory timeframes in 2013–14, up from 89 per cent the previous year.
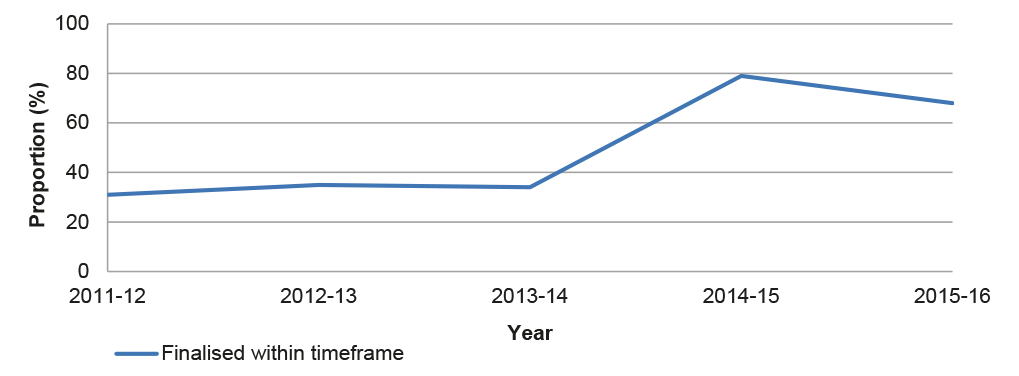
3.10 The ANAO re-calculated the APVMA’s timeframe performance for the three years prior to July 2014 based on the current elapsed time methodology, illustrated in Figure 3.2. In the pre-reform period the proportion of applications finalised within the elapsed timeframe ranged between 31–35 per cent, and rose to 79 per cent in the first year of the post-reform period.
Figure 3.2: Timeliness of assessments based on elapsed time, 2011–2016
Note: The methodology adopted by the ANAO includes all applications from the period of the commencement of assessment to the finalisation of an application (date of regulatory decision) and is consistent with the methodology adopted by the APVMA’s published application duration statistics produced by its internal auditors, available at: <http://apvma.gov.au/sites/default/files/publication/20246-apvma-application-duration-statistics_oakton.pdf>, [accessed 22 March 2017].
Source: ANAO analysis of APVMA information.
3.11 However, the APVMA’s timeframe performance in the post-reform period has been influenced by fewer submissions received for application categories with a timeframe greater than six months, and fewer applications overall. The number of applications submitted for assessment in the post-reform period has declined, from an average of 260 per month prior to July 2014 to 201 per month after, as illustrated in Figure 3.3.
Figure 3.3: Total applications submitted per month, July 2012 to January 2017
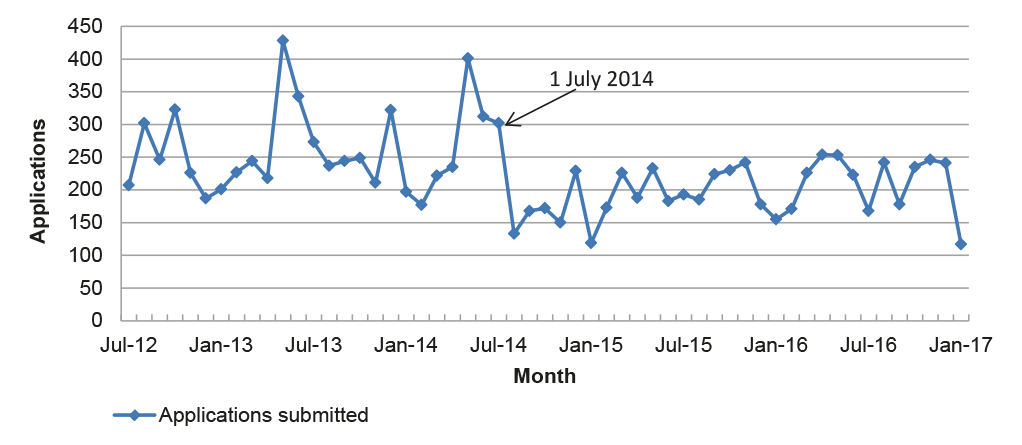
Note: Excludes notifiable variations and pre-application assistance.
Source: ANAO analysis of APVMA information.
3.12 Against the backdrop of fewer applications for assessment and improved timeliness in the post-reform period, the number of overdue assessments at the end of each month has fluctuated between 123 and 213 until late 2016 when there was a rapid increase to 344 at the end of January 2017, as illustrated in Figure 3.4. This suggests an accumulation of overdue assessments that are yet to be finalised at the end of each month.35
Figure 3.4: Overdue assessments by month, July 2014 to January 2017
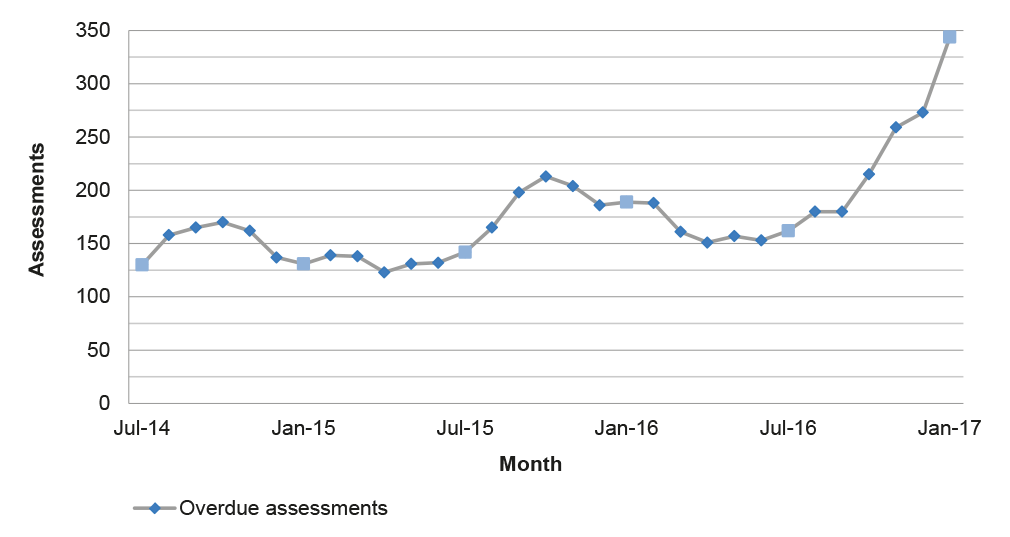
Note: Includes products, chemicals and transitional applications submitted to the APVMA from 1 July 2012. Overdue assessments were calculated using the ‘clock time’ methodology between 1 July 2012 and 30 June 2014 and the elapsed time methodology after 1 July 2014.
Source: ANAO analysis of APVMA information.
3.13 The APVMA’s reported timeframe performance in the six months to March 2017 indicates a decline in timeliness from 83 to 42 per cent of product applications and 77 to 62 per cent of all applications (products, chemicals and permits).
3.14 Overall, data trends on the timeliness of assessments suggest an initial improvement in performance compared with the pre-reform period. This has been followed by fluctuations in the level of assessments completed on time and a recent decline, in the context of fewer applications to be assessed and an increasing backlog of overdue assessments.
Self-assessment against the Regulator Performance Framework
3.15 The APVMA has also reviewed its performance under the Australian Government’s Regulator Performance Framework. In its first self-assessment of performance under the framework, covering the period 2015–16, the Authority outlined activities undertaken in relation to the framework’s six indicators, and the results achieved.36 The APVMA is yet to externally validate its self-assessment through stakeholder input, as required by the framework, prior to publication.
3.16 The APVMA’s reported progress towards effective and efficient regulation included regular interactions with industry and improved performance against timeframes. The report focussed on examples of success and did not clearly identify areas for improvement against targets or provide a reliable indication of the extent of overall improvement over time.
Are regulatory activities being delivered more efficiently?
The APVMA has not demonstrated greater efficiencies in the delivery of regulatory activities following its implementation of the regulatory reform program. While the Authority has not established a robust means to assess the extent to which efficiencies have been achieved, the ANAO’s analysis of available performance data and industry feedback indicates a decline in efficiency since 2014.
3.17 As outlined earlier, the APVMA has not established a robust performance measurement framework that, among other things, would assist the Authority to determine the extent to which efficiencies in its delivery of regulatory activities are being achieved. Such a framework could include performance measures with reference to resourcing levels and outputs over time, the ratio of corporate expenditure to expenditure on regulatory activities or benchmarking with similar regulatory bodies.
Assessment completion and resourcing
3.18 In the absence of a robust performance measurement framework to assess the impact of the 2014 reforms, the ANAO compared actual assessment completion durations (outputs) with assessment fees charged to industry (inputs) as a proxy measure of efficiency over time. The assessment period in which the APVMA is required to finalise an application varies depending on the complexity of the application. According to the Australian Government Cost Recovery Guidelines 2014, application fees should be set so that revenue (fees) generated from the assessment is equal to the expenses (resource time cost) incurred in undertaking the assessment. The APVMA’s fee structure generally correlates less complex assessments with shorter timeframes and lower fees.
3.19 To derive a proxy efficiency index, the ANAO divided the APVMA’s total actual assessment times by the total fees for those assessments over six month periods (elapsed time per application dollar). The lower the time per dollar, the greater the efficiency being achieved. In the six months to June 2012, the APVMA finalised 1224 assessments that had taken a total time of 3828 months to complete and which attracted fees of $2 858 429, equating to about 59 elapsed minutes per application dollar. The index of elapsed minutes per application dollar then fluctuated between 39–50 over the pre- and post-reform period until the end of 2014, when the index doubled from 39 to 80 over the following 12 months, prior to a decline to 60. This suggests an overall decrease in efficiency in the post-reform period compared with the pre-reform period, as illustrated in Figure 3.5.
Figure 3.5: Elapsed time per application dollar
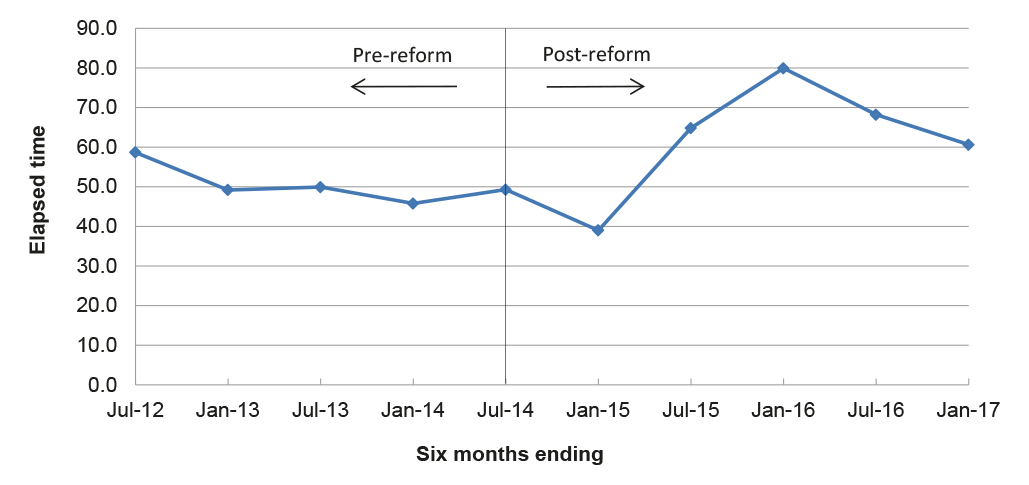
Note: The analysis includes products, chemicals and transitional applications, based on the actual application fees applied where cost data was available, and an average item fee for modular assessments as a proxy indicator of fees in the absence of APVMA data on the actual fees applied to these applications. Of the 11 862 completed assessments included in the analysis, the proxy average item fee was applied to 87 modular assessments in the post-reform period and 1550 in the pre-reform period. The analysis excludes withdrawn applications, pre-application assistance and notifiable variations.
Source: ANAO analysis of the APVMA information.
3.20 On the basis of the limited data available, the elapsed time for assessments per application dollar has increased over the two and a half years following the implementation of reform. In order to better understand the cost of assessments and identify potential areas for efficiency savings, there is scope for the APVMA to develop systems to collect and analyse accurate and comprehensive information about its assessment related activities.
Notifiable variations
3.21 A factor in the decline of the overall number of applications was the introduction of the Notifiable Variations Scheme on 1 January 2015. The Notifiable Variation Scheme removed the requirement for regulated entities to formally apply for low risk minor variations. Notifiable variations are deemed to be granted when they are lodged with the APVMA after checks are conducted. There are currently 13 types of notifiable variations, such as changes to the name of a product and to the safety instructions. In 2015–16, 736 variation notices were lodged with the APVMA and 696 variations were issued.37 The introduction of notifiable variations would likely have resulted in time and cost savings, given that, under the pre-reform system these variations would have been lodged and processed as applications. The APVMA has not, however, retained data to enable the extent of any efficiency improvements to be assessed.
Corporate expenditure
3.22 Analysis of the APVMA’s proportion of expenditure on corporate, or non-regulatory activities, does not indicate a trend towards greater organisational efficiency—that is, a reduction in the ratio of corporate expenses to regulatory expenses. In 2014, the APVMA estimated38 that 14.6 per cent of its total expenditure was attributed to corporate functions—equating to $4 570 384 in total or $25 676 per staff member in 2012–13.39 The Authority ranked 20th compared with the 90 other entities at that time and second out of 12 regulators based on the proportion of its expenditure directed towards corporate functions.
3.23 The ANAO’s analysis of expenditure data indicates that the proportion of total expenditure that the APVMA attributed to corporate services over the previous four financial years (2012–13 to 2015–16) ranged from 35–50 per cent. The Authority’s expenditure on corporate services of $12 770 435 in 2015–16 was 38 per cent of total expenditure, equating to $64 497 per staff member, which would have placed the Authority at 87 out of 91 entities and 11 out of 12 regulators on the basis of the 2014 assessment, assuming the other entities had maintained the same proportion of corporate expenditure as in 2012–13.
Benchmarking
3.24 Benchmarking regulatory activities against mature regulators in other jurisdictions or other sectors can assist in assessing whether improvements to efficiency and effectiveness have been achieved. The APVMA does not benchmark its performance against international agvet chemical regulators. The Authority informed the ANAO that most internationally comparable jurisdictions do not regulate the supply of both agricultural and veterinary medicines in the one agency or assess efficacy of products and chemicals, and operate within significantly different legislative regimes.
Industry perspectives on regulator efficiency
3.25 While the 2014 reforms were designed to improve the efficiency and effectiveness of regulation and reduce regulatory burden on industry, the APVMA and regulated entities informed the ANAO that some of the 2014 reforms have had the opposite effect. For example, restrictions on changes to applications at the preliminary assessment stage and limits on time applicants spend on responding to APVMA requests for additional information, were considered to have added to burden on industry.
3.26 A large proportion of regulated entity survey respondents, 44 per cent, disagreed or strongly disagreed with the statement that ‘the APVMA appears to be more efficient based on their experiences with the Authority over the previous 12 months’. A further 18 per cent agreed or strongly agreed with the statement, while the remaining respondents did not express a view on the matter.
3.27 Overall, the APVMA’s performance measures and its reporting against those measures do not enable a robust and well-rounded assessment of performance against its objectives, including its achievement of greater effectiveness and efficiency over time. Given the industry and public interest in the success of current and future reform of agvet chemical regulation, there is merit in the Authority further developing its performance measures, targets and reporting, to help demonstrate the extent to which it is achieving its objectives.
Recommendation No.2
3.28 The Australian Pesticides and Veterinary Medicines Authority should establish and monitor an appropriate set of measures and targets to assess the extent to which it is improving the effectiveness and efficiency of its regulatory activities through its ongoing reform agenda.
Australian Pesticides and Veterinary Medicines Authority’s response: Agreed.
3.29 The APVMA has a range of performance measures in its corporate and regulator performance plans and has reported against these indicators in the 2015–16 Annual Report. The APVMA also publishes quarterly a detailed report of timeframe performance and regulatory activity.
3.30 The APVMA agrees that a review of the performance measures is required to ensure they best reflect the regulatory framework within which it operates and to account for expectations under the government’s Agricultural Competitiveness White Paper initiatives and the relocation of the APVMA to Armidale.
3.31 The APVMA notes that the Department of Agriculture and Water Resources has responsibility for the legislative framework for agvet chemicals and for designing regulatory reform measures and will work with the department on future reforms to ensure performance measures are clearly defined.
3.32 The APVMA notes the methods for calculating timeframe performance changed with the implementation of the legislation, making direct comparison of efficiency before and after the legislation difficult. Nevertheless, the APVMA showed significantly improved performance over 2016 with over 80 per cent of product applications being completed within legislated timeframes in the September quarter, despite a challenging operating environment relating to the announcement of the relocation of the APVMA to Armidale.
Has the regulatory burden on industry been reduced?
Overall, feedback provided by industry stakeholders indicates that the regulatory burden on industry has not reduced following the implementation of regulatory reform, while noting some improvements in relation to individual measures such as pre-application assistance and online lodgement. The absence of specific performance measures to assess regulatory burden makes it more difficult for the APVMA to ascertain changes in regulatory burden arising from the roll-out of reform initiatives.
Industry perspectives on regulatory burden
3.33 In the absence of an appropriate APVMA measurement framework, the ANAO has drawn on its interviews with industry and survey results to consider the extent to which the APVMA has reduced regulatory burden on industry. A key message arising from the ANAO’s interviews with industry stakeholders (regulated entities, regulatory consultants and peak bodies) was that, more than two years after the commencement of the 2014 reforms, regulatory burden on industry had remained the same or increased. Examples of areas where burden had increased included: the larger and more complex applications; uncertainty about application requirements; and the perceived need to ‘over-prepare’ applications to avoid potential requests for additional information once assessments had commenced.
3.34 The overall number of applications in the post-reform period is similar to the pre-reform period if the lower regulatory notifications are taken into account. There has been, however, a reduction in the number of major applications—which require product, chemical and label assessments—with these applications generally more complex and higher risk.40 The rate of major applications per month is illustrated in Figure 3.6.
Figure 3.6: Major applications submitted, July 2012 to January 2017
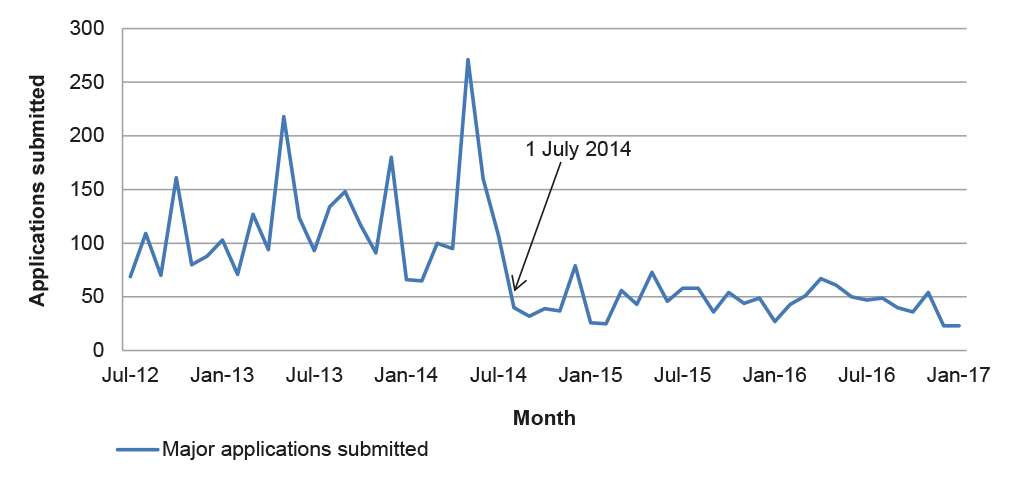
Note Major applications are those submitted under items 1–6, 10, 14 and 27 of Schedule 6 of the Agvet Code Regulations 1995.
Source: ANAO analysis of the APVMA information.
3.35 Only 13 per cent of the responses to the ANAO’s survey indicated that respondents would be more likely to bring forward chemical or agvet products for registration due to the regulatory reforms. On the basis of this feedback, and post-reform application trends, there is no evidence to suggest that legislative and operational changes have supported the reform bill’s aim to ‘encourage the development of newer and safer chemicals’.
3.36 The survey responses from regulated entities indicated that changes in regulatory costs with respect to making applications had remained about the same or had increased over the previous 12 months. In particular, the majority of respondents indicated that staff hours attributed to preparing applications, responding to issues during the application process and overall costs had remained about the same, as outlined in Table 3.3.
Table 3.3: Regulated entity views on the change in regulatory compliance costs
|
Category |
Respondents who reported a decrease in costs (%) |
Respondents who considered that costs were about the same (%) |
Respondents who reported an increase in costs (%) |
|
Staff hours spent preparing applications |
8.5 |
55.9 |
35.6 |
|
Staff hours spent responding to issues during the application process |
7.3 |
63.3 |
29.4 |
|
Overall costs |
6.2 |
50.9 |
42.9 |
Source: ANAO survey of agvet chemical regulated entities.
3.37 In contrast, when asked about the impact of specific elements of reform over the 12 months to October 2016, the views of regulated entities appeared more balanced. Online application lodgement, pre-application assistance, and the introduction of prescribed notifiable variations were identified as measures that had the greatest impact on reducing time and costs for regulated entities, as outlined in Table 3.4.
Table 3.4: Responses from regulated entities on compliance costs (by percentage)
|
Reform element |
Significantly reduced time/costs for my organisation (%) |
Somewhat reduced time/costs for my organisation (%) |
No impact (%) |
Somewhat increased time/costs for my organisation (%) |
Significantly increased time/costs for my organisation (%) |
N/A or Not sure (%) |
|
Revised written guidance (website, Section 6A Guidelines) |
0.6 |
14.4 |
33.9 |
12.8 |
10.6 |
27.8 |
|
Pre-application assistance |
1.7 |
22.8 |
32.2 |
10.6 |
8.9 |
23.9 |
|
Online application lodgement |
6.7 |
31.1 |
22.8 |
9.4 |
10.6 |
19.4 |
|
Case management of applications |
2.2 |
20.6 |
36.7 |
7.8 |
9.4 |
23.3 |
|
Preliminary assessment |
2.8 |
16.7 |
40.0 |
7.8 |
7.8 |
25.0 |
|
The introduction of prescribed/ notifiable variations |
6.7 |
22.2 |
30.0 |
7.2 |
5.0 |
28.9 |
|
Revised arrangements for the Chemical Review Program, if applicable |
1.1 |
9.4 |
35.6 |
2.2 |
6.1 |
45.6 |
Source: ANAO survey of agvet chemical regulated entities.
4. Governance arrangements
Areas examined
The ANAO examined whether the Australian Pesticides and Veterinary Medicines Authority (APVMA or the Authority) established sound governance arrangements to support the implementation of the reform program, including effective oversight, appropriate planning and sound management of risks. The ANAO also considered whether the APVMA effectively engaged industry stakeholders during the implementation process.
Conclusion
The APVMA's governance of the delivery of the reform program was not effective, with significant weaknesses in oversight, planning and risk management arrangements. In particular, the absence of an up to date implementation plan meant that committees established to oversee the implementation of the reform program were not well placed to effectively monitor the progress of projects and hold project managers to account. Further, while implementation risks were considered at an individual project level, they were not aggregated and integrated into an overarching risk management framework. Additionally, engagement with industry in relation to the reform program was undertaken when operational changes were yet to be finalised, which ultimately limited its effectiveness.
Areas for improvement
The ANAO has made two recommendations aimed at improving the APVMA's arrangements for the implementation of major reform initiatives and strengthening its approach to identifying and responding to emerging business risks.
The ANAO also made a number of suggestions for improvement, including that the APVMA develop an overarching implementation strategy or plan to coordinate and monitor the delivery of future change programs, which includes specific deliverables, timeframes, accountabilities and monitoring arrangements.
Were effective oversight arrangements established to monitor the implementation of the reform program?
The oversight arrangements to monitor the implementation of the reform program were not effective. The APVMA's governance forums lacked continuity and did not facilitate assurance on the progress of work, ultimately contributing to poor or incomplete implementation outcomes for some projects.
4.1 The key elements of the APVMA's governance arrangements to oversee the implementation of the reform program included executive management forums, internal reform focused committees and external reviews.
Executive Management
Executive Leadership Team
4.2 The APVMA Executive Leadership Team (ELT) comprises of the senior executives of the Authority and was formed in January 2013 with the commencement of the new CEO, replacing the former Executive Management Meeting. Chaired by the Chief Executive Officer (CEO), the ELT had oversight of the implementation of the reforms and core business activities. The ELT did not meet in a formal capacity in the ten weeks prior to the commencement of key reform elements in July 2014.41 Since July 2014, the ELT oversaw the implementation of the Authority's continuing reform related work and business-as-usual.
Advisory Board
4.3 An APVMA Advisory Board was created in November 2012 for a three-year term and not renewed in 2015.42 The Board was tasked with providing strategic advice and making recommendations to the CEO. The Board received updates on the implementation of various reform elements from the CEO each quarter until 1 July 2014 and provided comments and suggestions relating to reform initiatives.43 Meeting papers indicate that the Board did not provide views on the APVMA's overall preparedness to implement the reforms, although it did comment on some elements of the reform program.44
Reform oversight
Better Regulation Taskforce
4.4 In January 2011, the CEO established the Better Regulation Taskforce (BeRT) to develop and implement reform projects, and to report on project progress to the ELT.45 Key activities of the BeRT included project related decision-making, approving resource allocation, identifying critical issues, ensuring the completion of tasks and communicating with key stakeholders. The BeRT was abolished in August 2013 following concerns from the APVMA executive that reform projects were 'running in isolation and without adequate consideration or communication with other projects'.
Project Implementation Committee
4.5 A Project Implementation Committee (PIC) was subsequently established to replace the BeRT in September 2013. The committee's role was to oversee and coordinate reform projects, with an emphasis on ensuring an integrated rollout.46 Project reports that were prepared by the committee and provided to the ELT included information on key risks and challenges to implementation. The reports also outlined the progress of individual projects (in terms of percentage completed). However, the quality of reporting was variable47 and the reported progress of some projects over time decreased in some instances.48
4.6 As was the case with the BeRT, the PIC did not effectively coordinate and progress reform projects. Roles and responsibilities for some reform projects were not clearly defined. The minutes from committee meetings indicated that committee members were concerned about a 'lack of commitment and prioritisation by key project staff' and gaps in information sharing across teams and from senior management, leading to a 'number of issues' (the nature of these issues was not captured in meeting minutes). In February 2014, when the implementation of the reforms was estimated to be 42 per cent complete, the ELT discussed actions to 're-invigorate PIC', emphasising the need for commitment by senior management.
CEO project tracking
4.7 In the period from May to June 2014, the CEO co-chaired weekly meetings with a consultant to review the status of individual projects and to identify critical issues to be resolved. The APVMA informed the ANAO that the meetings were attended by executive staff and senior leaders, most of whom were members of the PIC. A project implementation stocktake was updated for each meeting49, although deliberations and decisions made at those meetings were not recorded.
Major Projects Board
4.8 In October 201550, a Major Projects Board (MPB) was established by the CEO to oversee key projects and to support the Authority's objective to become a 'contemporary world class regulator'. The Board comprises ELT members, executive managers and project leaders and meets monthly. Of the projects under the oversight of the Board, two relate to the 2014 reforms: the Lower Regulatory Approaches to Registration Project and the Internal Portals Project.
Additional oversight
4.9 In August 2013, the APVMA commissioned a review to assess its business processes and identify improvements to regulatory governance, organisational structure and supporting systems. The review found that activities were being undertaken in an uncoordinated manner and noted that responsibility for business improvement outcomes was 'diffused through the APVMA'. The review identified the 'need for more effective coordination of effort', including the resolution of 'differences between the managers of individual process components'. In response to this review, a restructure was undertaken in October 2014, which was designed to group common functions together and promote cooperation across the organisation.51
4.10 A follow-up review conducted in March 2016, found that the leadership team was not 'united, purposeful or effective in driving and supporting change'. The reviewer identified a number of specific issues contributing to this finding:
- Executive Directors have not followed through on implementation of agreed changes;
- weak processes and systems for monitoring, reporting and follow-up;
- governance committees have blocked rather than facilitated change; and
- critical decisions have been deferred or avoided.
4.11 The review identified eight 'major achievements' against outcomes of the 2013 review and eight areas that required further work, including an integrated end-to-end registration process, peer review of decision-making and capability development.
Audit Committee
4.12 The Audit Committee received periodic, high level updates on the implementation of the reform program. In February 2014, the APVMA advised the committee that major reform initiatives, including the internal and external portals for electronic application management, were on track for completion by July 2014. The committee noted that the status of the reform project was estimated at 40 per cent complete with four months remaining. According to the minutes of the meeting:
The Chair noted the lack of a formal, written change management strategy, and indicated it was unclear how all three streams of the transformation strategy (i.e. systems, processes, and regulatory) would come together as an integrated solution (i.e. future state model). The Chair also raised a concern about the capacity of the APVMA to deliver the regulatory reforms by the end of June 2014.
4.13 The APVMA's governance arrangements for the implementation of reform did not effectively facilitate assurance on the progress of work, ultimately contributing to poor or incomplete implementation outcomes for some projects, as discussed in Chapter 2. For future implementation of major reform initiatives, such as the work required to support the relocation of the Authority in 2019, the APVMA should strengthen its governance arrangements.
Recommendation No.3
4.14 The Australian Pesticides and Veterinary Medicines Authority should improve its governance of the implementation of major reforms, including the maintenance of an oversight body with clearly defined responsibilities and robust project monitoring arrangements.
Australian Pesticides and Veterinary Medicines Authority's Response: Agreed.
4.15 The APVMA notes the governance arrangements for the implementation of the legislation in 2014 were inadequate and recognises that ongoing effort is required to ensure appropriate governance arrangements are in place for major initiatives.
4.16 Following the experiences in 2014, the APMVA established a Project Board, along with a dedicated team, to provide oversight of the key reform projects being progressed under the Agricultural Competitiveness White Paper. This provides for a coordinated approach to implementation planning preparation of project documentation, identification and management of risks, anticipated benefits and budget management. The Project Board reports to the executive leadership team on a monthly basis.
4.17 Governance arrangements for the relocation of the APVMA to Armidale are in place with a dedicated executive leadership team and a steering committee established. There is also the APVMA Relocation Advisory Committee which meets monthly to provide advice on various aspects of the relocation with members drawn from industry, the Armidale Council, the University of New England and also the Department of Agriculture and Water Resources.
Were appropriate planning processes established for the implementation of the reform program?
The APVMA did not establish an effective planning framework to guide the implementation of the reform program. In the absence of such a framework, the numerous governance committees that were established over the course of reform program implementation lacked an appropriate basis on which to oversee progress and hold project managers to account.
Implementation planning
4.18 In July 2011, the BeRT developed a high-level implementation plan for the anticipated 2014 reforms. The plan defined the roles and responsibilities of staff involved, identified broad outputs, outlined risks, and provided for the plan to be updated as necessary. Key outputs to be delivered under the plan included:
- amendments to legislation (act, regulations);
- the development of legislative support tools;
- the introduction of a re-registration scheme;
- documentation of principles and procedures for re-registration priorities and intervals;
- documentation of a risk analysis framework;
- revised business processes to reject poor quality applications; and
- changes to assessment timeframes.
4.19 The APVMA did not update this plan or develop a consolidated strategy for the implementation of the reform program once the revised legislation was passed in June 2013. The absence of an up to date overarching plan for the implementation of the reform program limited the effectiveness of governance committees tasked with overseeing the progress of multiple individual projects. These oversight committees operated without a structured 'roadmap' outlining interim milestones, identifying cross-organisational dependencies and aligning diverse activities with broader reform objectives.
4.20 It was not until November 2013 that the APVMA produced a single, consolidated list of projects to identify and track the implementation of reform related work (as discussed in Chapter 2). Significant revisions were made to this list of projects on two occasions as the Authority reconsidered its priorities (see Table 2.1).52
4.21 The absence of an appropriate planning framework also resulted in the progression of individual reform projects without adequate planning. Of the 13 key reform projects overseen by the PIC, plans of some form, were developed for eight.53 A consolidated document setting out the purpose, scope, deliverables, roles and responsibilities, risk assessment, monitoring and reporting and timeframe was not produced for any of the projects. Further, in a number of cases, the plans were not endorsed by the Executive. The APVMA did not develop plans for the remaining five initiatives.54
4.22 To better support the achievement of major reform objectives, the APVMA should establish an effective planning framework to clearly articulate planning requirements.
Were implementation risks appropriately managed?
The risks to the effective implementation of the reform program were poorly managed by the APVMA. While an appropriate risk management framework is in place, high level business risk reviews were not conducted regularly and risks to the implementation of the reform program were not adequately managed. Further, risk management at the individual project level was inconsistent and treatments for risks in relation to the retention of staff capability have not been effective.
Risk management
Organisational business risk management
4.23 The APVMA's risk management policy is based on the Standards Australia/Standards New Zealand Standard Committee's Risk Management Standard—ISO 31000:2009 (Risk Management).55 The policy was updated in 2015 to reflect the requirements of the Commonwealth Risk Management Policy under the Public Governance, Performance and Accountability Act 2013. However, the Authority is yet to articulate its risk appetite and tolerances as required by the Commonwealth Risk Management Policy.
4.24 The APVMA's Risk Management Plans for 2013–2014 and 2015–2017 set out an appropriate framework for the identification, assessment and monitoring of risks and treatments. Further, the organisational business risk register template contained a suitable range of considerations, including risk descriptions, responsible officers, consequences, likelihood, ratings and treatments.
Managing the risks to implementing the reform program
4.25 The Authority considered risks in relation to the implementation of reform as part of its risk management process. Of the 39 risks listed in the Authority's register from February 2013 to May 2015, the following related to the APVMA's ability to deliver the reform program:
unable to (fully) implement new legislation (by 1 July 2014); Re-registration scheme not repealed; maintenance of core services while implementing new legislation.56
4.26 The treatment established to manage this risk was the development of a contingency plan in the event that the re-registration and re-approval legislation was not repealed.57 This risk was rated 'Moderate', increased to 'Extreme' (February 2014 to September 2014), reduced to 'High' (September 2014 to May 2015) and subsequently to 'Low' in May 2015. The framing of this risk, to include three separate components, made it more difficult for the APVMA to assess each component or establish and monitor the implementation of appropriate and specific mitigation measures.
4.27 The identification and monitoring of risks relating to individual projects was outlined in project status reports to the PIC and ELT, but these risk updates did not include information on mitigation strategies. For five of the key projects, the risks identified often related to general issues such as inadequate resourcing and timeframes, dependencies on other projects and IT capability. Project and application risks were not assessed as part of either the internal and external portals project, particularly the risk of system and data integrity when migrating the legacy systems to a new environment.58
4.28 Overall, the APVMA's documented review of reform implementation risks was incomplete and irregular, with no documentation retained by the Authority to evidence the conduct of risk reviews in the 18 months to December 2016. The risk register was developed in February 2013 and was updated quarterly from November 2013 to May 2015.59 In the May 2015 version of the register, three of the sixteen 'Extreme' or 'High' risks had not been assigned treatments. Due to the limited records retained by the APVMA, it was not clear that the Executive had considered the risks on a regular basis.
4.29 In January 2017, the APVMA informed the ANAO that risks had been carefully considered as part of its business management processes and acknowledged that it had not been documented since 2015. In the absence of relevant documentation, the APVMA is unable to demonstrate that a regular and structured approach to the monitoring of risk and the implementation of mitigating strategies was in place.
Relocation risk management
4.30 The Australian Government committed, as part of the 2016 election, to relocate the APVMA to Armidale, NSW, consistent with its policy of promoting employment growth in regional communities.60 In July 2015, the CEO advised the Minister for Agriculture that, with only 10 per cent of staff willing to relocate, including seven regulatory scientists, 'the magnitude of the expected loss of expertise and experience is such that I would have concerns about my ability to perform my legislative responsibilities should the APVMA relocate'.61
4.31 The 2016 cost benefit and risk analysis of the potential relocation, commissioned by the Department of Agriculture and Water Resources, noted the risk of retaining staff during the relocation process:
The most significant risk identified through the analysis relates to the ability of the APVMA to relocate, or to recruit and replace, key APVMA executive, management and technical assessment staff within the first two years of relocation. Critically, the loss of technical assessment staff (regulatory scientists) has the potential to seriously disrupt the ability of the APVMA to successfully fulfil its purpose and achieve its objectives in the short and medium term.62
4.32 In 2016, the APVMA developed a relocation strategy to inform Government decision-making in relation to the proposed relocation. This strategy was provided to the Government as an attachment to the department's submission seeking the Government's agreement to relocate the Authority. Key business continuity risks associated with the relocation were covered in the submission. The mitigating measures outlined in the strategy included:
- a multi-year phased approach to relocation to be completed in 2019;
- a transition team led by a senior executive, overseen by a Relocation Advisory Committee, with members from government, industry and the Armidale community;
- the development of a new business model to identify key business processes and how they will be performed by the APVMA in Armidale, including a new management structure;
- the implementation of a new digital strategy to better manage data, enable a fully mobile digital workplace and streamline and automate business systems; and
- staff retention and recruitment strategies such as:
- providing additional incentives for regulatory scientists to move to Armidale;
- establishing mechanisms for regulatory scientists to work remotely (from their homes) if they do not move to Armidale;
- providing incentives for regulatory scientists who intend to retire or transfer to other employment opportunities in Canberra to stay at the APVMA until the relocation occurs to train new recruits and/or continue undertaking assessments; and
- establishing approaches to source regulatory expertise from now until the APVMA is fully operational in Armidale.
4.33 Notwithstanding these plans, the CEO advised the Minister that 'there will be a reduction in the APVMA's scientific capability over the next few years while we rebuild capacity and implement a new business model'.
Staff retention
4.34 The APVMA's ability to maintain regulatory activities largely depends on its retention and recruitment of staff as it transitions to Armidale. The rate of staff departures from the APVMA has increased since 2014, coinciding with speculation about the relocation of the Authority in advance of the Government's decision in November 2016. In 2015–16, the Authority's staff departure rate grew to 18.7 per cent, more than double the Australian Public Service average of 7.1 per cent, as illustrated in Figure 4.1.
Figure 4.1: APVMA staff departure rates, 2013–14 to 2015–16
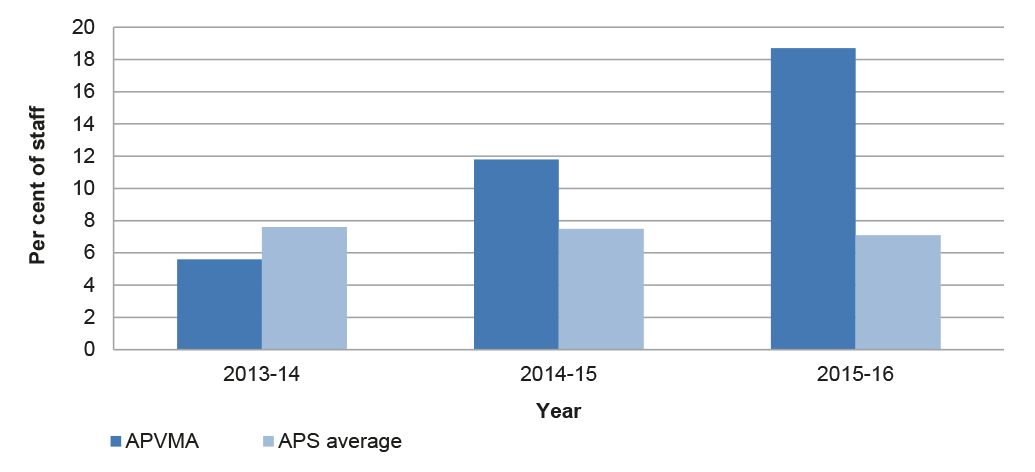
Source: ANAO analysis of APVMA and Australian Public Service Commission information.
4.35 In late 2016, the CEO informed the Government that the Authority was experiencing a 'higher than normal rate of staff departures' and sought feedback from industry peak bodies on options to prioritise the APVMA's assessment workload. The CEO noted that the Authority had exhausted all avenues to fill regulatory scientist vacancies in its residues team and that there were staffing pressures in the pesticides, health, environment and chemical review teams.63 Almost one quarter (48) of the Authority's workforce had departed in the period July 2016 to February 2017, including 20 regulatory scientists. Over the same period, the Authority reported that 57 staff commenced, most being non-ongoing, particularly in IT and case management and administration.
4.36 While the APVMA considered risks related to staff capability in its business risk register, it did not consider the risks associated with the proposed relocation. The November 2013 version of the register included one risk relating to the potential 'failure to maintain a sufficiently capable motivated base of human resources' and 'maintain or access contemporary scientific skills'.64 This was rated 'Moderate' until late 2014, after which it was upgraded to 'High', due to a raised likelihood rating. One existing control was identified—strong networks with other regulators and the scientific community. Three treatments were proposed between November 2013 and November 2014, relating to the examination of external employment contracts, increased recruitment via tender, and renewed outreach to universities. In May 2015, one of these measures had been marked complete65 while one had been discontinued.66 As at December 2016, the APVMA's primary approach to maintaining staff capability is through rolling recruitment campaigns.
4.37 Given identified weaknesses in the APVMA's risk management practices (noted at paragraph 4.26–4.28), the Authority is not well positioned to manage risks associated with staff retention as it prepares to relocate to Armidale. To address its changing risk profile, the APVMA should implement a robust risk management framework and oversight arrangements to support its treatment strategies and manage the impact of business continuity threats.
Recommendation No.4
4.38 The Australian Pesticides and Veterinary Medicines Authority should implement a structured and systematic approach to identifying and responding to emerging business risks.
Australian Pesticides and Veterinary Medicines Authority's response: Agreed.
4.39 The APVMA has undertaken a review of its approach to managing business risk, resulting in a revised risk management framework and an updated strategic enterprise risk profile, which is reviewed at monthly executive leadership meetings.
4.40 Risks relating to reform activities being progressed under the Agricultural Competitiveness White Paper risks are addressed in each project plan and monitored by the Project Board.
4.41 Risk management relating to the relocation of the APVMA has been identified as a high priority with specific resources being engaged to identify, monitor and mitigate relocation-related risks. The APVMA Relocation Advisory Committee has a standing agenda item relating to risk and the Relocation Steering Committee has direct oversight of risk management, reporting monthly to the APVMA executive leadership team.
Were industry stakeholders effectively engaged during the implementation of the reform program?
Industry stakeholders were engaged during the implementation of the reform program via a range of information and training sessions, but delays in the finalisation of reform projects limited the extent to which specific changes at the operational level were communicated. The feedback provided by industry stakeholders to the ANAO on the APVMA's engagement approach was mixed.
Stakeholder engagement
4.42 The effective implementation of reform relies on sound stakeholder engagement and feedback. As discussed in Chapter 2, the APVMA undertook public consultation on its draft regulatory guidelines in 2014, prior to the commencement of the reform program. The Authority also undertook consultation on other reform related activities in 2014–15 and 2015–16.67
Industry information and training
4.43 APVMA delivered a range of industry information and training programs in preparation for the legislative reforms, including:
- February and March 2014—six industry information sessions were conducted. Over 400 representatives attended the sessions, which provided a high-level overview of the changes to regulation under the reform program;68
- April 2014—an industry forum that covered a range of topics, including the long term reform agenda for the APVMA;
- June 2014—two training workshops were conducted to provide practical assistance to industry representatives before the commencement of the reform program. The workshops were attended by 190 industry representatives;
- September 2014—a two-day series of online services training sessions was conducted for industry and completed by 62 participants. The sessions included training on how to navigate the new systems and provided information on planned improvements;
- 2015—three industry information and education events were held in Sydney, Melbourne and Canberra. These events were developed in consultation with industry and were intended to provide a working knowledge of changes under the reform program, including pre-application assistance, compliance and chemical review; and
- 2016—two information and education sessions were held in Canberra and Melbourne to brief industry on ongoing reform.
4.44 While the APVMA delivered information and training to industry early, the APVMA's information and training for industry was delivered when reform related projects were incomplete, including pre-application assistance and the application portal. Further, transitional arrangements had not been fully determined.69 Consequently, APVMA systems and processes lacked maturity at this time to appropriately inform training content for industry stakeholders. While early training sessions related to relevant legislative changes and were intended to provide a high-level overview only, the APVMA was not well positioned to respond to queries on operational changes and new requirements.
Industry views
4.45 Initial feedback from industry on the APVMA information and training sessions was positive. According to the results of a survey conducted by the APVMA on the information sessions held in February and March 2014, 93 per cent of the 179 respondents considered the session to have been useful. The APVMA reported that 94 per cent of participants who had attended the training workshops in June 2014 believed the training had improved their readiness for the legislative changes. Feedback on the online services training in September 2014 was also generally positive.
4.46 Of the responses to the ANAO's survey of regulated entities, 33 per cent of respondents indicated that they had the opportunity to provide feedback on APVMA's operational policies and practices under the reforms. Of these, 17 per cent considered that their feedback had been used to help shape the APVMA's initiatives to give effect to regulatory reform. Industry responses on the quality of the APVMA's communications about reform were mixed, with 22–35 per cent indicating that they were satisfied or very satisfied, while 19–26 per cent of respondents indicated that they were dissatisfied or very dissatisfied.70
Appendices
Appendix 1 Entity response


Footnotes
1 The scheme commenced in 1995 following an inter-governmental agreement between Australian, state and territory government agriculture ministers.
2 Reviews include: Productivity Commission, Chemicals and Plastics Regulation Research Report, 2008; Department of Agriculture, Fisheries and Forestry, Better Regulation of Agricultural and Veterinary Chemicals, 2011; Gibbs, C., Harris-Adams, K. and Davidson, A., Review of Selected Regulatory Burdens on Agriculture and Forestry Businesses, 2013, Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra.
3 The department received 92 submissions in response to the discussion paper.
4 The reforms also included the introduction of a mandatory scheme for re-approval and re-registration of registered products. These provisions were repealed by the subsequent Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Act 2014.
5 Australian Government, Agricultural Competitiveness While Paper, 2015, p. 37–38.
6 The agreed funding for the relocation of the APVMA excludes the cost of implementing a new digital strategy for the Authority. The final costs and offsets for the digital strategy are to be agreed with the Minister for Finance. On 9 June 2017 the Senate Finance and Public Administration Committee reported on its inquiry into the Operation, effectiveness, and consequences of the Public Governance, Performance and Accountability (Location of Corporate Commonwealth Entities) Order 2016.
7 Productivity Commission, Regulation of Australian Agriculture, Report, 2017, p. 303. The 2014 legislative reforms also removed impediments to the APVMA’s use of international evidence.
8 The review was required by regulation 80E of the Agricultural and Veterinary Chemicals Code Regulations 1995.
9 Safe Work Australia is a national body, comprising 15 members, including representatives of the Commonwealth, all states and territories and industry associations and trade unions, responsible for developing uniform work health and safety legislation.
10 The ANAO invited 708 agvet chemical product registrants, and active, permit, and licence holders who had made applications to APVMA in the period July 2014 to June 2016, to participate in its voluntary online survey of regulated entities. The ANAO received 203 responses (28 per cent response rate) to the survey, including responses completed by regulatory consultants on behalf of their clients.
11 The funding was allocated for a scientific review panel and business system enhancements, including the establishment of an electronic records management system.
12 Debate in the House of Representatives on the Bill was adjourned to March 2013 and May 2013.
13 The APVMA’s governance arrangements for the implementation of the reform program and the various modifications to its approach over time are examined further in Chapter 4.
14 Legal Interaction, Expert Advice, Risk Framework, Overseas Assessment, Science Panel, Re-registration, Compliance and Business Improvement.
15 When the public consultation period closed in April 2014, the APVMA had received 19 submissions on the guidelines, published summaries on its website and outlined its intended response.
16 The APVMA did not develop a work plan to follow up on the findings of this review or monitor implementation.
17 Missing or incomplete project documentation included: the project business case; project plan; risk management; requirement gathering; user and stakeholder engagement; budget and cost management; stakeholder review; approval and sign-off.
18 The CEBRA was established 2013, through an agreement between the University of Melbourne, Agriculture and the New Zealand Ministry for Primary Industries, to help ensure policy and regulatory response to biosecurity risk is underpinned by world-class research.
19 The introduction of notifiable variations is discussed further in Chapter 3.
20 The Crop Groupings Project, which commenced in November 2015, is expected to continue until late 2017. Progress on the second project, the Permits to Labels Project that was established in July 2015, has been limited.
21 New legislative requirements included the preparation of work plans for all new and current reconsiderations and the introduction of prescribed timeframes for each reconsideration, with a maximum period of 57 months.
22 The APVMA also introduced a risked-based nomination and screening process for developing its future chemical review work program, although it is yet to accept nominations under the new arrangements.
23 For an example of better practice collection and analysis of compliance intelligence, see ANAO, Administration of the Domestic Fishing Compliance Program, Audit Report No. 20, 2012–2013.
24 Toxicology assessments of products include the toxicity of the active constituent and the other ingredients in the context of the whole product formulation.
25 The ANAO examined the APVMA’s assessment of 100 ‘major’ applications. The ANAO’s examination included reviewing preliminary assessments; technical assessments such as chemistry, efficacy and toxicology reports; key decision documents; and notices.
26 The application relating to the misplaced confidential commercial information was initially refused by the APVMA, as it was assumed there was insufficient data to support the application. The applicant wrote to the APVMA outlining its concerns over the missing data and requested that the APVMA return the missing CD and provide assurance that the commercially sensitive data was secured.
27 Scheduled or unscheduled leave was cited in the records relating to 15 assessments.
28 The majority of evaluations commenced on the day payment was received from the applicant or a few days after, although there were six evaluations that commenced between three and five weeks after payment was received.
29 An application is categorised as requiring assessments in line with a particular item of the Agvet Code Regulations, and where required, it is assigned particular tests, such as a comprehensive chemistry assessment. At any time after preliminary assessment, the APVMA may determine to recategorise an application to the appropriate item, assessment test, or testing level. A recategorisation of an application may change the cost, duration and number of tests required to be undertaken for the assessment.
30 The Quality Decision Making Project was suspended until April 2017 due to a reallocation of resources towards the Future State Process Mapping Project as part of the Digital Strategy. The Digital Strategy is discussed further in Chapter 4.
31 The Regulator Performance Framework is intended to increase the transparency and accountability of Commonwealth regulators. The framework has applied from 1 July 2015. Further information about the framework and the KPIs is available from: < https://www.cuttingredtape.gov.au/resources/rpf/reviewing-performance> [accessed 23 February 2017].
32 The 2016–17 Operational Plan listed six major areas of focus in place of the previous nine key result areas, and includes key activities and performance measures, noting not all measures include performance targets. The ANAO focused its review on the APVMA’s Operational Plan 2015–16 due to the alignment of that plan with its Corporate Plan 2015–20 and annual performance statement.
33 Governance arrangements are discussed further in Chapter 4.
34 Under the ‘clock time’ methodology, the ‘clock’ was ‘stopped’ when a request for additional material was made by the APVMA. Performance against assessment timeframes was calculated using days on which the clock was ‘on’.
35 These overdue assessments primarily involve the following application categories: items 7 and 10, registration of a chemical product containing an approved chemical, and approval of the product label; and 21, minor use permits.
36 The six indicators, matching six of the nine key result areas in the 2015–19 Corporate Plan, were: Unnecessary impediments to the efficient operation of regulated entities are removed; Communication with regulated entities is clear, targeted and effective; Actions undertaken by regulators are proportionate to the regulatory risk being managed; Compliance and monitoring approaches are streamlined and coordinated; Regulators are open and transparent in their dealings with regulated entities; Regulators actively contribute to the continuous improvement of regulatory frameworks.
37 Previously, the APVMA did not collect separate data on variation notices.
38 These estimates were included in data provided to the Australian Government’s National Commission of Audit. The APVMA did not record the method by which it had calculated its estimated corporate expenditure.
39 The Commission collected data from 91 entities on eight corporate functions: human resources; finance; legal; communications; general management; compliance; procurement; and information and communication technology (ICT).
40 The APVMA informed the ANAO that changes in the number of major applications submitted in the post-reform period primarily relate to changes in industry use of item 14 applications (under of Schedule 6 of the Agvet Code Regulations 1995, particularly for veterinary applications, changes to chemicals or overseas manufacturers, subject to Agvet Code Regulations changes.
41 One informal meeting was held on 1 May 2014 to discuss the implementation of the legislation, however, no record was retained of decisions made during this meeting.
42 The Board consisted of nine part-time external members. In 2015, the Minister for Agriculture and Water Resources announced the Government’s intention to abolish the Board as part of broader deregulation measures packaged as the Spring Repeal Day. The Agvet (Administration) Act 1992 is yet to be amended to formally abolish the Board.
43 Namely the risk based assessment framework, the regulatory guidelines and the compliance and enforcement strategy.
44 In February 2014, the Board noted ‘excellent progress’ in developing the guidelines and provided a number of suggestions for improvements to the guidelines and online application tools.
45 BeRT consisted of a Steering Group, a Change Agent Group and a Taskforce and was sponsored by the CEO who attended Steering Group meetings. The Steering Group comprised of Executive Managers from affected business areas, project leaders and other staff.
46 PIC was chaired by the Executive Director, Legal and Strategic Coordination Program and comprised program managers from across the APVMA who were responsible for delivering reform projects.
47 The ANAO assessed a sample of five projects that had been identified as priorities by the APVMA. In the progress reports, a methodology or justification was not provided to demonstrate how the percentage completion of each project was calculated. In some instances, this percentage decreased over time without explanation. Further, most reports did not make clear if/how previously reported risks had been addressed.
48 Reports did not indicate whether this was due to an expansion of project scope or a previous overestimation of project progress.
49 Stocktakes were updated for 9 May, 14 May, 22 May, 29 May, 5 June, 19 June and 26 June 2014.
50 Regular meetings commenced in December 2015.
51 Other recommendations of the review included the establishment of an executive committee to ensure coordination between the registration process and the science quality, legal, compliance and corporate functions. This recommendation was noted for consideration but was not implemented.
52 The Project Implementation Schedule was revised on 10 December 2013, 6 March 2014, 28 March 2014, 8 April 2014, 15 April 2014 and 15 May 2014; however, most changes were minor (for example, to accommodate the addition of deliverables).
53 Project plans were developed for the Regulatory Guidelines Content, Website Redevelopment and Practice and Procedures Projects (however they did not include plans for evaluation or review). Other planning documents were prepared for Legislative Reform Training Project, Chemical Review, Consultation Project, Case Management and Reform Communication Implementation.
54 Compliance, Legal Implementation, Pre-Application Assistance, Registration Application Assessment and Permits.
55 Standards Australia/Standards New Zealand Standard Committee, AS/NZS ISO 31000:2009, Risk Management-Principles and Guidelines, November 2009.
56 After May 2014 ‘Re-registration scheme not repealed’ was removed from the wording and ‘implement’ was changed to ‘fully implement’.
57 This treatment had no status.
58 Project risk management is now included within the Major Projects Board’s oversight responsibilities, although meeting papers indicate that in practice the consideration of risk is inconsistent.
59 As at December 2016, the risk register had not been updated since May 2015.
60 The Australian Government’s decision to relocate the APVMA in November 2016 ended two years of public speculation about the future of the Authority.
61 The CEO also noted her willingness to work with the Minister to give effect to any government policy order in line with her statutory obligations as the Accountable Authority.
62 EY, Cost benefit and risk analysis of the potential relocation of the APVMA, 2016, p. 4.
63 In response to the CEO, agvet industry peak bodies requested that the APVMA continue with its current queue system based on first in, first served.
64 Later reworded to ‘failure to maintain a sufficient pool of scientific advisers’.
65 ‘Examine professional indemnity insurance in contractors employment contracts’
66 ‘Reinvigorate the vacation scholars program’.
67 For example, consultation on the re-design of the Pre-Application Assistance Program.
68 The industry information sessions were held in in Melbourne, Perth, Canberra, Adelaide, Brisbane and Sydney.
69 The APVMA estimated that the portal work was 60 per cent complete at 20 March 2014.
70 The ANAO asked separate questions regarding the APVMA’s communication on reforms included the background, purpose, main features, timeframes, plans for implementation and impact on industry of the reforms. The number of respondents who reported that they were satisfied or very satisfied ranged from 22 to 35 per cent.